Red Cell Distribution Width (RDW), Platelets and Platelet Index MPV/PLT Ratio as Specific Time Point Predictive Variables of Survival Outcomes in COVID-19 Hospitalized Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Base Analysis
2.2. Statistical Analysis
3. Results
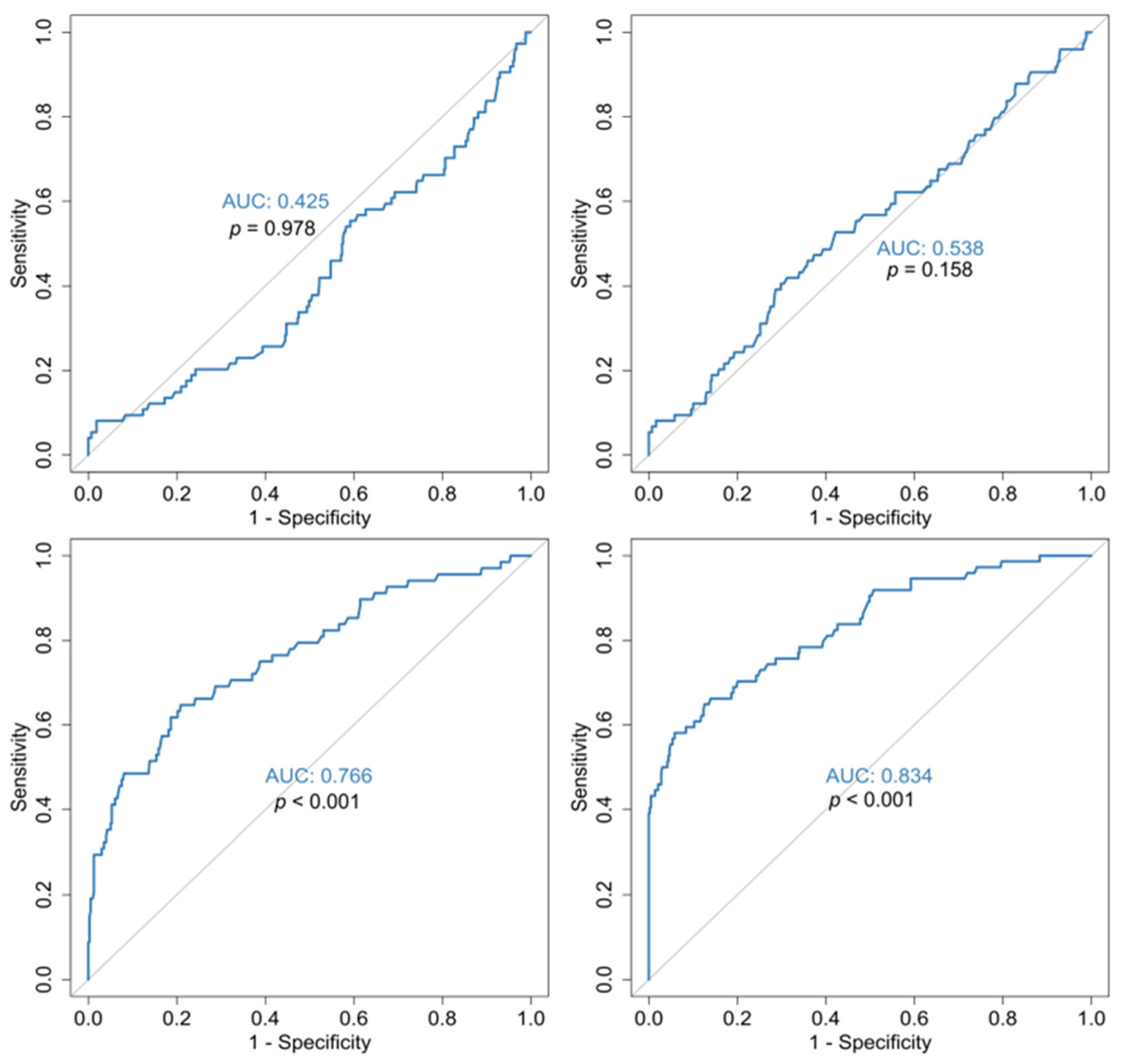
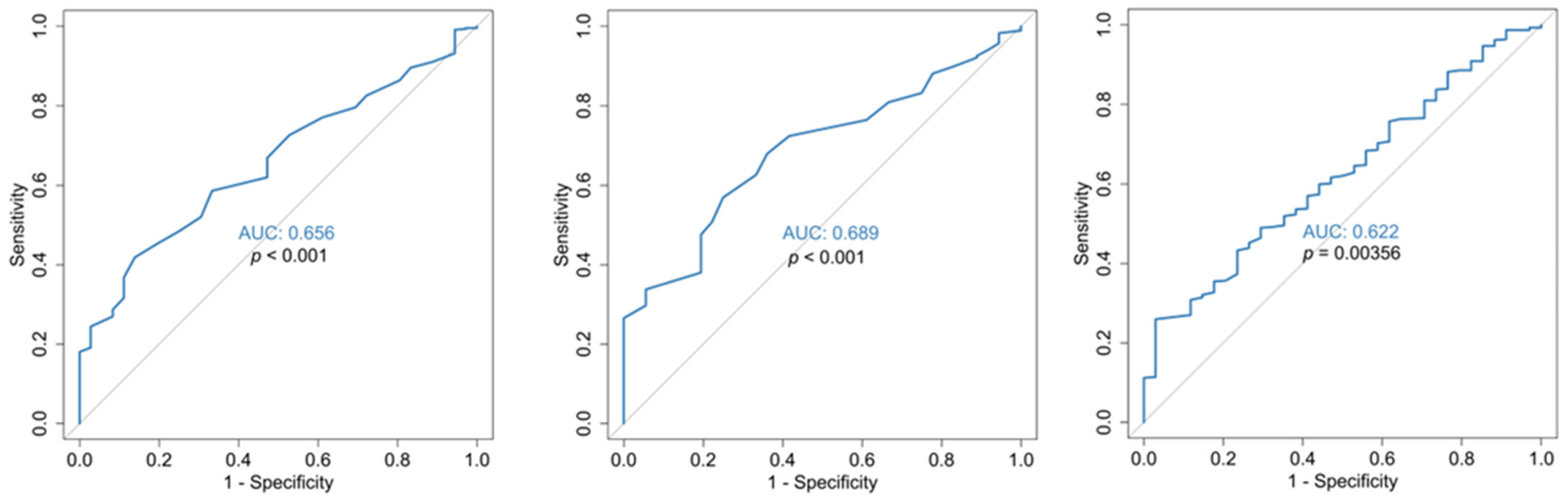
3.1. Outcome Analysis (Survival vs. Mortality)
3.2. Disease Severity Analysis (Mild/Moderate vs. Severe/Critical)
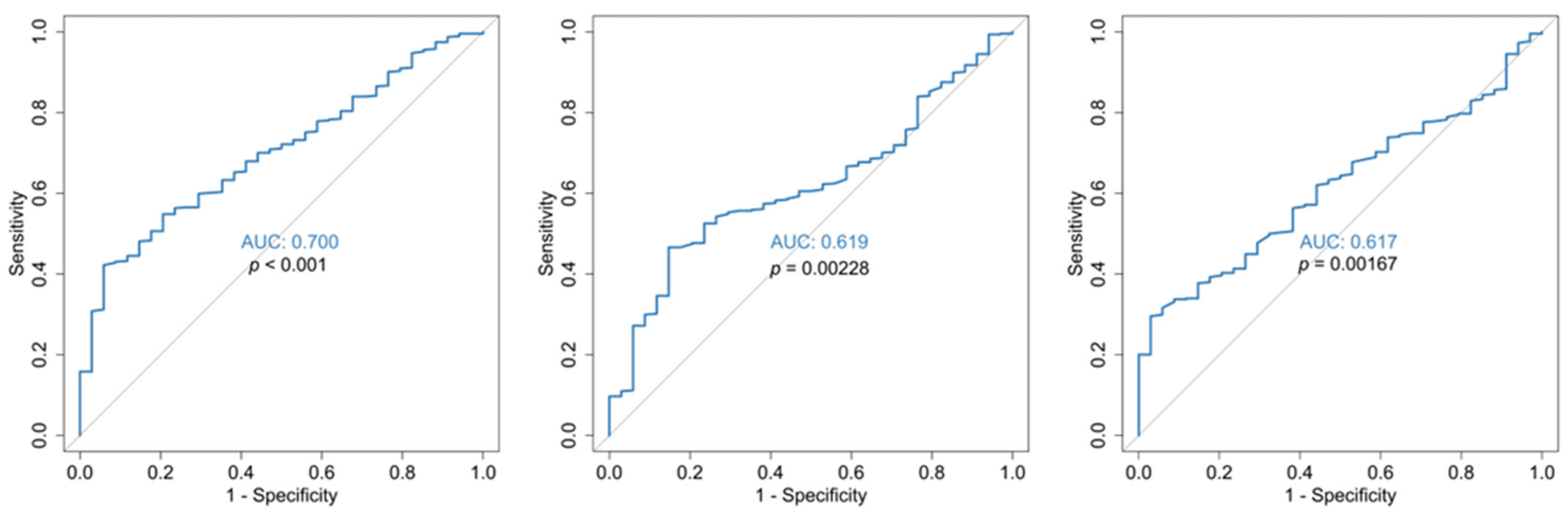
3.3. Multivariable Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Characteristics | Death (N = 74) | Survival (N = 432) | p-Value |
|---|---|---|---|
| Age | |||
| Mean (SD) | 84.9 (9.10) | 64.6 (14.7) | <0.001 MW |
| Median [Min, Max] | 88.0 [37.0, 96.0] | 65.0 [22.0, 99.0] | |
| Gender | |||
| Male | 29 (39.2%) | 233 (53.9%) | 0.0264 x2 |
| Female | 45 (60.8%) | 199 (46.1%) | |
| Disease Severity | |||
| Critical | 2 (2.7%) | 13 (3.0%) | 0.0327 fx |
| Severe | 72 (97.3%) | 383 (88.7%) | |
| Moderate | 0 (0%) | 34 (7.9%) | |
| Mild | 0 (0%) | 2 (0.5%) | |
| Days of hospitalization | |||
| Mean (SD) | 24.2 (12.7) | 16.9 (7.98) | <0.001 MW |
| Median [Min, Max] | 21.0 [6.00, 72.0] | 15.0 [5.00, 59.0] | |
| FiO2 | |||
| Mean (SD) | 76.3% (25.7%) | 43.5% (18.6%) | <0.001 MW |
| Median [Min, Max] | 90% [35%, 100%] | 40% [21%, 100%] | |
| 2-Level Disease Severity | |||
| Severe/Critical | 74 (100%) | 396 (91.7%) | 0.0197 x2 |
| Mild/Moderate | 0 (0%) | 36 (8.3%) | |
| Comorbidities | |||
| Cardiovascular disease (CVS) | 41 (55.4%) | 90 (20.8%) | <0.001 x2 |
| Arterial Hypertension | 33 (44.6%) | 262 (60.6%) | 0.0139 x2 |
| Diabetes Mellitus | 25 (33.8%) | 71 (16.4%) | <0.001 x2 |
| Malignancy | 20 (27.0%) | 20 (4.6%) | <0.001 x2 |
| Immunosuppression | 3 (4.1%) | 4 (0.9%) | 0.0679 fx |
| COPD | 7 (9.5%) | 31 (7.2%) | 0.653 x2 |
| Sleep Apnea | 2 (2.7%) | 4 (0.9%) | 0.214 fx |
| Bronchial Asthma | 1 (1.4%) | 22 (5.1%) | 0.227 fx |
| Chronic kidney Disease | 4 (5.4%) | 1 (0.2%) | 0.00189 fx |
| Neurological Disease | 29 (39.2%) | 55 (12.7%) | <0.001 x2 |
| Psychiatric Disease | 8 (10.8%) | 55 (12.7%) | 0.786 x2 |
| Morbid obesity | 1 (1.4%) | 11 (2.5%) | 1 fx |
| Autoimmune Disease | 1 (1.4%) | 8 (1.9%) | 1 fx |
| ECOG 4 | 10 (13.5%) | 7 (1.6%) | <0.001 fx |


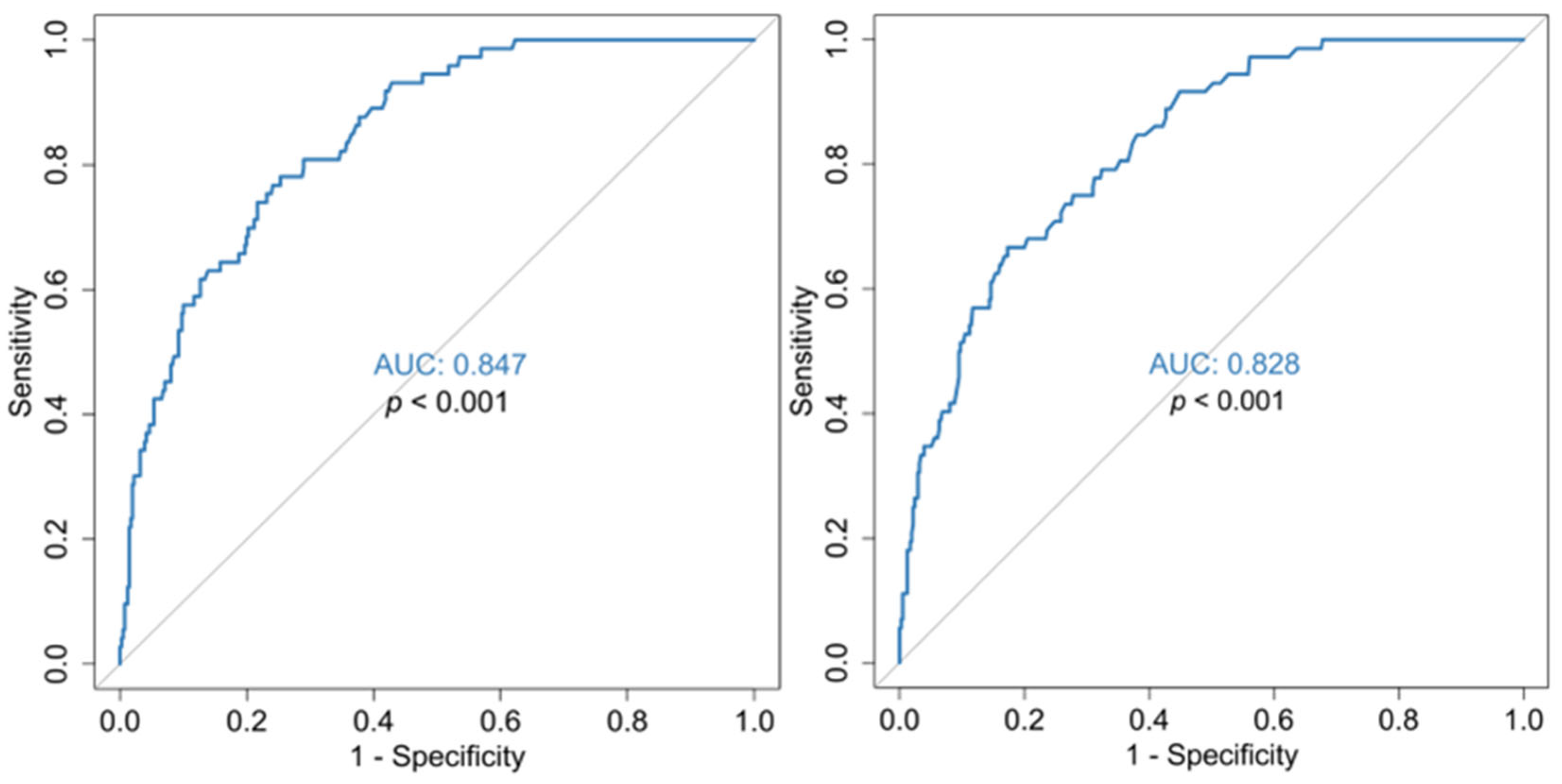
| Mean absolute lymphocyte count | 897/μL (AUC 0.808, sensitivity 0.743, specificity 0.733) |
| Nadir lymphocyte count | 505/μL (AUC 0.838, sensitivity 0.757, specificity 0.756) |
| Percentage % of lymphocytes nadir | 7.5% (AUC 0.823, sensitivity 78.6, specificity 73.1) |
| Mean DD | 1.125 (mg/L) (AUC 0.828, sensitivity 73.6, specificity 72.7) |
| Zenith DD value | 2.055 (mg/L) (AUC0.847, sensitivity 0.767, specificity 0.759) |
| Mean CRP value | 45 (mg/L) (AUC 0.805, sensitivity 74, specificity 73.8) |
| Characteristics | Mild/Moderate (N = 36) | Severe/Critical (N = 475) | p-Value |
|---|---|---|---|
| Age | 65.5 [37.0, 95.0] | 68.0 [22.0, 99.0] | 0.865 MW |
| Gender | |||
| Male | 9 (25.0%) | 255 (53.7%) | 0.00165 x2 |
| Female | 27 (75.0%) | 220 (46.3%) | |
| FiO2 | |||
| Mean (SD) | 22.3% (2.7%) | 50.8% (22.8%) | <0.001 MW |
| Median [Min, Max] | 21% [21%, 28%] | 40% [21%, 100%] | |
| Comorbidities | |||
| CVS disease | 11 (30.6%) | 121 (25.5%) | 0.635 x2 |
| Arterial hypertension | 7 (19.4%) | 208 (43.8%) | 0.00742 x2 |
| Diabetes Mellitus | 4 (11.1%) | 93 (19.6%) | 0.304 x2 |
| Malignancy | 5 (13.9%) | 37 (7.8%) | 0.204 fx |
| Immunosuppression | 0 (0%) | 8 (1.7%) | 1 fx |
| COPD | 1 (2.8%) | 38 (8.0%) | 0.508 fx |
| Sleep Apnea | 0 (0%) | 6 (1.3%) | 1 fx |
| Bronchial Asthma | 0 (0%) | 23 (4.8%) | 0.395 fx |
| Chronic kidney Disease | 0 (0%) | 5 (1.1%) | 1 fx |
| Neurological Disease | 12 (33.3%) | 74 (15.6%) | 0.0119 x2 |
| Psychiatric Disease | 12 (33.3%) | 51 (10.7%) | <0.001 fx |
| Morbid obesity | 0 (0%) | 12 (2.5%) | 1 fx |
| Autoimmune Disease | 2 (5.6%) | 7 (1.5%) | 0.127 fx |
| ECOG 4 | 0 (0%) | 17 (3.6%) | 0.623 fx |
| Days in hospital | 14.5 [6.00, 48.0] | 15.0 [5.00, 72.0] | 0.646 MW |
| Outcome | |||
| Improvement | 36 (100%) | 390 (82.1%) | 0.0148 fx |
| Death | 0 (0%) | 74 (15.6%) | |
| Discharge with O2 support | 0 (0%) | 6 (1.3%) | |
| Death | 0 (0%) | 74 (15.6%) | 0.0197 x2 |
References
- Chorba, T. The Concept of the Crown and Its Potential Role in the Downfall of Coronavirus. Emerg. Infect. Dis. 2020, 26, 2302–2305. [Google Scholar] [CrossRef]
- Perlstein, T.S.; Weuve, J.; Pfeffer, M.A.; Beckman, J.A. Red blood cell distribution width and mortality risk in a community-basedprospectivecohort. Arch. Intern. Med. 2009, 169, 588–594. [Google Scholar] [CrossRef]
- Salvagno, G.L.; Sanchis-Gomar, F.; Picanza, A.; Lippi, G. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit. Rev. Clin. Lab. Sci. 2015, 52, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Filippozzi, L.; Montagnana, M.; Salvagno, G.L.; Franchini, M.; Guidi, G.C.; Targher, G. Clinical usefulness of measuring red blood cell distribution width on admission in patients with acute coronary syndromes. Clin. Chem. Lab. Med. 2009, 47, 353–357. [Google Scholar] [CrossRef]
