Association of TNF-R1 with Exercise Capacity in Asymptomatic Hypertensive Heart Disease—Mediating Role of Left Ventricular Diastolic Function Deterioration
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Protocol
2.3. Blood Assay
2.4. Echocardiography
2.5. 24 h ABPM
2.6. Cardiopulmonary Exercise Testing and Exercise BP
2.7. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Echocardiographic Characteristics
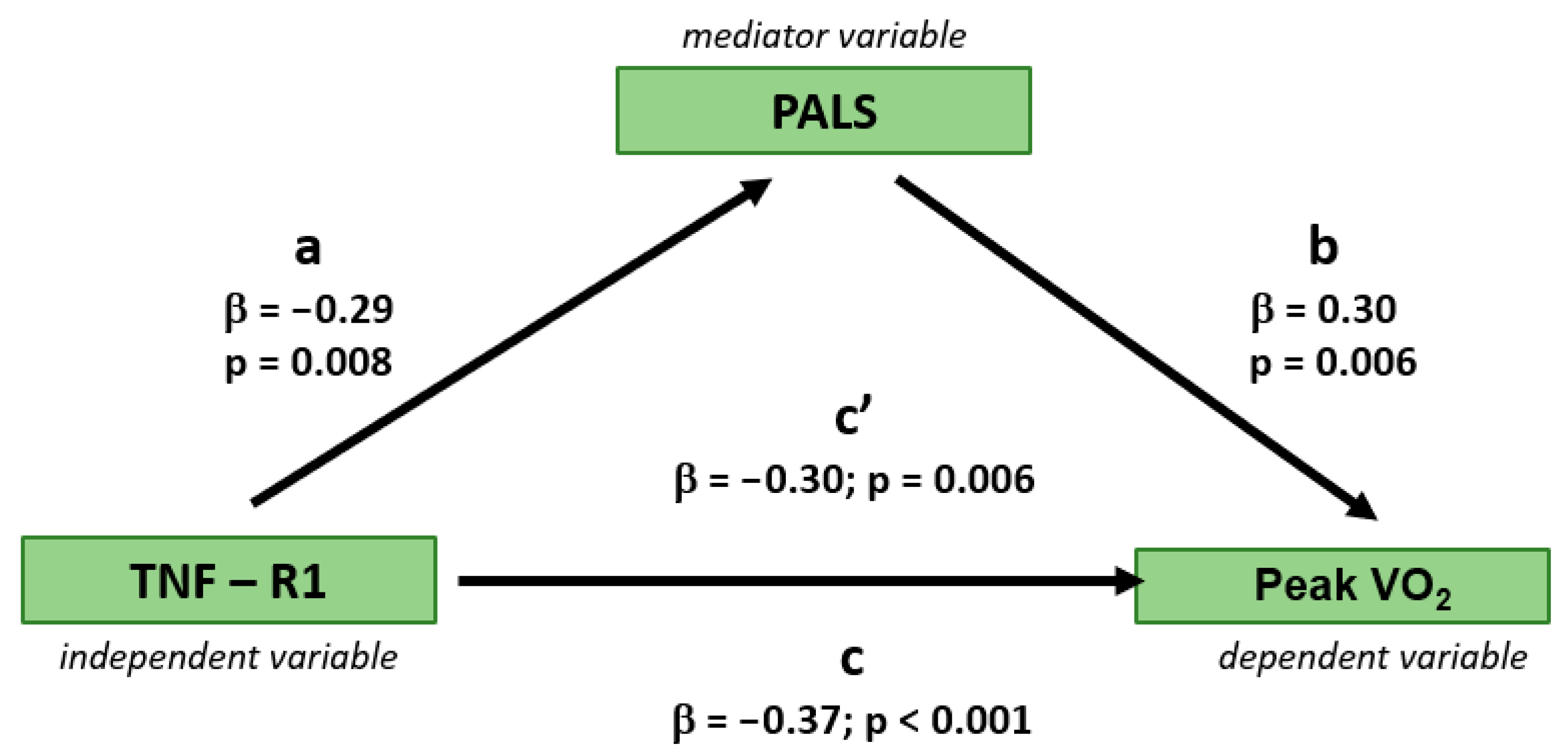
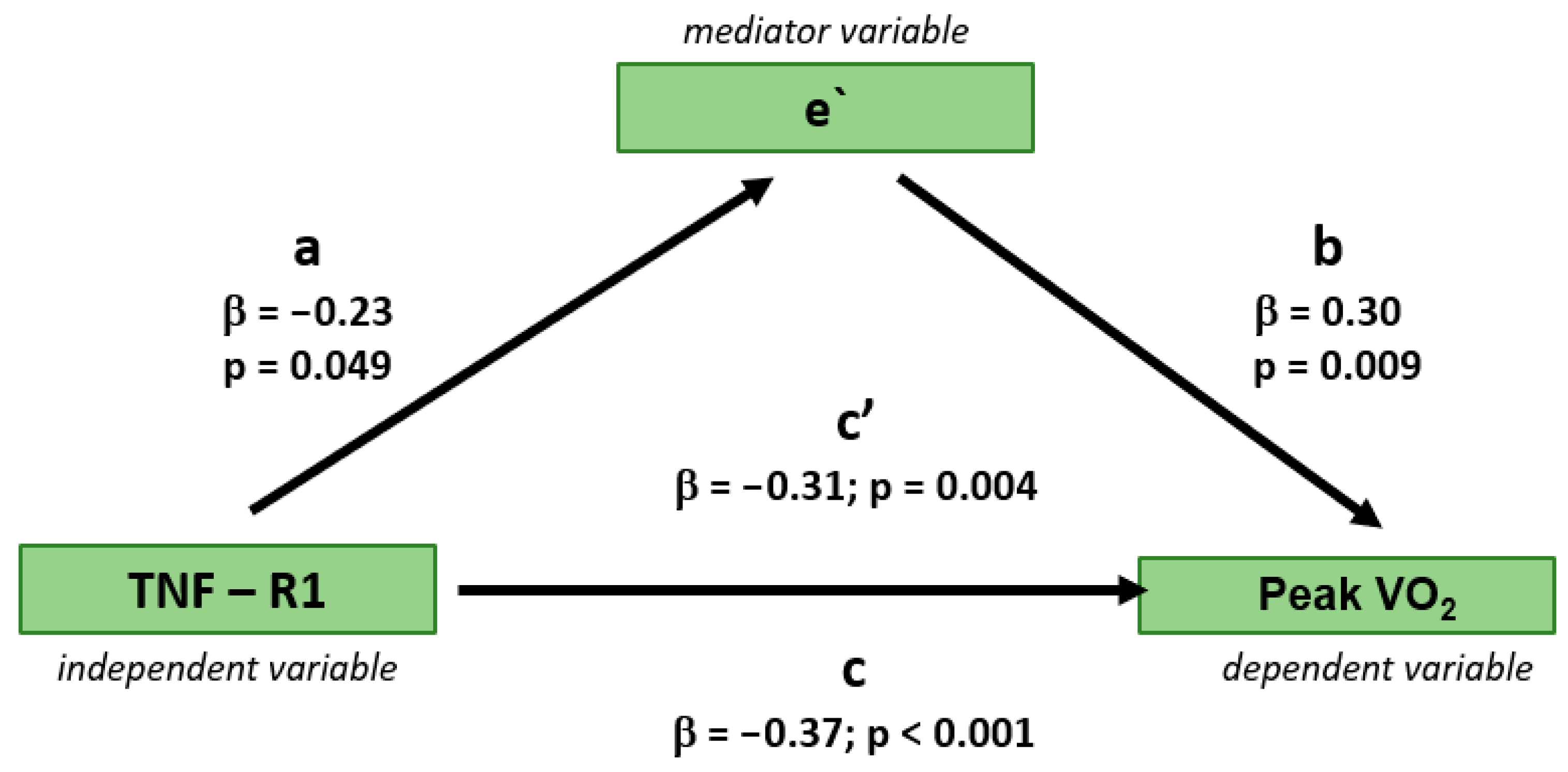
3.3. Independent Determinants of Peak VO2
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABPM | Ambulatory Blood Pressure Monitoring |
| eGFR | Estimated Glomerular Filtration Rate |
| HHD | Hypertensive Heart Disease |
| HF | Heart Failure |
| HFpEF | Herat Failure with preserved Ejection Fraction |
| HFrEF | Herat Failure with reduced Ejection Fraction |
| GLS | Global Longitudinal Strain |
| LVEF | Left Ventricle Ejection Fraction |
| LAVI | Left Atrial Volume Index |
| NT-proBNP | N-terminal prohormone of brain natriuretic peptide |
| PACS | Peak Atrial Contraction Strain |
| PALS | Peak Atrial Longitudinal Strain |
| Peak VO2 | Peak Oxygen Uptake |
| TNF-R1 | Tumour necrosis factor receptor-1 |
References
- Tadic, M.; Cuspidi, C.; Marwick, T.H. Phenotyping the hypertensive heart. Eur. Heart J. 2022, 43, 3794–3810. [Google Scholar] [CrossRef]
- Díez, J.; González, A.; López, B.; Querejeta, R. Mechanisms of disease: Pathologic structural remodeling is more than adaptive hypertrophy in hypertensive heart disease. Nat. Clin. Pract. Cardiovasc. Med. 2005, 2, 209–216. [Google Scholar] [CrossRef]
- Dos Passos, R.R.; Santos, C.V.; Priviero, F.; Briones, A.M.; Tostes, R.C.; Webb, R.C.; Bomfim, G.F. Immunomodulatory activity of cytokines in hypertension: A vascular perspective. Hypertension 2024, 81, 1411–1423. [Google Scholar] [CrossRef] [PubMed]
- Rolski, F.; Błyszczuk, P. Complexity of TNF-α signaling in heart disease. J. Clin. Med. 2020, 9, 3267. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 2018, 36, 1953–2041. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, S.B.; Abhayaratna, W.P.; Leano, R.; Stowasser, M.; Sharman, J.E. Waiting a few extra minutes before measuring blood pressure has potentially important clinical and research ramifications. J. Hum. Hypertens. 2014, 28, 56–61. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef]
