Right Ventricular Structure and Function in Patients with Primary Aldosteronism: A Cardiac Magnetic Resonance Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
- (i)
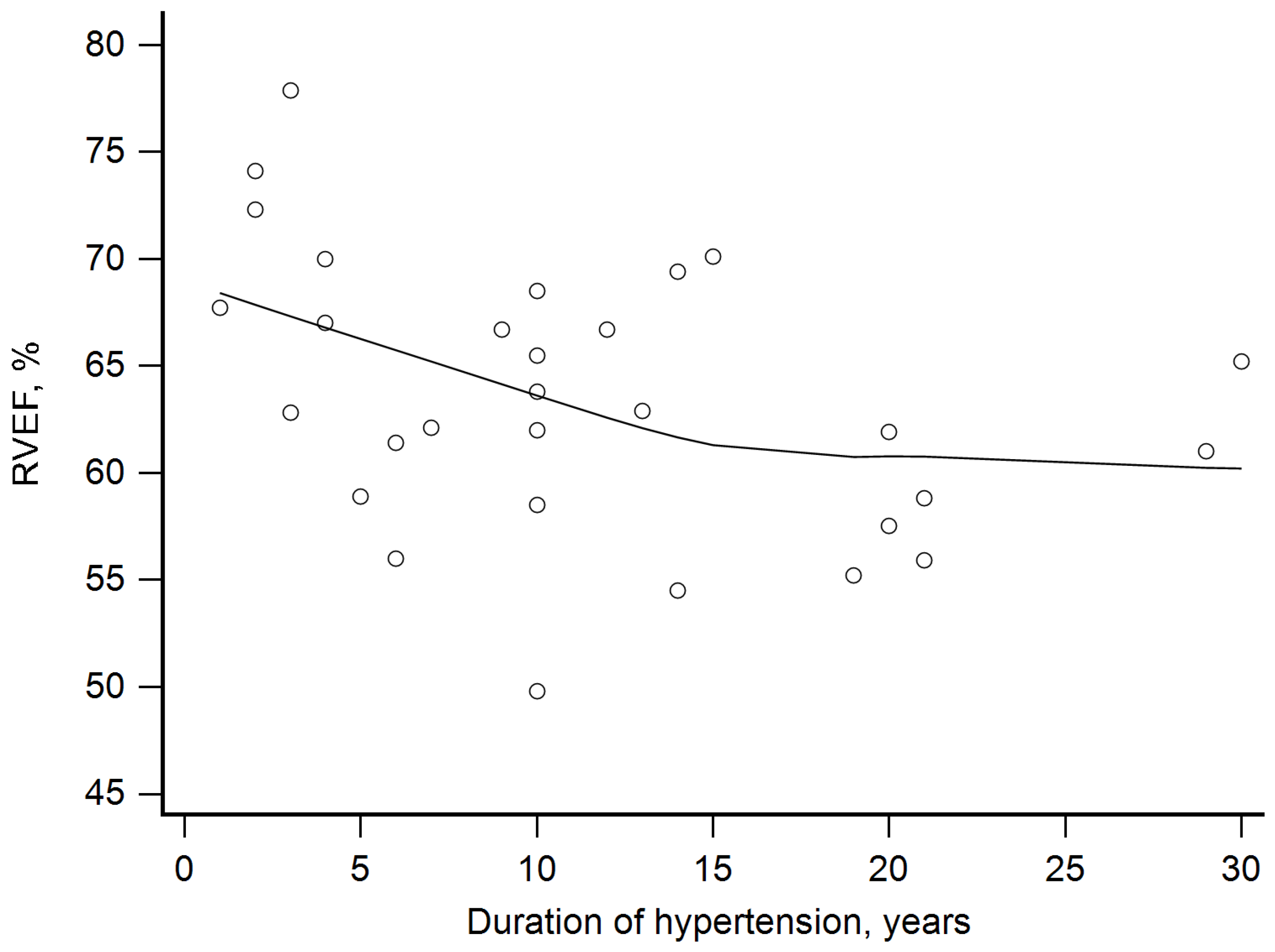
- The longer duration of hypertension, the higher the observed RVEDVi, and the longer duration of hypertension, the lower RVEF was noted.