- Azab, B.; Torbey, E.; Hatoum, H.; Singh, J.; Khoueiry, G.; Bachir, R.; McGinn, J.T., Jr.; McCord, D.; Lafferty, J. Usefulness of red cell distribution width in predicting all-cause long-term mortality after non-ST-elevation myocardial infarction. Cardiology 2011, 119, 72–80. [Google Scholar] [CrossRef]
- Uyarel, H.; Ergelen, M.; Cicek, G.; Kaya, M.G.; Ayhan, E.; Turkkan, C.; Yıldırım, E.; Kırbas, V.; Onturk, E.T.; Erer, H.B.; et al. Red cell distribution width as a novel prognostic marker in patients undergoing primary angioplasty for acute myocardial infarction. Coron. Artery. Dis. 2011, 22, 138–144. [Google Scholar] [CrossRef]
- Borné, Y.; Smith, J.G.; Melander, O.; Engström, G. Red cell distribution width in relation to incidence of coronary events and case fatality rates: A population-based cohort study. Heart 2014, 100, 1119–1124. [Google Scholar] [CrossRef]
- Kurtul, A.; Murat, S.N.; Yarlioglues, M.; Duran, M.; Karadeniz, M.; Ergun, G.; Akyel, A.; Mendi, M.A.; Oksuz, F. The association of red cell distribution width with in-stent restenosis in patients with stable coronary artery disease. Platelets 2015, 26, 48–52. [Google Scholar] [CrossRef]
- Yao, H.M.; Sun, T.W.; Zhang, X.J.; Shen, D.L.; Du, Y.Y.; Wan, Y.D.; Zhang, J.Y.; Li, L.; Zhao, L.S. Red Blood Cell Distribution Width and Long-Term Outcome in Patients Undergoing Percutaneous Coronary Intervention in the Drug-Eluting Stenting Era: A Two-Year Cohort Study. Lipinski M, editor. PLoS ONE 2014, 9, e94887. [Google Scholar] [CrossRef]
- Lippi, G.; Turcato, G.; Cervellin, G.; Sanchis-Gomar, F. Red blood cell distribution width in heart failure: A narrative review. World J. Cardiol. 2018, 10, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Emans, M.E.; Gaillard, C.A.; Pfister, R.; Tanck, M.W.; Boekholdt, S.M.; Wareham, N.J.; Khaw, K.T. Red cell distribution width is associated with physical inactivity and heart failure, independent of established risk factors, inflammation or iron metabolism; the EPIC-Norfolk study. Int. J. Cardiol. 2013, 168, 3550–3555. [Google Scholar] [CrossRef]
- Jackson, C.E.; Dalzell, J.R.; Bezlyak, V.; Tsorlalis, I.K.; Myles, R.C.; Spooner, R.; Ford, I.; Petrie, M.C.; Cobbe, S.M.; McMurray, J.J. Red cell distribution width has incremental prognostic value to B-type natriuretic peptide in acute heart failure. Eur. J. Heart Fail. 2009, 11, 1152–1154. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Jia, J.; Chen, J.; Qin, S.; Tao, H.; Kong, Q.; Xue, Q.; Zhang, D. Comparison of prognostic value of red cell distribution width and NT-proBNP for short-term clinical outcomes in acute heart failure patients. Int. Heart J. 2014, 55, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Xanthopoulos, A.; Giamouzis, G.; Dimos, A.; Skoularigki, E.; Starling, R.C.; Skoularigis, J.; Triposkiadis, F. Red Blood Cell Distribution Width in Heart Failure: Pathophysiology, Prognostic Role, Controversies and Dilemmas. J. Clin. Med. 2022, 11, 1951. [Google Scholar] [CrossRef]
- Kitai, T.; Xanthopoulos, A.; Tang, W.H.W.; Kaji, S.; Furukawa, Y.; Oishi, S.; Akiyama, E.; Suzuki, S.; Yamamoto, M.; Kida, K.; et al. Validation of the Larissa Heart Failure Risk Score for risk stratification in acute heart failure. Int. J. Cardiol. 2020, 307, 119–124. [Google Scholar] [CrossRef]
- Adler, E.D.; Voors, A.A.; Klein, L.; Macheret, F.; Braun, O.O.; Urey, M.A.; Zhu, W.; Sama, I.; Tadel, M.; Campagnari, C.; et al. Improving risk prediction in heart failure using machine learning. Eur. J. Heart Fail. 2020, 22, 139–147. [Google Scholar] [CrossRef]
- Ertaş, G.; Aydin, C.; Sönmez, O.; Erdoğan, E.; Turfan, M.; Tasal, A.; Bacaksiz, A.; Vatankulu, M.A.; Uyarel, H.; Ergelen, M.; et al. Red cell distribution width predicts new-onset atrial fibrillation after coronary artery bypass grafting. Scand. Cardiovasc. J. 2013, 47, 132–135. [Google Scholar] [CrossRef]
- Kurt, M.; Tanboga, I.H.; Buyukkaya, E.; Karakas, M.F.; Akçay, A.B.; Sen, N. Relation of red cell distribution width with CHA2DS2-VASc score in patients with nonvalvular atrial fibrillation. Clin. Appl. Thromb. Hemost. 2014, 20, 687–692. [Google Scholar] [CrossRef]
- Malavasi, V.L.; Proietti, M.; Spagni, S.; Valenti, A.C.; Battista, A.; Pettorelli, D.; Colella, J.; Vitolo, M.; Lip, G.Y.; Boriani, G. Usefulness of Red Cells Distribution Width to Predict Worse Outcomes in Patients with Atrial Fibrillation. Am. J. Cardiol. 2019, 124, 1561–1567. [Google Scholar] [CrossRef]
- Arkew, M.; Gemechu, K.; Haile, K.; Asmerom, H. Red Blood Cell Distribution Width as Novel Biomarker in Cardiovascular Diseases: A Literature Review. J. Blood Med. 2022, 13, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Rezende, S.M.; Lijfering, W.M.; Rosendaal, F.R.; Cannegieter, S.C. Hematologic variables and venous thrombosis: Red cell distribution width and blood monocyte count are associated with an increased risk. Haematologica 2014, 99, 194–200. [Google Scholar] [CrossRef]
- Zöller, B.; Melander, O.; Svensson, P.; Engström, G. Red cell distribution width and risk for venous thromboembolism: A population-based cohort study. Thromb. Res. 2014, 133, 334–339. [Google Scholar] [CrossRef]