- Schultz, M.G.; Sharman, J.E. Exercise hypertension. Pulse 2014, 1, 161–176. [Google Scholar] [CrossRef]
- Stergiou, G.S.; Giovas, P.P.; Gkinos, C.P.; Tzamouranis, D.G. Validation of the A&D UM-101 professional hybrid device for office blood pressure measurement according to the International Protocol. Blood Press. Monit. 2008, 13, 37–42. [Google Scholar] [CrossRef]
- Myers, J.; Arena, R.; Franklin, B.; Pina, I.; Kraus, W.E.; McInnis, K.; Balady, G.J. American Heart Association Committee on Exercise, Cardiac Rehabilitation, and Prevention of the Council on Clinical Cardiology, the Council on Nutrition, Physical Activity, and Metabolism, and the Council on Cardiovascular Nursing. Recommendations for clinical exercise laboratories: A scientific statement from the American Heart Association. Circulation 2009, 119, 3144–3161. [Google Scholar]
- Wei, W.; Tölle, M.; Zidek, W.; van der Giet, M. Validation of the mobil-O-Graph: 24 h-blood pressure measurement device. Blood Press. Monit. 2010, 15, 225–228. [Google Scholar] [CrossRef]
- Gozdzik, A.; Marwick, T.H.; Przewlocka-Kosmala, M.; Jankowska, E.A.; Ponikowski, P.; Kosmala, W. Comparison of left ventricular longitudinal systolic function parameters in the prediction of adverse outcome in heart failure with preserved ejection fraction. ESC Heart Fail. 2021, 8, 1531–1540. [Google Scholar] [CrossRef]
- Jasic-Szpak, E.; Marwick, T.H.; Donal, E.; Przewlocka-Kosmala, M.; Huynh, Q.; Gozdzik, A.; Woznicka, A.K.; Jankowska, E.A.; Ponikowski, P.; Kosmala, W. Prediction of AF in Heart Failure With Preserved Ejection Fraction: Incremental Value of Left Atrial Strain. JACC Cardiovasc. Imaging 2021, 14, 131–144. [Google Scholar] [CrossRef]
- Kleinbongard, P.; Schulz, R.; Heusch, G. TNFα in myocardial ischemia/reperfusion, remodeling, and heart failure. Heart Fail. Rev. 2011, 16, 49–69. [Google Scholar] [CrossRef] [PubMed]
- Hamid, T.; Gu, Y.; Ortines, R.V.; Bhattacharya, C.; Wang, G.; Xuan, Y.T.; Prabhu, S.D. Divergent tumor necrosis factor receptor-related remodeling responses in heart failure: Role of nuclear factor-kappaB and inflammatory activation. Circulation 2009, 119, 1386–1397. [Google Scholar] [CrossRef]