- (ii)
- Although RVMi was within the normal range in all but two patients, it was greater in PA patients than in controls.
- (iii)
- Patients with PA exhibited unfavorable remodeling confirmed by higher RV mass-to-volume ratio when compared to healthy volunteers.
- (iv)
- PA patients exhibit subclinical RV systolic dysfunction expressed as impaired RV global longitudinal strain.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BP | Blood pressure |
| BSA | Body surface area |
| CMR | Cardiac magnetic resonance |
| eGFR | eGFR, estimated glomerular filtration |
| IQR | Interquartile range |
| LV | Left ventricular |
| PA | Primary aldosteronism |
| RV | Right ventricular |
| RVEDVi | Right ventricular end-diastolic volume index |
| RVEF | Right ventricular ejection fraction |
| RVESVi | Right ventricular end-systolic volume index |
| RVGLS | Right ventricular global longitudinal strain |
| RVMi | Right ventricular mass index |
| RVSVi | Right ventricular stroke volume index |
| SD | Standard deviation |
References
- Mancia, G.; Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2023, 41, 1874–2071. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Kocjan, T.; Theiler-Schwetz, V.; Trummer, C. Primary aldosteronism 2.0: An update for clinicians on diagnosis and treatment. Pol. Arch. Intern. Med. 2023, 133, 16585. [Google Scholar] [CrossRef] [PubMed]
- Januszewicz, A.; Mulatero, P.; Dobrowolski, P.; Monticone, S.; Van der Niepen, P.; Sarafidis, P.; Reincke, M.; Rexhaj, E.; Eisenhofer, G.; Januszewicz, M.; et al. Cardiac Phenotypes in Secondary Hypertension: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 80, 1480–1497. [Google Scholar] [CrossRef] [PubMed]
- Freel, E.M.; Mark, P.B.; Weir, R.A.; McQuarrie, E.P.; Allan, K.; Dargie, H.J.; McClure, J.D.; Jardine, A.G.; Davies, E.; Connell, J.M. Demonstration of blood pressure-independent noninfarct myocardial fibrosis in primary aldosteronism: A cardiac magnetic resonance imaging study. Circ. Cardiovasc. Imaging 2012, 5, 740–747. [Google Scholar] [CrossRef]
- Imiela, A.M.; Siedliński, M.; Dobrowolski, P.; Pręgowska-Chwała, B.; Kabat, M.; Oliveira Silva, R.N.; Koshy, A.M.; Wróbel, A.; Cendrowska-Demkow, I.; Januszewicz, M.; et al. Altered monocytic phenotypes are linked to a hypertension form: A clinical observational study. Kardiol. Pol. 2022, 80, 346–349. [Google Scholar] [CrossRef]
- Imiela, A.M.; Mikołajczyk, T.P.; Siedliński, M.; Dobrowolski, P.; Konior-Rozlachowska, A.; Wróbel, A.; Biernat-Kałuża, E.; Januszewicz, M.; Guzik, B.; Guzik, T.J.; et al. Th17/Treg imbalance in patients with primary hyperaldosteronism and resistant hypertension. Pol. Arch. Intern. Med. 2022, 132, 16171. [Google Scholar] [CrossRef]
- Imiela, A.M.; Mikołajczyk, T.P.; Kołodziejczyk-Kruk, S.; Kądziela, J.; Śpiewak, M.; Januszewicz, M.; Kabat, M.; Sterliński, I.; Wąs, M.; WróBel, A.; et al. Plasmacytoid dendritic cell content is associated with plasma aldosterone concentration in patients with primary aldosteronism. Am. J. Hypertens. 2025, hpaf019. [Google Scholar] [CrossRef]