- Hammons, L.; Filopei, J.; Steiger, D.; Bondarsky, E. A narrative review of red blood cell distribution width as a marker for pulmonary embolism. J. Thromb. Thrombolysis 2019, 48, 638–647. [Google Scholar] [CrossRef]
- Zinellu, A.; Mangoni, A.A. The Emerging Clinical Significance of the Red Cell Distribution Width as a Biomarker in Chronic Obstructive Pulmonary Disease: A Systematic Review. J. Clin. Med. 2022, 11, 5642. [Google Scholar] [CrossRef]
- Seretis, C.; Seretis, F.; Lagoudianakis, E.; Gemenetzis, G.; Salemis, N.S. Is red cell distribution width a novel biomarker of breast cancer activity? Data from a pilot study. J. Clin. Med. Res. 2013, 5, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.L.; Zhou, X.; Xiao, D.C. Is red blood cell distribution width a prognostic factor for colorectal cancer? A meta-analysis. Front. Surg. 2022, 9, 945126. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, Y.; Zhou, K.; Li, J.; Che, G. Prognostic value of pre-treatment red blood cell distribution width in lung cancer: A meta-analysis. Biomarkers 2020, 25, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.M.; Zhu, K.P.; Guo, Z.W.; Yi, W.; He, Y.; Du, G.C. Is red cell distribution width a prognostic factor in patients with breast cancer? A meta-analysis. Front. Surg. 2023, 10, 1000522. [Google Scholar] [CrossRef]
- Lippi, G.; Targher, G.; Montagnana, M.; Salvagno, G.L.; Zoppini, G.; Guidi, G.C. Relationship between red blood cell distribution width and kidney function tests in a large cohort of unselected outpatients. Scand. J. Clin. Lab. Investig. 2008, 68, 745–748. [Google Scholar] [CrossRef]
- Lou, Y.; Wang, M.; Mao, W. Clinical usefulness of measuring red blood cell distribution width in patients with hepatitis B. PLoS ONE 2012, 7, e37644. [Google Scholar] [CrossRef]
- Fan, X.; Deng, H.; Wang, X.; Fu, S.; Liu, Z.; Sang, J.; Zhang, X.; Li, N.; Han, Q.; Li, Z. Association of red blood cell distribution width with severity of hepatitis B virus-related liver diseases. Clin. Chim. Acta. 2018, 482, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Aslam, H.; Oza, F.; Ahmed, K.; Kopel, J.; Aloysius, M.M.; Ali, A.; Dahiya, D.S.; Aziz, M.; Perisetti, A.; Goyal, H. The Role of Red Cell Distribution Width as a Prognostic Marker in Chronic Liver Disease: A Literature Review. Int. J. Mol. Sci. 2023, 24, 3487. [Google Scholar] [CrossRef]
- Veeranna, V.; Zalawadiya, S.K.; Panaich, S.S.; Ramesh, K.; Afonso, L. The association of red cell distribution width with glycated hemoglobin among healthy adults without diabetes mellitus. Cardiology 2012, 122, 129–132. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, P.; Yan, Z.; Liu, Z.; Ma, Q.; Zhang, Z.; Wang, Y.; Su, Y. The Relationship between Erythrocytes and Diabetes Mellitus. J. Diabetes Res. 2021, 2021, 6656062. [Google Scholar] [CrossRef]
- Blaslov, K.; Kruljac, I.; Mirošević, G.; Gaćina, P.; Kolonić, S.O.; Vrkljan, M. The prognostic value of red blood cell characteristics on diabetic retinopathy development and progression in type 2 diabetes mellitus. Clin. Hemorheol. Microcirc. 2019, 71, 475–481. [Google Scholar] [CrossRef]
- Kim, C.H.; Park, J.T.; Kim, E.J.; Han, J.H.; Han, J.S.; Choi, J.Y.; Han, S.H.; Yoo, T.H.; Kim, Y.S.; Kang, S.W.; et al. An increase in red blood cell distribution width from baseline predicts mortality in patients with severe sepsis or septic shock. Crit. Care 2013, 17, R282. [Google Scholar] [CrossRef]
- Bazick, H.S.; Chang, D.; Mahadevappa, K.; Gibbons, F.K.; Christopher, K.B. Red cell distribution width and all-cause mortality in critically ill patients. Crit. Care Med. 2011, 39, 1913–1921. [Google Scholar] [CrossRef]
- Wu, H.; Liao, B.; Cao, T.; Ji, T.; Huang, J.; Ma, K. Diagnostic value of RDW for the prediction of mortality in adult sepsis patients: A systematic review and meta-analysis. Front. Immunol. 2022, 13, 997853. [Google Scholar] [CrossRef] [PubMed]
- Foy, B.H.; Carlson, J.C.T.; Reinertsen, E.; Padros Valls, R.I.; Pallares Lopez, R.; Palanques-Tost, E.; Mow, C.; Westover, M.B.; Aguirre, A.D.; Higgins, J.M. Association of Red Blood Cell Distribution Width with Mortality Risk in Hospitalized Adults with SARS-CoV-2 Infection. JAMA Netw. Open 2020, 3, e2022058. [Google Scholar] [CrossRef]
- Karampitsakos, T.; Akinosoglou, K.; Papaioannou, O.; Panou, V.; Koromilias, A.; Bakakos, P.; Loukides, S.; Bouros, D.; Gogos, C.; Tzouvelekis, A. Increased Red Cell Distribution Width Is Associated with Disease Severity in Hospitalized Adults with SARS-CoV-2 Infection: An Observational Multicentric Study. Front. Med. 2020, 7, 616292. [Google Scholar] [CrossRef] [PubMed]
- Lagadinou, M.; Gkentzi, D.; Marangos, M.N.; Paliogianni, F.; Solomou, E.E. Red Blood Cell Distribution Width: Another Prognostic Factor for COVID-19? Clin. Hematol. Int. 2021, 3, 69–71. [Google Scholar] [CrossRef]
- Soni, M.; Gopalakrishnan, R. Significance of RDW in predicting mortality in COVID-19-An analysis of 622 cases. Int. J. Lab. Hematol. 2021, 43, O221–O223. [Google Scholar] [CrossRef]
- Banon, T.; Wortsman, J.; Ben Moshe, S.; Gazit, S.; Peretz, A.; Ben Tov, A.; Chodick, G.; Perez, G.; Patalon, T. Evaluating red blood cell distribution width from community blood tests as a predictor of hospitalization and mortality in adults with SARS-CoV-2: A cohort study. Ann. Med. 2021, 53, 1410–1418. [Google Scholar] [CrossRef]