- Vasan, R.S.; Sullivan, L.M.; Roubenoff, R.; Dinarello, C.A.; Harris, T.; Benjamin, E.J.; Sawyer, D.B.; Levy, D.; Wilson, P.W.; D’Agostino, R.B. Framingham Heart Study. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: The Framingham Heart Study. Circulation 2003, 107, 1486–1491. [Google Scholar] [CrossRef] [PubMed]
- Glezeva, N.; Baugh, J.A. Role of inflammation in the pathogenesis of heart failure with preserved ejection fraction and its potential as a therapeutic target. Heart Fail. Rev. 2014, 19, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Nwabuo, C.C.; Vasan, R.S. Pathophysiology of hypertensive heart disease: Beyond left ventricular hypertrophy. Curr. Hypertens. Rep. 2020, 22, 11. [Google Scholar] [CrossRef]
- Kane, G.C.; Karon, B.L.; Mahoney, D.W.; Redfield, M.M.; Roger, V.L.; Burnett, J.C., Jr.; Jacobsen, S.J.; Rodeheffer, R.J. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA 2011, 306, 856–863. [Google Scholar] [CrossRef]
- Sanders-van Wijk, S.; Tromp, J.; Beussink-Nelson, L.; Hage, C.; Svedlund, S.; Saraste, A.; Swat, S.A.; Sanchez, C.; Njoroge, J.; Tan, R.S.; et al. Proteomic evaluation of the comorbidity-inflammation paradigm in heart failure with preserved ejection fraction: Results from the PROMIS-HFpEF study. Circulation 2020, 142, 2029–2044. [Google Scholar] [CrossRef]
- Cesari, M.; Penninx, B.W.; Newman, A.B.; Kritchevsky, S.B.; Nicklas, B.J.; Sutton-Tyrrell, K.; Rubin, S.M.; Ding, J.; Simonsick, E.M.; Harris, T.B.; et al. Inflammatory markers and onset of cardiovascular events: Results from the Health ABC study. Circulation 2003, 108, 2317–2322. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Penninx, B.W.; Newman, A.B.; Kritchevsky, S.B.; Nicklas, B.J.; Sutton-Tyrrell, K.; Tracy, R.P.; Rubin, S.M.; Harris, T.B.; Pahor, M. Inflammatory markers and cardiovascular disease (The Health, Aging and Body Composition [Health ABC] Study). Am. J. Cardiol. 2003, 92, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Kalogeropoulos, A.; Georgiopoulou, V.; Psaty, B.M.; Rodondi, N.; Smith, A.L.; Harrison, D.G.; Liu, Y.; Hoffmann, U.; Bauer, D.C.; Newman, A.B.; et al. Health ABC Study Investigators. Inflammatory markers and incident heart failure risk in older adults: The Health ABC (Health, Aging, and Body Composition) study. J. Am. Coll. Cardiol. 2010, 55, 2129–2137. [Google Scholar] [CrossRef] [PubMed]