- Ferreira, N.S.; Tostes, R.C.; Paradis, P.; Schiffrin, E.L. Aldosterone, Inflammation, Immune System, and Hypertension. Am. J. Hypertens. 2021, 34, 15–27. [Google Scholar] [CrossRef]
- Grotevendt, A.; Wallaschofski, H.; Reincke, M.; Adolf, C.; Quinkler, M.; Nauck, M.; Hoffmann, W.; Rettig, R.; Hannemann, A. Associations of aldosterone and renin concentrations with inflammation-the Study of Health in Pomerania and the German Conn’s Registry. Endocrine 2017, 57, 298–307. [Google Scholar] [CrossRef]
- Tsai, C.H.; Pan, C.T.; Chang, Y.Y.; Chen, Z.W.; Wu, V.C.; Hung, C.S.; Lin, Y.H. Left ventricular remodeling and dysfunction in primary aldosteronism. J. Hum. Hypertens. 2021, 35, 131–147. [Google Scholar] [CrossRef]
- Wu, T.; Ren, Y.; Wang, W.; Cheng, W.; Zhou, F.; He, S.; Liu, X.; Li, L.; Tang, L.; Deng, Q.; et al. Left Ventricular Remodeling in Patients with Primary Aldosteronism: A Prospective Cardiac Magnetic Resonance Imaging Study. Korean J. Radiol. 2021, 22, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Marzano, L.; Colussi, G.; Sechi, L.A.; Catena, C. Adrenalectomy is comparable with medical treatment for reduction of left ventricular mass in primary aldosteronism: Meta-analysis of long-term studies. Am. J. Hypertens. 2015, 28, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Cuspidi, C.; Tadic, M.; Sala, C.; Quarti-Trevano, F.; Gherbesi, E.; Mancia, G.; Grassi, G. Regression of left ventricular hypertrophy in primary aldosteronism after adrenalectomy: A meta-analysis of echocardiographic studies. J. Hypertens. 2021, 39, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, M.; Rudski, L.G.; Addetia, K.; Afilalo, J.; D’Alto, M.; Freed, B.H.; Friend, L.B.; Gargani, L.; Grapsa, J.; Hassoun, P.M.; et al. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults and Special Considerations in Pulmonary Hypertension: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2025, 38, 141–186. [Google Scholar] [CrossRef]
- Kramer, C.M.; Barkhausen, J.; Bucciarelli-Ducci, C.; Flamm, S.D.; Kim, R.J.; Nagel, E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 17. [Google Scholar] [CrossRef]
- Schulz-Menger, J.; Bluemke, D.A.; Bremerich, J.; Flamm, S.D.; Fogel, M.A.; Friedrich, M.G.; Kim, R.J.; von Knobelsdorff-Brenkenhoff, F.; Kramer, C.M.; Pennell, D.J.; et al. Standardized image interpretation and post-processing in cardiovascular magnetic resonance—2020 update: Society for Cardiovascular Magnetic Resonance (SCMR): Board of Trustees Task Force on Standardized Post-Processing. J. Cardiovasc. Magn. Reson. 2020, 22, 19. [Google Scholar] [CrossRef]
- Funder, J.W.; Carey, R.M.; Mantero, F.; Murad, M.H.; Reincke, M.; Shibata, H.; Stowasser, M.; Young, W.F., Jr. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 1889–1916. [Google Scholar] [CrossRef]
- Galiè, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [CrossRef]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC Task Force for the Management of Arterial Hypertension. J. Hypertens. 2018, 36, 2284–2309. [Google Scholar] [CrossRef]