- Kaufmann, C.C.; Ahmed, A.; Brunner, U.; Jäger, B.; Aicher, G.; Equiluz-Bruck, S.; Spiel, A.O.; Funk, G.C.; Gschwantler, M.; Fasching, P.; et al. Red Cell Distribution Width Upon Hospital Admission Predicts Short-Term Mortality in Hospitalized Patients With COVID-19: A Single-Center Experience. Front. Med. 2021, 8, 652707. [Google Scholar] [CrossRef]
- Barrett, J.; Bilaloglu, S.; Cornwell, M.; Burgess, H.M.; Virginio, V.W.; Drenkova, K.; Ibrahim, H.; Yuriditsky, E.; Aphinyanaphongs, Y.; Lifshitz, M.; et al. Platelets contribute to disease severity in COVID-19. J. Thromb. Haemost. 2021, 19, 3139–3153. [Google Scholar] [CrossRef] [PubMed]
- Ulgen, A.; Cetin, S.; Cetin, M.; Sivgin, H.; Li, W. A composite ranking of risk factors for COVID-19 time-to-event data from a Turkish cohort. Comput. Biol. Chem. 2022, 98, 107681. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.Q.; Huang, Q.F.; Xie, W.M.; Lv, C.; Quan, X.Q. The association between severe COVID-19 and low platelet count: Evidence from 31 observational studies involving 7613 participants. Br. J. Haematol. 2020, 190, e29–e33. [Google Scholar] [CrossRef]
- Braekkan, S.K.; Mathiesen, E.B.; Njølstad, I.; Wilsgaard, T.; Størmer, J.; Hansen, J.B. Mean platelet volume is a risk factor for venous thromboembolism: The Tromsø Study, Tromsø, Norway. J. Thromb. Haemost. 2010, 8, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.B.; Jakubowski, J.A.; Quinn, P.G.; Deykin, D.; Valeri, C.R. Platelet size and age determine platelet function independently. Blood 1984, 63, 1372–1375. [Google Scholar] [CrossRef][Green Version]
- Huang, H.L.; Chen, C.H.; Kung, C.T.; Li, Y.C.; Sung, P.H.; You, H.L.; Lin, Y.H.; Huang, W.T. Clinical utility of mean platelet volume and immature platelet fraction in acute coronary syndrome. Biomed. J. 2019, 42, 107–115. [Google Scholar] [CrossRef]
- Sharma, G.; Berger, J.S. Platelet activity and cardiovascular risk in apparently healthy individuals: A review of the data. J. Thromb. Thrombolysis 2011, 32, 201–208. [Google Scholar] [CrossRef]
- Hille, L.; Lenz, M.; Vlachos, A.; Grüning, B.; Hein, L.; Neumann, F.J.; Nührenberg, T.G.; Trenk, D. Ultrastructural, transcriptional, and functional differences between human reticulated and non-reticulated platelets. J. Thromb. Haemost. 2020, 18, 2034–2046. [Google Scholar] [CrossRef]
- Lippi, G.; Henry, B.M.; Favaloro, E.J. Mean Platelet Volume Predicts Severe COVID-19 Illness. Semin. Thromb. Hemost. 2021, 47, 456–459. [Google Scholar] [CrossRef]
- Du, F.; Liu, B.; Zhang, S. COVID-19: The role of excessive cytokine release and potential ACE2 down-regulation in promoting hypercoagulable state associated with severe illness. J. Thromb. Thrombolysis 2021, 51, 313–329. [Google Scholar] [CrossRef]
- Manne, B.K.; Denorme, F.; Middleton, E.A.; Portier, I.; Rowley, J.W.; Stubben, C.; Petrey, A.C.; Tolley, N.D.; Guo, L.; Cody, M.; et al. Platelet gene expression and function in patients with COVID-19. Blood 2020, 136, 1317–1329. [Google Scholar] [CrossRef]
- Daniels, S.; Wei, H.; van Tongeren, M.; Denning, D.W. Are platelet volume indices of clinical use in COVID-19? A systematic review. Front. Cardiovasc. Med. 2022, 9, 1031092. [Google Scholar] [CrossRef] [PubMed]
- Hottz, E.D.; Azevedo-Quintanilha, I.G.; Palhinha, L.; Teixeira, L.; Barreto, E.A.; Pao, C.R.R.; Righy, C.; Franco, S.; Souza, T.M.L.; Kurtz, P.; et al. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood 2020, 136, 1330–1341. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, W.; Guo, Y.; Chen, L.; Zhang, L.; Zhao, S.; Long, D.; Yu, L. Association between platelet parameters and mortality in coronavirus disease 2019: Retrospective cohort study. Platelets 2020, 31, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Thachil, J. What do monitoring platelet counts in COVID-19 teach us? J. Thromb. Haemost. 2020, 18, 2071–2072. [Google Scholar] [CrossRef]
- Azab, B.; Torbey, E.; Singh, J.; Akerman, M.; Khoueiry, G.; McGinn, J.T.; Widmann, W.D.; Lafferty, J. Mean platelet volume/platelet count ratio as a predictor of long-term mortality after non-ST-elevation myocardial infarction. Platelets 2011, 22, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.H.; Rhee, S.Y.; Jeon, H.J.; Park, J.Y.; Kang, S.W.; Oh, J. An Increase in Mean Platelet Volume/Platelet Count Ratio Is Associated with Vascular Access Failure in Hemodialysis Patients. PLoS ONE 2017, 12, e0170357. [Google Scholar] [CrossRef]
- Oh, G.H.; Chung, S.P.; Park, Y.S.; Hong, J.H.; Lee, H.S.; Chung, H.S.; You, J.S.; Park, J.W.; Park, I. Mean Platelet Volume to Platelet Count Ratio as a Promising Predictor of Early Mortality in Severe Sepsis. Shock 2017, 47, 323–330. [Google Scholar] [CrossRef]
- Ray, B.; Tinsley, L.; Ford, L.; Thompson, D.M.; Sidorov, E.V.; Bohnstedt, B.N. Trends of Platelet Volume Index Predicts Delayed Cerebral Ischemia After Subarachnoid Hemorrhage. World Neurosurg. 2018, 111, e624–e631. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://eody.gov.gr/wp-content/uploads/2021/12/covid_19_algorithmos-nosileuomenon_20211227.pdf (accessed on 27 December 2021).