- Bakhshi, H.; Varadarajan, V.; Ambale-Venkatesh, B.; Meyghani, Z.; Ostovaneh, M.R.; Durda, P.; Wu, C.O.; Tracy, R.P.; Cushman, M.; Bluemke, D.A.; et al. Association of soluble interleukin-2 receptor α and tumour necrosis factor receptor 1 with heart failure: The Multi-Ethnic Study of Atherosclerosis. ESC Heart Fail. 2020, 7, 639–644. [Google Scholar] [CrossRef]
- Ueland, T.; Kjekshus, J.; Frøland, S.S.; Omland, T.; Squire, I.B.; Gullestad, L.; Dickstein, K.; Aukrust, P. Plasma levels of soluble tumor necrosis factor receptor type I during the acute phase following complicated myocardial infarction predict survival in high-risk patients. J. Am. Coll. Cardiol. 2005, 46, 2018–2021. [Google Scholar] [CrossRef]
- Stenemo, M.; Nowak, C.; Byberg, L.; Sundström, J.; Giedraitis, V.; Lind, L.; Ingelsson, E.; Fall, T.; Ärnlöv, J. Circulating proteins as predictors of incident heart failure in the elderly. Eur. J. Heart Fail. 2018, 20, 55–62. [Google Scholar] [CrossRef]
- Marti, C.N.; Khan, H.; Mann, D.L.; Georgiopoulou, V.V.; Bibbins-Domingo, K.; Harris, T.; Koster, A.; Newman, A.; Kritchevsky, S.B.; Kalogeropoulos, A.P.; et al. Health ABC Study. Soluble tumor necrosis factor receptors and heart failure risk in older adults: Health, Aging, and Body Composition (Health ABC) Study. Circ. Heart Fail. 2014, 7, 5–11. [Google Scholar] [CrossRef]
- Hage, C.; Michaëlsson, E.; Linde, C.; Donal, E.; Daubert, J.C.; Gan, L.M.; Lund, L.H. Inflammatory biomarkers predict heart failure severity and prognosis in patients with heart failure with preserved ejection fraction: A holistic proteomic approach. Circ. Cardiovasc. Genet. 2017, 10, e001633. [Google Scholar] [CrossRef]
- Pastore, M.C.; Cavigli, L.; Olivoni, G.; Morrone, F.; Amati, F.; Imbalzano, E.; Rinaldi, A.; Liga, R.; Mattioli, A.V.; Scicchitano, P.; et al. Working Group on Hypertension, Prevention and Peripheral Circulation of the Italian Society of Cardiology. Physical exercise in hypertensive heart disease: From the differential diagnosis to the complementary role of exercise. Int. J. Cardiol. 2024, 410, 1322–1332. [Google Scholar] [CrossRef]
- Franssen, C.; Chen, S.; Unger, A.; Korkmaz, H.I.; De Keulenaer, G.W.; Tschöpe, C.; Leite-Moreira, A.F.; Musters, R.; Niessen, H.W.; Linke, W.A.; et al. Myocardial microvascular inflammatory endothelial activation in heart failure with preserved ejection fraction. JACC Heart Fail. 2016, 4, 312–324. [Google Scholar] [CrossRef]