- Lindholm, A.; Kjellström, B.; Rådegran, G.; Arheden, H.; Ostenfeld, E. Right ventricular dyssynchrony predicts outcome in pulmonary arterial hypertension when assessed in multiple cardiac magnetic resonance views. J. Cardiovasc. Magn. Reson. 2024, 26, 101103. [Google Scholar] [CrossRef]
- Barison, A.; Ceolin, R.; Palmieri, A.; Tamborrino, P.P.; Todiere, G.; Grigoratos, C.; Gueli, I.A.; De Gori, C.; Clemente, A.; Pistoia, L.; et al. Biventricular Tissue Tracking with Cardiovascular Magnetic Resonance: Reference Values of Left- and Right-Ventricular Strain. Diagnostics 2023, 13, 2912. [Google Scholar] [CrossRef]
- Śpiewak, M.; Kołodziejczyk-Kruk, S.; Sokołowska, D.; Waś, J.; Januszewicz, A.; Marczak, M. Relationships of markers of heart injury, overload, and inflammation with cardiac fibrosis in patients with primary aldosteronism. Pol. Arch. Intern. Med. 2025; Online ahead of print. [Google Scholar] [CrossRef]
- Zhan, Y.; Friedrich, M.G.; Dendukuri, N.; Lu, Y.; Chetrit, M.; Schiller, I.; Joseph, L.; Shaw, J.L.; Chuang, M.L.; Riffel, J.H.; et al. Meta-Analysis of Normal Reference Values for Right and Left Ventricular Quantification by Cardiovascular Magnetic Resonance. Circ. Cardiovasc. Imaging 2024, 17, e016090. [Google Scholar] [CrossRef] [PubMed]
- Tadic, M.; Cuspidi, C. Right Ventricle in Arterial Hypertension: Did We Forget Something? J. Clin. Med. 2022, 11, 6257. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Vriz, O.; Motoji, Y.; Ferrara, F.; Bossone, E.; Naeije, R. The Right Heart-Pulmonary Circulation Unit in Systemic Hypertension. Heart Fail. Clin. 2018, 14, 247–253. [Google Scholar] [CrossRef]
- Vriz, O.; Argiento, P.; D’Alto, M.; Ferrara, F.; Vanderpool, R.; Naeije, R.; Bossone, E. Increased pulmonary vascular resistance in early stage systemic hypertension: A resting and exercise stress echocardiography study. Can. J. Cardiol. 2015, 31, 537–543. [Google Scholar] [CrossRef]
- Brilla, C.G.; Pick, R.; Tan, L.B.; Janicki, J.S.; Weber, K.T. Remodeling of the rat right and left ventricles in experimental hypertension. Circ. Res. 1990, 67, 1355–1364. [Google Scholar] [CrossRef]
- Weber, K.T.; Brilla, C.G. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation 1991, 83, 1849–1865. [Google Scholar] [CrossRef]
- Lombès, M.; Alfaidy, N.; Eugene, E.; Lessana, A.; Farman, N.; Bonvalet, J.P. Prerequisite for cardiac aldosterone action. Mineralocorticoid receptor and 11 beta-hydroxysteroid dehydrogenase in the human heart. Circulation 1995, 92, 175–182. [Google Scholar] [CrossRef]
- Patel, R.B.; Li, E.; Benefield, B.C.; Swat, S.A.; Polsinelli, V.B.; Carr, J.C.; Shah, S.J.; Markl, M.; Collins, J.D.; Freed, B.H. Diffuse right ventricular fibrosis in heart failure with preserved ejection fraction and pulmonary hypertension. ESC Heart Fail. 2020, 7, 253–263. [Google Scholar] [CrossRef]
- Pennell, D.J. Ventricular volume and mass by CMR. J. Cardiovasc. Magn. Reson. 2002, 4, 507–513. [Google Scholar] [CrossRef]