- Levi, M.; Toh, C.H.; Thachil, J.; Watson, H.G. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br. J. Haematol. 2009, 145, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Lu, H.; Zhang, W. Clinical observation and management of COVID-19 patients. Emerg. Microbes Infect. 2020, 9, 687–690. [Google Scholar] [CrossRef]
- Available online: https://eody.gov.gr/wp-content/uploads/2022/04/covid_19_algorithmos-mi-nosileuomenon_20220404.pdf (accessed on 4 April 2022).
- R Foundation. The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 12 January 2025).
- Rothman, K.J. No adjustments are needed for multiple comparisons. Epidemiology 1990, 1, 43–46. [Google Scholar] [CrossRef]
- Althouse, A.D. Adjust for Multiple Comparisons? It’s Not That Simple. Ann. Thorac. Surg. 2016, 101, 1644–1645. [Google Scholar] [CrossRef]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef]
- Kuhn, M. Building predictive models in R using the caret package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef]
- Higgins, J.M.; Mahadevan, L. Physiological and pathological population dynamics of circulating human red blood cells. Proc. Natl. Acad. Sci. USA 2010, 107, 20587–20592. [Google Scholar] [CrossRef]
- Patel, H.H.; Patel, H.R.; Higgins, J.M. Modulation of red blood cell population dynamics is a fundamental homeostatic response to disease. Am. J. Hematol. 2015, 90, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Jelkmann, W. Proinflammatory cytokines lowering erythropoietin production. J. Interferon Cytokine Res. 1998, 18, 555–559. [Google Scholar] [CrossRef]
- Lee, J.J.; Montazerin, S.M.; Jamil, A.; Jamil, U.; Marszalek, J.; Chuang, M.L.; Chi, G. Association between red blood cell distribution width and mortality and severity among patients with COVID-19: A systematic review and meta-analysis. J. Med. Virol. 2021, 93, 2513–2522. [Google Scholar] [CrossRef]
- Gavriilaki, E.; Brodsky, R.A. Severe COVID-19 infection and thrombotic microangiopathy: Success does not come easily. Br. J. Haematol. 2020, 189, e227–e230. [Google Scholar] [CrossRef]
- Henry, B.M.; Benoit, J.L.; Benoit, S.; Pulvino, C.; Berger, B.A.; Olivera, M.H.S.; Crutchfield, C.A.; Lippi, G. Red Blood Cell Distribution Width (RDW) Predicts COVID-19 Severity: A Prospective, Observational Study from the Cincinnati SARS-CoV-2 Emergency Department Cohort. Diagnostics 2020, 10, 618. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Pérez, L.; Fortes, J.; Soto, C.; Vidal-Gonzalez, A.; Alonso-Riano, M.; Lafarga, M.; Cortti, M.J.; Lazaro-Garcia, A.; Pérez-Tanoira, R.; Trascasa, A.; et al. Histiocytic hyperplasia with hemophagocytosis and acute alveolar damage in COVID-19 infection. Mod. Pathol. 2020, 33, 2139–2146. [Google Scholar] [CrossRef]
- Sarkar, S.; Kannan, S.; Khanna, P.; Singh, A.K. Role of red blood cell distribution width, as a prognostic indicator in COVID-19: A systematic review and meta-analysis. Rev. Med. Virol. 2022, 32, e2264. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Stefanoni, D.; Dzieciatkowska, M.; Issaian, A.; Nemkov, T.; Hill, R.C.; Francis, R.O.; Hudson, K.E.; Buehler, P.W.; Zimring, J.C.; et al. Evidence of Structural Protein Damage and Membrane Lipid Remodeling in Red Blood Cells from COVID-19 Patients. J. Proteome Res. 2020, 19, 4455–4469. [Google Scholar] [CrossRef]
- Angileri, F.; Légaré, S.; Marino Gammazza, A.; Conway de Macario, E.; Macario, A.J.L.; Cappello, F. Is molecular mimicry the culprit in the autoimmune haemolytic anaemia affecting patients with COVID-19? Br. J. Haematol. 2020, 190, e92–e93. [Google Scholar] [CrossRef]
- Lippi, G.; Pavesi, F.; Bardi, M.; Pipitone, S. Lack of harmonization of red blood cell distribution width (RDW). Evaluation of four hematological analyzers. Clin. Biochem. 2014, 47, 1100–1103. [Google Scholar] [CrossRef]
- Atik, D.; Kaya, H.B. Evaluation of the relationship of MPV, RDW and PVI parameters with disease severity in COVID-19 patients. Acta Clin. Croat. 2021, 60, 103–114. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, K.; Zuo, P.; Liu, Y.; Zhang, M.; Xie, S.; Zhang, H.; Chen, X.; Liu, C. Early decrease in blood platelet count is associated with poor prognosis in COVID-19 patients-indications for predictive, preventive, and personalized medical approach. EPMA J. 2020, 11, 139–145. [Google Scholar] [CrossRef]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Q.; Wang, Y.; Wu, Y.; Xu, J.; Yu, Y.; Shang, Y. Thrombocytopenia and its association with mortality in patients with COVID-19. J. Thromb. Haemost. 2020, 18, 1469–1472. [Google Scholar] [CrossRef]
- Wang, C.; Deng, R.; Gou, L.; Fu, Z.; Zhang, X.; Shao, F.; Wang, G.; Fu, W.; Xiao, J.; Ding, X.; et al. Preliminary study to identify severe from moderate cases of COVID-19 using combined hematology parameters. Ann. Transl. Med. 2020, 8, 593. [Google Scholar] [CrossRef]