- Faselis, C.; Doumas, M.; Kokkinos, J.P.; Panagiotakos, D.; Kheirbek, R.; Sheriff, H.M.; Hare, K.; Papademetriou, V.; Fletcher, R.; Kokkinos, P. Exercise capacity and progression from prehypertension to hypertension. Hypertension 2012, 60, 333–338. [Google Scholar] [CrossRef]
- Maffeis, C.; Inciardi, R.M.; Khan, M.S.; Tafciu, E.; Bergamini, C.; Benfari, G.; Setti, M.; Ribichini, F.L.; Cicoira, M.; Butler, J.; et al. Determinants of exercise intolerance symptoms considered non-specific for heart failure in patients with stage A and B: Role of the left atrium in the transition phase to overt heart failure. Int. J. Cardiovasc. Imaging 2022, 38, 103–112. [Google Scholar] [CrossRef]
- Kosmala, W.; Rojek, A.; Przewlocka-Kosmala, M.; Mysiak, A.; Karolko, B.; Marwick, T.H. Contributions of nondiastolic factors to exercise intolerance in heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 2016, 67, 659–670. [Google Scholar] [CrossRef]
- El Missiri, A.M.; Alzurfi, A.S.; Keddeas, V.W. The relationship between tumor necrosis factor alpha and left ventricular diastolic function. J. Cardiovasc. Echogr. 2020, 30, 62–67. [Google Scholar] [CrossRef]
- Jasic-Szpak, E.; Serafin, A.; Marwick, T.H.; Kosowski, W.; Woznicka, A.K.; Kotwica, T.; Przewlocka-Kosmala, M.; Ponikowski, P.; Kosmala, W. Association of reduced left atrial reserve with exercise intolerance and outcome in hypertension. J. Am. Soc. Echocardiogr. 2024, 37, 872–883. [Google Scholar] [CrossRef] [PubMed]
- von Roeder, M.; Rommel, K.P.; Kowallick, J.T.; Blazek, S.; Besler, C.; Fengler, K.; Lotz, J.; Hasenfuß, G.; Lücke, C.; Gutberlet, M.; et al. Influence of left atrial function on exercise capacity and left ventricular function in patients with heart failure and preserved ejection fraction. Circ. Cardiovasc. Imaging 2017, 10, e005467. [Google Scholar] [CrossRef] [PubMed]
- Telles, F.; Nanayakkara, S.; Evans, S.; Patel, H.C.; Mariani, J.A.; Vizi, D.; William, J.; Marwick, T.H.; Kaye, D.M. Impaired left atrial strain predicts abnormal exercise hemodynamics in heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2019, 21, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Bandera, F.; Generati, G.; Alfonzetti, E.; Bussadori, C.; Guazzi, M. Left atrial function dynamics during exercise in heart failure: Pathophysiological implications on the right heart and exercise ventilation inefficiency. JACC Cardiovasc. Imaging 2017, 10, 1253–1264. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Q.; Han, J.; Lu, Y.; Zhang, Y.; Song, W.; Cheng, Y.; Cong, T.; Liu, Y.; Jiang, Y. Left atrial stiffness index as a marker of early target organ damage in hypertension. Hypertens. Res. 2021, 44, 299–309. [Google Scholar] [CrossRef]
- Santos, A.B.; Roca, G.Q.; Claggett, B.; Sweitzer, N.K.; Shah, S.J.; Anand, I.S.; Fang, J.C.; Zile, M.R.; Pitt, B.; Solomon, S.D.; et al. Prognostic relevance of left atrial dysfunction in heart failure with preserved ejection fraction. Circ. Heart Fail. 2016, 9, e002763. [Google Scholar] [CrossRef]
- Thomas, L.; Marwick, T.H.; Popescu, B.A.; Donal, E.; Badano, L.P. Left atrial structure and function, and left ventricular diastolic dysfunction: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2019, 73, 1961–1977. [Google Scholar] [CrossRef]
- Dalen, H.; Letnes, J.M.; Hoydal, M.A.; Wisløff, U. Diastolic function and dysfunction in athletes. Eur. Heart J. Cardiovasc. Imaging 2024, 25, 1537–1545. [Google Scholar] [CrossRef]
- Tadic, M.; Grassi, G.; Cuspidi, C. Cardiorespiratory fitness in patients with type 2 diabetes: A missing piece of the puzzle. Heart Fail. Rev. 2021, 26, 301–308. [Google Scholar] [CrossRef]
- Tschöpe, C.; Van Linthout, S.; Kherad, B. Heart failure with preserved ejection fraction and future pharmacological strategies: A glance in the crystal ball. Curr. Cardiol. Rep. 2017, 19, 70. [Google Scholar] [CrossRef]
- Mattos, B.R.; Bonacio, G.F.; Vitorino, T.R.; Garcia, V.T.; Amaral, J.H.; Dellalibera-Joviliano, R.; Franca, S.C.; Tanus-Santos, J.E.; Rizzi, E. TNF-α inhibition decreases MMP-2 activity, reactive oxygen species formation, and improves hypertensive vascular hypertrophy independent of its effects on blood pressure. Biochem. Pharmacol. 2020, 180, 114–121. [Google Scholar] [CrossRef]
| Parameter | All | Group 1 n = 40 | Group 2 n = 40 | p Value |
|---|---|---|---|---|
| Age, years (SD) | 56.0 (13.3) | 62.8 (1.7) | 49.1 (11.2) | <0.001 |
| Female, n (%) | 42 (53) | 26 (65) | 16 (40) | 0.026 |
| BMI, kg∙m−2 (SD) | 27.6 (4.1) | 28.2 (4.8) | 27.0 (3.1) | 0.191 |
| Smoking, n (%) | 12 (15) | 5 (13) | 7 (18) | 0.756 |
| Heart rate rest, 1/min (SD) | 77 (15) | 76 (14) | 77 (16) | 0.712 |