- Pattynama, P.M.; Lamb, H.J.; Van der Velde, E.A.; Van der Geest, R.J.; Van der Wall, E.E.; De Roos, A. Reproducibility of MRI-derived measurements of right ventricular volumes and myocardial mass. Magn. Reson. Imaging 1995, 13, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Espe, E.K.S.; Bendiksen, B.A.; Zhang, L.; Sjaastad, I. Analysis of right ventricular mass from magnetic resonance imaging data: A simple post-processing algorithm for correction of partial-volume effects. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H912–H922. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.M.; Schmieder, R.E. Aldosterone-induced cardiac damage: Focus on blood pressure independent effects. Am. J. Hypertens. 2003, 16, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Todiere, G.; Neglia, D.; Ghione, S.; Fommei, E.; Capozza, P.; Guarini, G.; Dell’Omo, G.; Aquaro, G.D.; Marzilli, M.; Lombardi, M.; et al. Right ventricular remodelling in systemic hypertension: A cardiac MRI study. Heart 2011, 97, 1257–1261. [Google Scholar] [CrossRef]
- Kochav, J.; Chen, J.; Nambiar, L.; Mitlak, H.W.; Kushman, A.; Sultana, R.; Horn, E.; RoyChoudhury, A.; Devereux, R.B.; Weinsaft, J.W.; et al. Novel Echocardiographic Algorithm for Right Ventricular Mass Quantification: Cardiovascular Magnetic Resonance and Clinical Prognosis Validation. J. Am. Soc. Echocardiogr. 2021, 34, 839–850.e831. [Google Scholar] [CrossRef]
- Lu, J.C.; Christensen, J.T.; Yu, S.; Donohue, J.E.; Ghadimi Mahani, M.; Agarwal, P.P.; Dorfman, A.L. Relation of right ventricular mass and volume to functional health status in repaired tetralogy of Fallot. Am. J. Cardiol. 2014, 114, 1896–1901. [Google Scholar] [CrossRef]
- Tadic, M.; Cuspidi, C.; Bombelli, M.; Grassi, G. Right heart remodeling induced by arterial hypertension: Could strain assessment be helpful? J. Clin. Hypertens. 2018, 20, 400–407. [Google Scholar] [CrossRef]
- Tadic, M.; Cuspidi, C.; Pencic, B.; Sljivic, A.; Ivanovic, B.; Neskovic, A.; Scepanovic, R.; Celic, V. High-normal blood pressure impacts the right heart mechanics: A three-dimensional echocardiography and two-dimensional speckle tracking imaging study. Blood Press. Monit. 2014, 19, 145–152. [Google Scholar] [CrossRef]
- Tadic, M.; Cuspidi, C.; Suzic-Lazic, J.; Andric, A.; Stojcevski, B.; Ivanovic, B.; Hot, S.; Scepanovic, R.; Celic, V. Is there a relationship between right-ventricular and right atrial mechanics and functional capacity in hypertensive patients? J. Hypertens. 2014, 32, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Anastasiou, V.; Papazoglou, A.S.; Moysidis, D.V.; Daios, S.; Tsalikakis, D.; Giannakoulas, G.; Karamitsos, T.; Delgado, V.; Ziakas, A.; Kamperidis, V. The prognostic value of right ventricular longitudinal strain in heart failure: A systematic review and meta-analysis. Heart Fail. Rev. 2023, 28, 1383–1394. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, S.; Orihara, Y.; Eguchi, A.; Pfeiffer, M.; Peterson, B.; Ruzieh, M.; Gao, Z.; Boehmer, J.; Gorcsan, J., 3rd; Wilson, R. Additive Value of Right Ventricular Global Longitudinal Strain to a Conventional Echocardiographic Parameter to Improve Prognostic Value in Intermediate-Risk Pulmonary Embolism. J. Am. Heart Assoc. 2025, 14, e036294. [Google Scholar] [CrossRef] [PubMed]