- Zhong, Q.; Peng, J. Mean platelet volume/platelet count ratio predicts severe pneumonia of COVID-19. J. Clin. Lab. Anal. 2021, 35, e23607. [Google Scholar] [CrossRef]
- Lippi, G.; Pavesi, F.; Pipitone, S. Evaluation of mean platelet volume with four hematological analyzers: Harmonization is still an unresolved issue. Blood Coagul. Fibrinolysis 2015, 26, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Elalamy, I.; Kastritis, E.; Sergentanis, T.N.; Politou, M.; Psaltopoulou, T.; Gerotziafas, G.; Dimopoulos, M.A. Hematological findings and complications of COVID-19. Am. J. Hematol. 2020, 95, 834–847. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Mengn, M.; Kumar, R.; Wu, Y.; Huang, J.; Deng, Y.; Weng, Z.; Yang, L. Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A systemic review and meta-analysis. Int. J. Infect. Dis. 2020, 96, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Wang, Q.; Zhang, D.; Ding, J.; Huang, Q.; Tang, Y.Q.; Wang, Q.; Miao, H. Lymphopenia predicts disease severity of COVID-19: A descriptive and predictive study. Signal Transduct. Target. Ther. 2020, 5, 33. [Google Scholar] [CrossRef]
- Bhatraju, P.K.; Ghassemieh, B.J.; Nichols, M.; Kim, R.; Jerome, K.R.; Nalla, A.K.; Greninger, A.L.; Pipavath, S.; Wurfel, M.M.; Evans, L.; et al. Covid-19 in Critically Ill Patients in the Seattle Region–Case Series. N. Engl. J. Med. 2020, 382, 2012–2022. [Google Scholar] [CrossRef] [PubMed]
- Tahir Huyut, M.; Huyut, Z.; İlkbahar, F.; Mertoğlu, C. What is the impact and efficacy of routine immunological, biochemical and hematological biomarkers as predictors of COVID-19 mortality? Int. Immunopharmacol. 2022, 105, 108542. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Cao, Y.Y.; Tan, G.; Dong, X.; Wang, B.C.; Lin, J.; Yan, Y.Q.; Liu, G.H.; Akdis, M.; Akdis, C.A.; et al. Clinical, radiological, and laboratory characteristics and risk factors for severity and mortality of 289 hospitalized COVID-19 patients. Allergy 2021, 76, 533–550. [Google Scholar] [CrossRef]
- International Committee for Standardization in Haematology; ICSH Expert Panel on Cytometry. ICSH recommendations for the analysis of red cell, white cell and platelet size distribution curves. Methods for fitting a single reference distribution and assessing its goodness of fit. Clin. Lab. Haematol. 1990, 12, 417–431. [Google Scholar] [CrossRef]
| Characteristics | N, % |
|---|---|
| Age | |
| Mean (SD) | 67.7 (15.7) |
| Median [Min, Max] | 68.0 [22.0–99.0] |
| Gender | |
| Male | 264 (51.7%) |
| Female | 247 (48.3%) |
| Other Nationalities | 38 (7.4%) |
| Severity | |
| Mild | 2 (0.4%) |
| Moderate | 34 (6.7%) |
| Critical | 19 (3.7%) |
| Severe | 456 (89.2%) |
| 2-Level Severity | |
| Mild/Moderate | 36 (7.0%) |
| Severe/Critical | 475 (93.0%) |
| ECOG 4 | |
| Yes | 17 (3.3%) |
| No | 494 (96.7%) |
| FiO2 | |
| Mean (SD) | 48.7% (0.232) |
| Median [Min, Max] | 40% [21%, 100%] |
| Missing | 5 (1.0%) |
| Days in hospital | |
| Mean (SD) | 17.9 (9.15) |
| Median [Min, Max] | 15.0 [5.00, 72.0] |
| No Remdesivir | 154 (30.1%) |
| R | 346 (67.7%) |
| R + A | 5 (1.0%) |
| R + T | 3 (0.6%) |
| R + B | 1 (0.2%) |
| T | 1 (0.2%) |
| A | 1 (0.2%) |
| Outcome | |
| Improvement | 426 (83.4%) |
| Death | 74 (14.5%) |
| Discharge with O2 support | 6 (1.2%) |
| Missing | 5 (1.0%) |
| Characteristics | Death (N = 74) | Survival (N = 432) | p-Value |
|---|---|---|---|
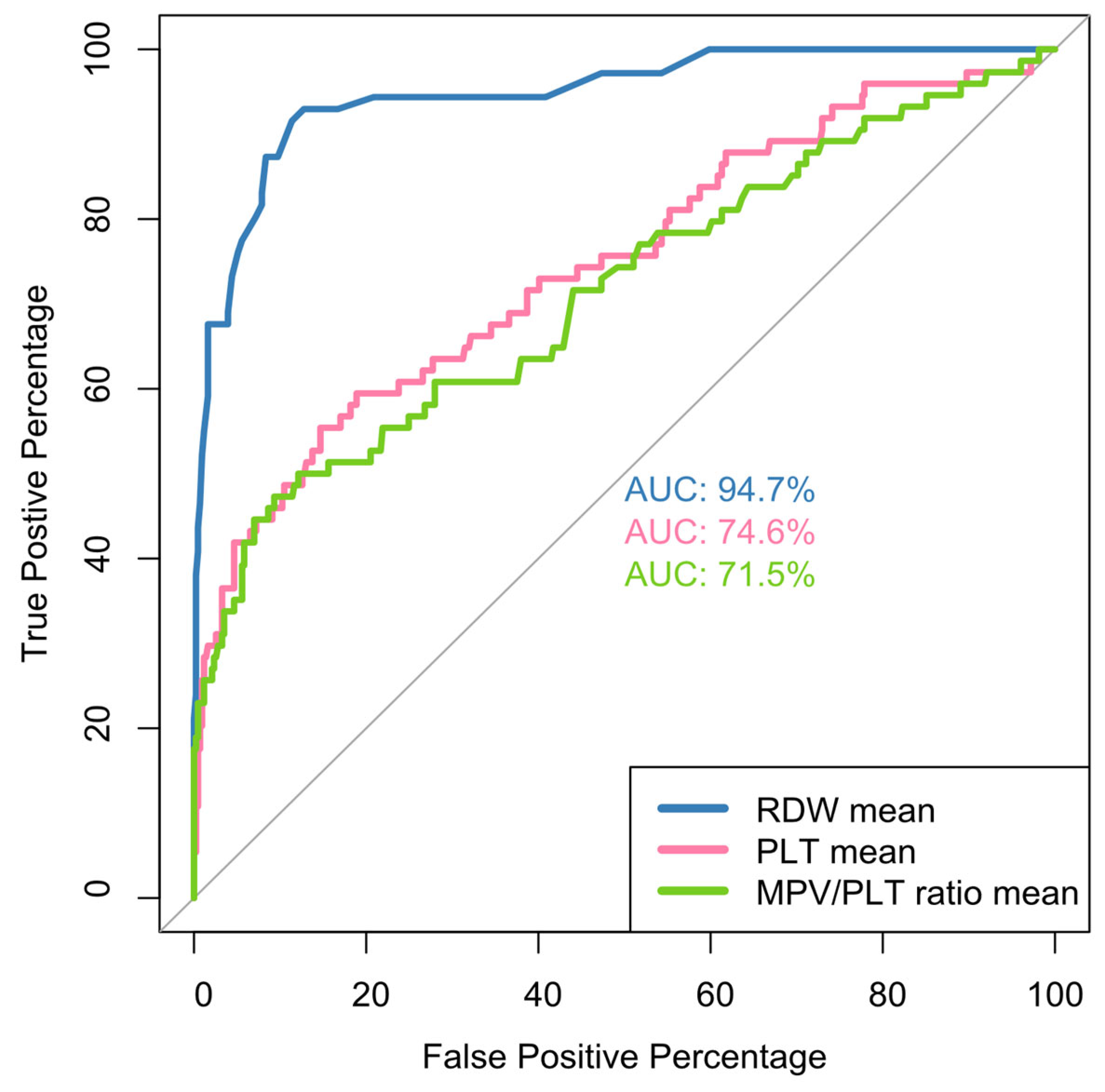
| RDW mean | |||
| Mean (SD) | 16.3 (1.98) | 13.4 (0.805) | <0.001 |
| Median (min, max) | 16.0 [13.2, 23.4] | 13.3 [11.4, 17.3] | |
| PLT mean | |||
| Mean (SD) | 207,000 (87,400) | 281,000 (83,500) | <0.001 |
| Median (min, max) | 189,000 [23,500, 490,000] | 269,000 [102,000, 616,000] | |
| MPV/PLT ratio mean | |||
| Mean (SD) | 7.80 (9.60) | 3.99 (1.39) | <0.001 |
| Median (min, max) | 5.42 [1.81, 73.1] | 3.85 [1.29, 9.60] | |
| Admission RDW | |||
| Mean (SD) | 15.8 (2.15) | 13.4 (0.876) | <0.001 |
| Median (min, max) | 15.4 [12.8, 22.3] | 13.4 [11.2, 17.2] | |
| Admission MPV/PLT ratio | |||
| Mean (SD) | 5.65 (4.15) | 5.63 (2.28) | 0.0392 |
| Median (min, max) | 4.89 [1.81, 27.1] | 5.46 [1.21, 15.3] | |
| Admission PLTs | |||
| Mean (SD) | 220,000 (89,400) | 209,000 (82,200) | 0.258 |
| Median (min, max) | 201,000 [43,000, 504,000] | 185,000 [66,000, 683,000] | |
| Discharge RDW | |||
| Mean (SD) | 17.0 (2.11) | 13.6 (0.984) | <0.001 |
| Median (min, max) | 16.7 [13.3, 24.0] | 13.4 [11.5, 18.0] | |
| RDW dif | |||
| Mean (SD) | 1.23 (1.75) | 0.163 (0.755) | <0.001 |
| Median (min, max) | 1.00 [−3.80, 6.80] | 0.100 [−2.10, 4.00 | |
| Discharge PLTs | |||
| Mean (SD) | 166,000 (110,000) | 322,000 (118,000) | <0.001 |