| Heart rate exercise, 1/min (SD) | 160 (18) | 153 (20) | 158 (16) | 0.001 |
| SBP rest, mmHg (SD) | 123 (14) | 123 (15) | 123 (13) | 0.886 |
| SBP exercise, mmHg (SD) | 178 (25) | 172 (20) | 183 (28) | 0.055 |
| DBP rest, mmHg (SD) | 78 (10) | 78 (9) | 78 (11) | 0.869 |
| DBP exercise, mmHg (SD) | 83 (14) | 83 (11) | 84 (15) | 0.904 |
| 24-Hour SBP mmHg (SD) | 118 (8) | 117 (9) | 120 (8) | 0.130 |
| 24-Hour DBP mmHg (SD) | 75 (7) | 72 (7) | 77 (6) | 0.007 |
| NT-proBNP, ng∙mL−1 (SD) | 4.06 (0.57) | 3.99 (0.67) | 4.13 (0.42) | 0.390 |
| Hemoglobin, g∙dL−1 (SD) | 13.95 (1.0) | 13.7 (1.0) | 14.2 (1.0) | 0.111 |
| eGFR, mL∙min−1∙1.73 m−2 (SD) | 79 (15) | 74 (11.7) | 85 (16) | 0.008 |
| TNF-R1, pg/mL (SD) | 1303 (379) | 1410 (471) | 1197 (216) | 0.011 |
| Peak VO2, kg/mL/min (SD) | 30.0 (8.7) | 22.7 (4.4) | 37.3 (5.0) | <0.001 |
| Exercise time, s (SD) | 558 (220) | 420 (167) | 695 (179) | <0.001 |
| Antihypertensive treatment | ||||
| ACEI and ARB, n (%) | 49 (61) | 28 (70) | 21 (52) | 0.169 |
| BB, n (%) | 17 (21) | 10 (25) | 7 (18) | 0.584 |
| CB, n (%) | 26 (33) | 11 (28) | 15 (38) | 0.177 |
| MRA, n (%) | 12 (15) | 5 (13) | 7 (18) | 0.754 |
| Diuretic, n (%) | 14 (18) | 8 (20) | 6 (15) | 0.694 |
| Parameter | All | Group 1 n = 40 | Group 2 n = 40 | p Value |
|---|---|---|---|---|
| LVDd, mm (SD) | 47.7 (4,2) | 48.4 (4.1) | 46.9 (4.2) | 0.111 |
| LVMI g∙m−2 (SD) | 90.0 (16.7) | 92.0 (18.2) | 88.0 (15.1) | 0.289 |
| LVEF, % (SD) | 59 (6) | 59 (7) | 59 (6) | 0.842 |
| GLS, % (SD) | 17.9 (3.0) | 18.0 (3.4) | 17.8 (2.6) | 0.711 |
| LAVI, mL∙m−2 (SD) | 30.5 (7.3) | 31.0 (8.1) | 29.9 (6.5) | 0.524 |
| E/A (SD) | 1.27 (0.42) | 1.17 (0.36) | 1.37 (0.45) | 0.033 |
| DT ms (SD) | 188 (47) | 191 (42) | 184 (51) | 0.515 |
| e’ septal, m∙s−1 (SD) | 0.087 (0.023) | 0.080 (0.024) | 0.094 (0.019) | 0.007 |
| e’ lateral, m∙s−1 (SD) | 0.113 (0.032) | 0.103 (0.031) | 0.123 (0.030) | 0.006 |
| E/e’ (SD) | 8.5 (3.0) | 9.3 (3.4) | 7.7 (2.1) | 0.008 |
| PALS, % (SD) | 26.3 (6.9) | 23.9 (7.4) | 28.7 (5.5) | 0.001 |
| PACS, % (SD) | 12.5 (4.0) | 12.1 (4.6) | 12.9 (3.4) | 0.376 |
| Parameter | Age | eGFR | TNF R1 | PALS | E/e’ | e’ Sept | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p | R | p | r | p | r | p | r | p | r | p | |
| Peak VO2 | −0.596 | <0.001 | 0.348 | 0.001 | −0.366 | 0.001 | 0.304 | 0.006 | −0.353 | 0.002 | 0.297 | 0.009 |