- Hulshof, H.G.; Eijsvogels, T.M.H.; Kleinnibbelink, G.; van Dijk, A.P.; George, K.P.; Oxborough, D.L.; Thijssen, D.H.J. Prognostic value of right ventricular longitudinal strain in patients with pulmonary hypertension: A systematic review and meta-analysis. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 475–484. [Google Scholar] [CrossRef]
- Tadic, M.; Suzic, J.; Sljivic, A.; Andric, A.; Vukomanovic, V.; Filipovic, T.; Celic, V.; Cuspidi, C. The Relationship Between Right Ventricular Longitudinal Strain and Adverse Outcome in Hypertensive Patients: 10-year Follow-up. High. Blood Press. Cardiovasc. Prev. 2024, 31, 631–638. [Google Scholar] [CrossRef]
- Tadic, M.; Filipovic, T.; Suzic, J.; Majstorovic, A.; Pencic, B.; Vukomanovic, V.; Cuspidi, C.; Celic, V. The Predictive Value of Global Longitudinal and Circumferential Strains in Hypertensive Patients: 10-Year Follow-Up. J. Clin. Med. 2024, 13, 5799. [Google Scholar] [CrossRef]
- Reiser, C.S.; Assuncao, A.N., Jr.; Araujo-Filho, J.A.B.; Dantas, R.N., Jr.; Bortolotto, L.A.; Parga-Filho, J.R. Left ventricle remodeling by CMR in treated patients with primary aldosteronism and primary systemic arterial hypertension. PLoS ONE 2024, 19, e0316140. [Google Scholar] [CrossRef]
- Zhou, F.; Wu, T.; Wang, W.; Cheng, W.; Wan, S.; Tian, H.; Chen, T.; Sun, J.; Ren, Y. CMR-Verified Myocardial Fibrosis Is Associated With Subclinical Diastolic Dysfunction in Primary Aldosteronism Patients. Front. Endocrinol. 2021, 12, 672557. [Google Scholar] [CrossRef]
- Wu, T.; Xu, C.; Tang, L.; Wu, X.; Peng, P.; Yue, X.; Cheng, W.; He, S.; Li, L.; Chen, Y.; et al. NT-pro-BNP Level is Related to Left Ventricular Remodeling in Patients With Primary Aldosteronism. Exp. Clin. Endocrinol. Diabetes 2024, 132, 562–569. [Google Scholar] [CrossRef]
- Redheuil, A.; Blanchard, A.; Pereira, H.; Raissouni, Z.; Lorthioir, A.; Soulat, G.; Vargas-Poussou, R.; Amar, L.; Paul, J.L.; Helley, D.; et al. Aldosterone-Related Myocardial Extracellular Matrix Expansion in Hypertension in Humans: A Proof-of-Concept Study by Cardiac Magnetic Resonance. JACC Cardiovasc. Imaging 2020, 13, 2149–2159. [Google Scholar] [CrossRef]
- Higuchi, S.; Ota, H.; Tezuka, Y.; Seiji, K.; Takagi, H.; Lee, J.; Lee, Y.W.; Omata, K.; Ono, Y.; Morimoto, R.; et al. Aldosterone-induced cardiac damage in primary aldosteronism depends on its subtypes. Endocr. Connect. 2021, 10, 29–36. [Google Scholar] [CrossRef]
- Karaye, K.M.; Bonny, A. Right ventricular dysfunction in systemic hypertension: A call to action. Int. J. Cardiol. 2016, 206, 51–53. [Google Scholar] [CrossRef]
- Marketou, M.; Anastasiou, I.; Nakou, H.; Kochiadakis, G.; Patrianakos, A.; Fragkiadakis, K.; Parthenakis, F. Right ventricular dysfunction in arterial hypertension: Still terra incognita? J. Hum. Hypertens. 2019, 33, 491–498. [Google Scholar] [CrossRef]

| Primary Aldosteronism Patients (n = 30) | Controls (n = 30) | p-Value | |
|---|---|---|---|