| Median (min, max) | 150,000 [9000, 508,000] | 303,000 [93,000, 744,000] | |
| Discharge MPV/PLT ratio | |||
| Mean (SD) | 14.8 (23.2) | 3.01 (1.42) | <0.001 |
| Median (min, max) | 6.56 [1.61, 128] | 2.66 [0.850, 8.40] | |
| PLTs 1w | |||
| Mean (SD) | 223,000 (91,800) | 245,000 (89,200) | 0.0467 |
| Median (min, max) | 206,000 [23,500, 493,000] | 222,000 [93,300, 616,000] | |
| MPV/PLT ratio 1w | 4.90 [1.83, 73.1] | 4.50 [1.29, 11.8] | 0.3 |
| PLT 2w | |||
| Mean (SD) | 234,000 (105,000) | 347,000 (113,000) | <0.001 |
| Median (min, max) | 220,000 [43,000, 570,000] | 337,000 [118,000, 744,000] | |
| MPV/PLT ratio 2w | |||
| Mean (SD) | 5.14 (3.48) | 2.86 (1.18) | <0.001 |
| Median (min, max) | 4.16 [1.36, 20.1] | 2.60 [0.850, 8.40] | |
| PLTs nadir | 119,000 [9000, 367,000] | 175,000 [66,000, 605,000] | <0.001 |
| MPV/PLT highest | |||
| Mean (SD) | 16.6 (23.2) | 5.97 (2.24) | <0.001 |
| Median (min, max) | 8.18 [2.67, 128] | 5.70 [1.49, 15.1] | |
| MPV/PLT lowest | |||
| Mean (SD) | 3.85 (3.66) | 2.41 (0.969) | <0.001 |
| Median (min, max) | 3.16 [1.09, 27.1] | 2.19 [0.850, 6.69] | |
| DD highest | |||
| Median (min, max) | 4.69 [0.790, 99.0] | 1.02 [0.190, 45.4] | <0.001 |
| Mean DD | <0.001 | ||
| Mean | 4.04(6.02) | 1.07(1.28) | |
| Median (min, max) | 2.32 [0.510, 33.8] | 0.720 [0.190, 14.2] | |
| Mean LYMP | |||
| Mean (SD) | 747 (341) | 1180 (412) | <0.001 |
| Median (min, max) | 694 [246, 1860] | 1130 [361, 2620] | |
| LYMP # lowest | |||
| Mean (SD) | 404 (241) | 749 (313) | <0.001 |
| Median (min, max) | 370 [80.0, 1280 | 700 [190, 2210] | |
| LYMP % lowest | |||
| Median (min, max) | 0.0400 [0.0100, 0.630] | 0.110 [0.0200, 0.360] | <0.001 |
| LYMP # lowest when | |||
| 1w | 27 (36.5%) | 341 (78.9%) | <0.001 fx |
| 2w | 16 (21.6%) | 63 (14.6%) | |
| 3w | 12 (16.2%) | 17 (3.9%) | |
| 4w | 10 (13.5%) | 4 (0.9%) | |
| 5w | 4 (5.4%) | 1 (0.2%) | |
| 8w | 1 (1.4%) | 0 (0%) | |
| 6w | 0 (0%) | 1 (0.2%) | |
| Mean CRP | 71.0 (36.0) | 33.5 (29.4) | <0.001 |
| CRP highest | 152 (78.0) | 82.7(67.4) | <0.001 |
| CPR highest at: | |||
| 1w | 29 (39.2%) | 369 (85.4%) | <0.001 fx |
| 2w | 9 (12.2%) | 38 (8.8%) | |
| 3w | 14 (18.9%) | 10 (2.3%) | |
| 4w | 11 (14.9%) | 3 (0.7%) | |
| 5w | 6 (8.1%) | 2 (0.5%) | |
| 6w | 2 (2.7%) | 0 (0%) | |
| 7w | 2 (2.7%) | 0 (0%) |
| Characteristics | Mild/Moderate (N = 36) | Severe/Critical (N = 475) | p-Value |
|---|---|---|---|
| RDW mean | |||
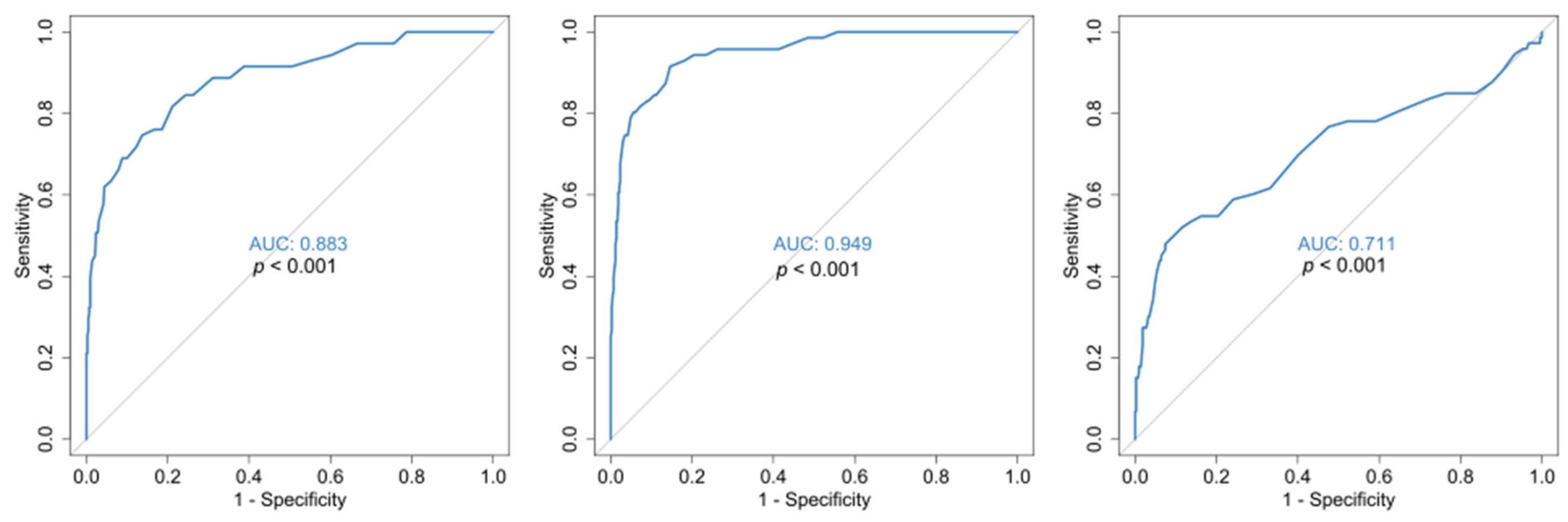
| Mean (SD) | 13.1 (0.570) | 13.9 (1.51) | <0.001 |
| Median [Min, Max] | 13.0 [11.9, 14.1] | 13.5 [11.4, 23.4] | |
| PLTs mean | |||
| Mean (SD) | 271,000 (73,300) | 269,000 (89,200) | 0.774 |
| Median [Min, Max] | 266,000 [169,000, 499,000] | 261,000 [23,500, 616,000] | |
| Admission MPV/PLT ratio | |||
| Mean (SD) | 4.14 (1.44) | 5.75 (2.66) | <0.001 |
| Median [Min, Max] | 4.18 [1.57, 7.70] | 5.49 [1.21, 27.1] | |
| MPV/PLT ratio mean | |||
| Mean (SD) | 3.55 (0.889) | 4.63 (4.22) | 0.0228 |
| Median [Min, Max] | 3.49 [1.55, 5.46] | 3.98 [1.29, 73.1] | |
| Admission RDW | |||
| Mean (SD) | 13.1 (0.687) | 13.8 (1.46) | 0.00175 |
| Median [Min, Max] | 13.1 [11.4, 14.7] | 13.5 [11.2, 22.3] | |
| Discharge RDW | |||
| Mean (SD) | 13.2 (0.604) | 14.1 (1.74) | <0.001 |
| Median [Min, Max] | 13.1 [12.3, 14.4] | 13.7 [11.5, 24.0] | |
| RDW diff | |||
| Mean (SD) | 0.0556 (0.466) | 0.336 (1.06) | 0.183 |
| Median [Min, Max] | 0 [−0.800, 0.900] | 0.200 [−3.80, 6.80] | |
| Admission PLTs | |||
| Mean (SD) | 244,000 (96,900) | 208,000 (81,700) | 0.0175 |
| Median [Min, Max] | 211,000 [131,000, 497,000] | 185,000 [43,000, 683,000] | |
| PLTs 1w | |||
| Mean (SD) | 245,000 (85,800) | 240,000 (90,100) | 0.774 |
| Median [Min, Max] | 221,000 [131,000, 499,000] | 221,000 [23,500, 616,000] | |
| PLTs 2w | |||
| Mean (SD) | 300,000 (95,100) | 331,000 (120,000) | 0.185 |
| Median [Min, Max] | 301,000 [156,000, 523,000] | 326,000 [43,000, 744,000] | |
| Discharge PLTs | |||
| Mean (SD) | 299,000 (99,800) | 298,000 (131,000) | 0.887 |
| Median [Min, Max] | 294,000 [151,000, 610,000] | 293,000 [9000, 744,000] | |
| PLT nadir | |||
| Mean (SD) | 206,000 (71,900) | 176,000 (71,000) | 0.0112 |
| Median [Min, Max] | 186,000 [120,000, 490,000] | 169,000 [9000, 605,000] | |
| MPV/PLT ratio 1w | |||
| Mean (SD) | 4.05 (1.33) | 5.05 (3.86) | 0.0207 |
| Median [Min, Max] | 4.08 [1.55, 7.70] | 4.70 [1.29, 73.1] | |
| MPV/PLT ratio 2w | |||
| Mean (SD) | 3.15 (1.16) | 3.22 (1.96) | 0.51 |
| Median [Min, Max] | 2.86 [1.31, 5.38] | 2.73 [0.850, 20.1] | |
| Discharge MPV/PLT ratio | |||
| Mean (SD) | 3.02 (1.12) | 4.88 (10.2) | 0.616 |
| Median [Min, Max] | 2.69 [1.25, 5.38] | 2.91 [0.850, 128] | |
| Missing | 2 (5.6%) | 1 (0.2%) | |
| DD highest | |||
| Mean (SD) | 1.29 (1.19) | 3.66 (8.66) | 0.0113 |