| Age | - | - | −0.286 | 0.035 | 0.307 | 0.006 | −0.387 | <0.001 | 0.462 | <0.001 | −0.511 | <0.001 |
| eGFR | - | - | −0.040 | 0.773 | 0.242 | 0.075 | −0.163 | 0.249 | 0.128 | 0.368 | ||
| TNF R1 | - | - | −0.291 | 0.009 | 0.207 | 0.071 | −0.225 | 0.049 | ||||
| PALS | - | - | −0.399 | <0.001 | 0.502 | <0.001 | ||||||
| E/e’ | - | - | −0.610 | <0.001 | ||||||||
| MODEL 1 Peak VO2 R2 = 0.42 | MODEL 2 Peak VO2 R2 = 0.28 | MODEL 3 Peak VO2 R2 = 0.25 | MODEL 4 Peak VO2 R2 = 0.24 | MODEL 5 Peak VO2 R2 = 0.18 | MODEL 6 Peak VO2 R2 = 0.18 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | p | Β | SE | p | β | SE | p | β | SE | p | β | SE | p | β | SE | p | |
| Age, | −0.49 | 0.09 | <0.001 | |||||||||||||||
| TNF-R1 | −0.21 | 0.09 | 0.025 | −0.31 | 0.10 | 0.003 | −0.31 | 0.13 | 0.017 | −0.31 | 0.11 | 0.004 | −0.31 | 0.11 | 0.004 | −0.30 | 0.11 | 0.006 |
| eGFR | 0.18 | 0.09 | 0.046 | 0.25 | 0.10 | 0.012 | 0.31 | 0.12 | 0.015 | 0.26 | 0.10 | 0.013 | ||||||
| E/e’ | −0.25 | 0.10 | 0.016 | |||||||||||||||
| e’ | 0.20 | 0.10 | 0.058 | 0.22 | 0.11 | 0.037 | ||||||||||||
| PALS | 0.16 | 0.11 | 0.131 | 0.22 | 0.11 | 0.049 | ||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gozdzik, A.T.; Obremska, M. Association of TNF-R1 with Exercise Capacity in Asymptomatic Hypertensive Heart Disease—Mediating Role of Left Ventricular Diastolic Function Deterioration. J. Clin. Med. 2025, 14, 5391. https://doi.org/10.3390/jcm14155391
Gozdzik AT, Obremska M. Association of TNF-R1 with Exercise Capacity in Asymptomatic Hypertensive Heart Disease—Mediating Role of Left Ventricular Diastolic Function Deterioration. Journal of Clinical Medicine. 2025; 14(15):5391. https://doi.org/10.3390/jcm14155391
Chicago/Turabian StyleGozdzik, Anna Teresa, and Marta Obremska. 2025. "Association of TNF-R1 with Exercise Capacity in Asymptomatic Hypertensive Heart Disease—Mediating Role of Left Ventricular Diastolic Function Deterioration" Journal of Clinical Medicine 14, no. 15: 5391. https://doi.org/10.3390/jcm14155391
APA StyleGozdzik, A. T., & Obremska, M. (2025). Association of TNF-R1 with Exercise Capacity in Asymptomatic Hypertensive Heart Disease—Mediating Role of Left Ventricular Diastolic Function Deterioration. Journal of Clinical Medicine, 14(15), 5391. https://doi.org/10.3390/jcm14155391