| Age, years | 51.0 (10.4) | 50.7 (10.6) | 0.88 |
| Sex, males | 50% | 50% | 1.0 |
| Duration of hypertension, years | 10.0 (5.0–15.0) | – | – |
| Aldosterone, pg/mL | 263.5 (175.0–473.0) | ||
| Renin, pg/mL | 1.8 (0.78–5.0) | ||
| Aldosterone renin ratio | 168 (58–587) | ||
| eGFR, mL/min/1.73 m2 | 100 (83–105) | ||
| Potassium, mmol/L | 3.63 (0.51) | ||
| Number of antihypertensive medications, n | 4 (3–5) | – | – |
| Medications at the time of CMR | – | – | |
| RAS blocker, n (%) | 28 (93.7) | – | – |
| ACE inhibitors, n (%) | 14 (46.7) | – | – |
| ARB, n (%) | 14 (46.7) | – | – |
| Calcium channel blocker, n (%) | 24 (80) | – | – |
| Beta-blocker, n (%) | 22 (73.3) | – | – |
| MRA, n (%) | 16 (53.3) | – | – |
| Diuretics, n (%) | 12 (40) | – | – |
| Thiazide/Thiazide-like, n (%) | 10 (33.3) | – | – |
| Loop, n (%) | 2 (6.7) | – | – |
| Alpha-blocker, n (%) | 11 (36.7) | – | – |
| Primary Aldosteronism Patients (n = 30) | Controls (n = 30) | p-Value | |
|---|---|---|---|
| RVEDVi, mL/m2 | 79.0 (16.0) | 72.0 (13.3) | 0.077 |
| RVEDVi exceeding the upper reference range, n (%) | 1 (3.3%) | 0 | 1.0 |
| RVESVi, mL/m2 | 29.4 (9.7) | 26.7 (8.7) | 0.27 |
| RVSVi, mL/m2 | 49.6 (8.5) | 45.4 (6.1) | 0.03 |
| RVEF, % | 63.5 (6.4) | 63.7 (6.2) | 0.93 |
| RVEF below the lower reference range, n (%) | 0 | 0 | 1.0 |
| RVMi, g/m2 | 18.9 (4.9) | 13.6 (3.2) | <0.0001 |
| RVMi exceeding the upper reference range, n (%) | 2 (6.7%) | 0 | 0.49 |
| RV mass-to-volume ratio, g/mL | 0.25 (0.08) | 0.19 (0.04) | 0.001 |
| RVGLS, % | −16.8 (2.5) | −19.6 (2.7) | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Śpiewak, M.; Kołodziejczyk-Kruk, S.; Kubik, A.; Łebek-Szatańska, A.; Szwench-Pietrasz, E.; Florczak, E.; Januszewicz, M.; Januszewicz, A.; Marczak, M. Right Ventricular Structure and Function in Patients with Primary Aldosteronism: A Cardiac Magnetic Resonance Study. J. Clin. Med. 2025, 14, 5367. https://doi.org/10.3390/jcm14155367
Śpiewak M, Kołodziejczyk-Kruk S, Kubik A, Łebek-Szatańska A, Szwench-Pietrasz E, Florczak E, Januszewicz M, Januszewicz A, Marczak M. Right Ventricular Structure and Function in Patients with Primary Aldosteronism: A Cardiac Magnetic Resonance Study. Journal of Clinical Medicine. 2025; 14(15):5367. https://doi.org/10.3390/jcm14155367
Chicago/Turabian StyleŚpiewak, Mateusz, Sylwia Kołodziejczyk-Kruk, Agata Kubik, Agnieszka Łebek-Szatańska, Elżbieta Szwench-Pietrasz, Elżbieta Florczak, Magdalena Januszewicz, Andrzej Januszewicz, and Magdalena Marczak. 2025. "Right Ventricular Structure and Function in Patients with Primary Aldosteronism: A Cardiac Magnetic Resonance Study" Journal of Clinical Medicine 14, no. 15: 5367. https://doi.org/10.3390/jcm14155367
APA StyleŚpiewak, M., Kołodziejczyk-Kruk, S., Kubik, A., Łebek-Szatańska, A., Szwench-Pietrasz, E., Florczak, E., Januszewicz, M., Januszewicz, A., & Marczak, M. (2025). Right Ventricular Structure and Function in Patients with Primary Aldosteronism: A Cardiac Magnetic Resonance Study. Journal of Clinical Medicine, 14(15), 5367. https://doi.org/10.3390/jcm14155367