| Median [Min, Max] | 0.830 [0.190, 5.70] | 1.24 [0.190, 99.0] | |
| Missing | 1 (2.8%) | 22 (4.6%) | |
| Mean DD | |||
| Mean (SD) | 0.811 (0.546) | 1.57 (2.89) | 0.0305 |
| Median [Min, Max] | 0.710 [0.190, 2.15] | 0.840 [0.190, 33.8] | |
| Missing | 1 (2.8%) | 24 (5.1%) | |
| LYMP # Lowest | |||
| Mean (SD) | 1060 (348) | 673 (310) | <0.001 |
| Median [Min, Max] | 1010 [520, 2210] | 620 [80.0, 1890] | |
| LYMP # lowest at: | |||
| 1w | 25 (69.4%) | 345 (72.6%) | 0.203 fx |
| 2 w | 7 (19.4%) | 72 (15.2%) | |
| 4 w | 3 (8.3%) | 11 (2.3%) | |
| 3 w | 0 (0%) | 31 (6.5%) | |
| 5 w | 0 (0%) | 5 (1.1%) | |
| 6 w | 0 (0%) | 1 (0.2%) | |
| 8 w | 0 (0%) | 1 (0.2%) | |
| LYMP % lowest | |||
| Mean (SD) | 0.245 (0.0742) | 0.110 (0.0737) | <0.001 |
| Median [Min, Max] | 0.250 [0.110, 0.360] | 0.0900 [0.0100, 0.630] | |
| Mean LYMP | |||
| Mean (SD) | 1400 (441) | 1100 (423) | <0.001 |
| Median [Min, Max] | 1350 [717, 2620] | 1050 [246, 2580] | |
| CRP highest | |||
| Mean (SD) | 27.4 (24.3) | 98.1 (73.5) | <0.001 |
| Median [Min, Max] | 22.2 [2.00, 103] | 76.9 [2.00, 440] | |
| CPR highest at: | |||
| 1 w | 27 (75.0%) | 373 (78.5%) | 0.61 fx |
| 2 w | 5 (13.9%) | 43 (9.1%) | |
| 3 w | 0 (0%) | 26 (5.5%) | |
| 4 w | 0 (0%) | 14 (2.9%) | |
| 5 w | 0 (0%) | 8 (1.7%) | |
| 6 w | 0 (0%) | 2 (0.4%) | |
| 7 w | 0 (0%) | 2 (0.4%) | |
| Mean CRP | |||
| Mean (SD) | 13.7 (13.5) | 41.1 (29.9) | <0.001 |
| Median [Min, Max] | 11.0 [2.00, 70.0] | 33.6 [1.24, 162] | |
| MPV/PLT highest | |||
| Mean (SD) | 4.72 (1.38) | 7.75 (10.1) | <0.001 |
| Median [Min, Max] | 4.57 [1.75, 8.55] | 6.14 [1.49, 128] | |
| MPV/PLT lowest | |||
| Mean (SD) | 2.48 (0.854) | 2.64 (1.78) | 0.905 |
| Median [Min, Max] | 2.32 [1.25, 4.21] | 2.30 [0.850, 27.1] |
| Characteristic | Estimate | Std Error | p-Value | OR (95% CI) |
|---|---|---|---|---|
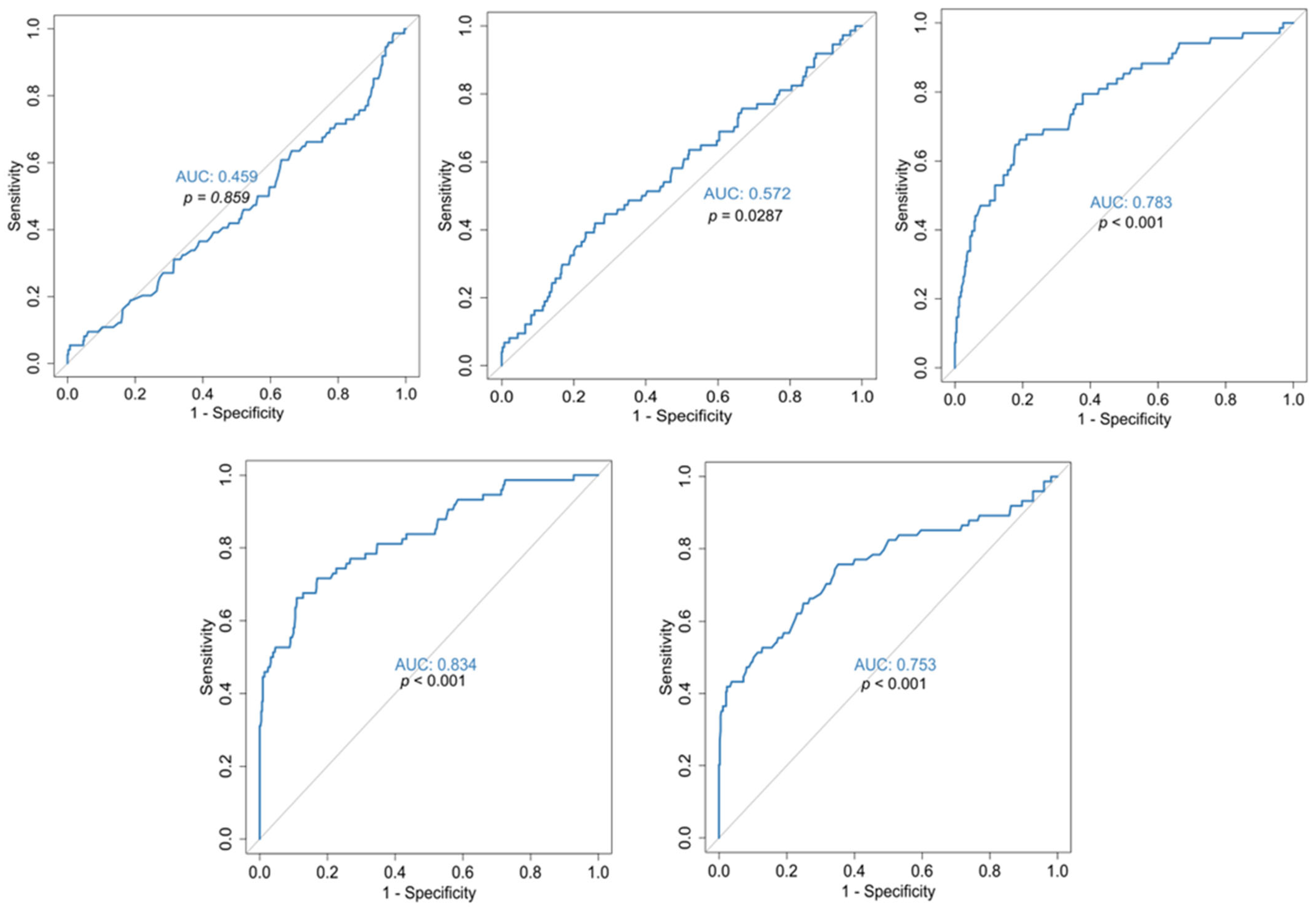
| Age | 0.0672 | 0.0263 | 0.0107 | 1.0696 (1.0157–1.1262) |
| Gender Female | 0.0844 | 0.4971 | 0.8652 | 1.088 (0.4107–2.8827) |
| RDW mean | 1.7723 | 0.2668 | <0.0001 | 5.8844 (3.4884–9.9263) |
| PLT mean | 0.0000 | 0.0000 | 0.9143 | 1 (1–1) |
| MPV/PLT ratio mean | 0.3873 | 0.2926 | 0.1856 | 1.473 (0.8301–2.6138) |
| PLT 2w | −0.000001 | 0.000007 | 0.8261 | 1 (1–1) |
| MPV/PLT ratio 2w | 0.0534 | 0.2619 | 0.8384 | 1.0549 (0.6314–1.7623) |
| Estimate | Std Error | p-Value | OR (95% CI) | |
|---|---|---|---|---|
| Age | −0.0226 | 0.0149 | 0.1276 | 0.9776 (0.9495–1.0065) |
| Gender Female | −1.5338 | 0.4468 | 0.0006 | 0.2157 (0.0899–0.5178) |
| RDW mean | 1.6300 | 0.5256 | 0.0019 | 5.1039 (1.8219–14.298) |
| Admission MPV/PLT ratio | 0.8461 | 0.2352 | 0.0003 | 2.3306 (1.4698–3.6956) |
| MPV/PLT ratio mean | −0.1815 | 0.1083 | 0.0939 | 0.834 (0.6745–1.0313) |
| Admission RDW | −0.2380 | 0.4427 | 0.5908 | 0.7882 (0.331–1.8769) |
| Admission PLTs | 0.000008 | 0.000004 | 0.0789 | 1 (1–1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgiadou, D.; Xanthos, T.; Komninaka, V.; Xatzikiriakou, R.; Baka, S.; Pouliakis, A.; Spyridaki, A.; Theodoridis, D.; Papapanagiotou, A.; Karida, A.; et al. Red Cell Distribution Width (RDW), Platelets and Platelet Index MPV/PLT Ratio as Specific Time Point Predictive Variables of Survival Outcomes in COVID-19 Hospitalized Patients. J. Clin. Med. 2025, 14, 5381. https://doi.org/10.3390/jcm14155381
Georgiadou D, Xanthos T, Komninaka V, Xatzikiriakou R, Baka S, Pouliakis A, Spyridaki A, Theodoridis D, Papapanagiotou A, Karida A, et al. Red Cell Distribution Width (RDW), Platelets and Platelet Index MPV/PLT Ratio as Specific Time Point Predictive Variables of Survival Outcomes in COVID-19 Hospitalized Patients. Journal of Clinical Medicine. 2025; 14(15):5381. https://doi.org/10.3390/jcm14155381
Chicago/Turabian StyleGeorgiadou, Despoina, Theodoros Xanthos, Veroniki Komninaka, Rea Xatzikiriakou, Stavroula Baka, Abraham Pouliakis, Aikaterini Spyridaki, Dimitrios Theodoridis, Angeliki Papapanagiotou, Afroditi Karida, and et al. 2025. "Red Cell Distribution Width (RDW), Platelets and Platelet Index MPV/PLT Ratio as Specific Time Point Predictive Variables of Survival Outcomes in COVID-19 Hospitalized Patients" Journal of Clinical Medicine 14, no. 15: 5381. https://doi.org/10.3390/jcm14155381
APA StyleGeorgiadou, D., Xanthos, T., Komninaka, V., Xatzikiriakou, R., Baka, S., Pouliakis, A., Spyridaki, A., Theodoridis, D., Papapanagiotou, A., Karida, A., Paliatsiou, S., Volaki, P., Barmparousi, D., Sakagianni, A., Tsagarakis, N. J., Alexandridou, M., Palla, E., Kanakaris, C., & Iacovidou, N. M. (2025). Red Cell Distribution Width (RDW), Platelets and Platelet Index MPV/PLT Ratio as Specific Time Point Predictive Variables of Survival Outcomes in COVID-19 Hospitalized Patients. Journal of Clinical Medicine, 14(15), 5381. https://doi.org/10.3390/jcm14155381








