1. Background
Full-thickness macular holes (MHs) are a significant cause of central vision impairment, with a prevalence ranging from 0.2% to 0.8% in the general population, affecting predominantly women and individuals over the age of 60 [
1,
2]. Despite considerable advances in vitreoretinal surgery, the management of large MHs (greater than 400 µm) remains a challenge, with suboptimal visual recovery even in anatomically successful cases.
The term “macular hole” has long been used in ophthalmic nomenclature, yet it may oversimplify the true pathomorphological nature of the condition. The word “hole” implies an empty space to be filled or plugged. In contrast, current understanding suggests that full-thickness MHs should be regarded as mechanical retinal ruptures, involving tissue discontinuity rather than simple absence of structure [
3,
4]. This conceptual shift carries important clinical implications, promoting a surgical strategy focused on active approximation and biological stabilization of retinal tissue, rather than passive sealing with exogenous materials.
Several mechanisms have been implicated in the formation of macular ruptures, including horizontal vitreoretinal traction (particularly tangential forces at the foveola), sudden posterior vitreous detachment (PVD), structural changes and fibrosis of the ILM, and secondary causes such as trauma, high myopia, diabetes, or previous ocular procedures [
4,
5,
6].
Conventional surgical techniques typically involve pars plana vitrectomy (PPV) with ILM peeling and gas or silicone oil tamponade. More advanced methods include the inverted ILM flap technique or the use of biological adjuvants such as PRP, amniotic membrane, or collagen matrix [
7,
8,
9]. Although these approaches have demonstrated high closure rates, they often leave non-physiological material on the retinal surface, potentially promoting gliotic scar formation and limiting neuroretinal recovery [
10,
11]. Moreover, PRP’s (platelet-rich plasma) high leukocyte and erythrocyte content has been linked to increased postoperative inflammation and delayed wound healing [
11,
12].
The present study introduces an alternative concept based on the use of ACP, a leukocyte-poor concentrate capable of forming a transparent fibrin membrane in situ. This membrane functions as a temporary biological scaffold that facilitates edge approximation and supports neuroepithelial regeneration without leaving foreign tissue or inducing chronic inflammation. Intraoperative optical coherence tomography (i-OCT) enables real-time visualization of the macular hole margins, allowing for precise control of membrane placement and tissue positioning [
3,
4,
10].
Therefore, the aim of this study is to evaluate the safety and efficacy of ACP in the treatment of large idiopathic macular holes (400–800 µm), with particular attention to early postoperative visual outcomes, surgical control, and the avoidance of pro-inflammatory sequelae associated with traditional techniques.
2. Materials and Methods
2.1. Study Design
This retrospective, single-center study included 50 patients (70 eyes) who underwent pars plana vitrectomy (PPV) combined with ACP application for the treatment of idiopathic full-thickness MHs between 2018 and 2024. The study adhered to the principles of the Declaration of Helsinki and received approval from the Local Bioethics Committee (approval number: AKBE/27/2025). Informed consent was obtained from all participants.
2.2. Statistical Methods
The paired Student’s t-test was applied to compare mean BCVA values measured one day before surgery, on postoperative day 7, and at the 12-month follow-up. A p-value < 0.001 was considered statistically significant based on the results obtained.
2.3. Inclusion and Exclusion Criteria
The inclusion criteria were as follows:
- (1)
Idiopathic full-thickness macular holes;
- (2)
Minimum linear diameter of the hole between 400 and 800 µm;
- (3)
Age ≥ 18 years;
- (4)
Complete preoperative and postoperative data available;
- (5)
Informed consent for surgery and participation.
The exclusion criteria were as follows:
- (1)
Secondary MHs due to trauma, high myopia, or retinal vascular diseases;
- (2)
Previous retinal surgery in the studied eye;
- (3)
Concomitant advanced glaucoma or uveitis;
- (4)
Retinal detachment.
2.4. Preoperative and Postoperative Assessment
All patients underwent a comprehensive ophthalmological examination including BCVA, intraocular pressure (IOP) measurement, slit-lamp biomicroscopy, dilated fundus examination, and spectral-domain optical coherence tomography (OCT). BCVA was measured using a decimal Snellen chart at a distance of 6 m under standardized ambient photopic conditions. Values were recorded in decimal format and converted into logMAR units for statistical evaluation. All measurements were performed by trained ophthalmic personnel under consistent clinical settings.
Follow-up visits were scheduled at day 1, day 7, 1 month, 3 months, 6 months, and 12 months postoperatively.
The primary outcome was defined as complete anatomical closure of the macular hole confirmed by OCT. The secondary outcome was defined as improvement in BCVA recorded on day 7 and at subsequent follow-up visits.
Intraoperative and postoperative complications such as retinal tears, elevated IOP, vitreous hemorrhage, fibrin reaction, or reopening of the MH were systematically documented.
2.5. Surgical Technique
A subtotal pars plana vitrectomy was performed using the CONSTELLATION® Vision System (Alcon, Fort Worth, TX, USA). The posterior hyaloid membrane was visualized with a suspension of triamcinolone acetonide and gently detached using a ULTRAVIT® 27+ vitreotome (Alcon, Fort Worth, TX, USA).
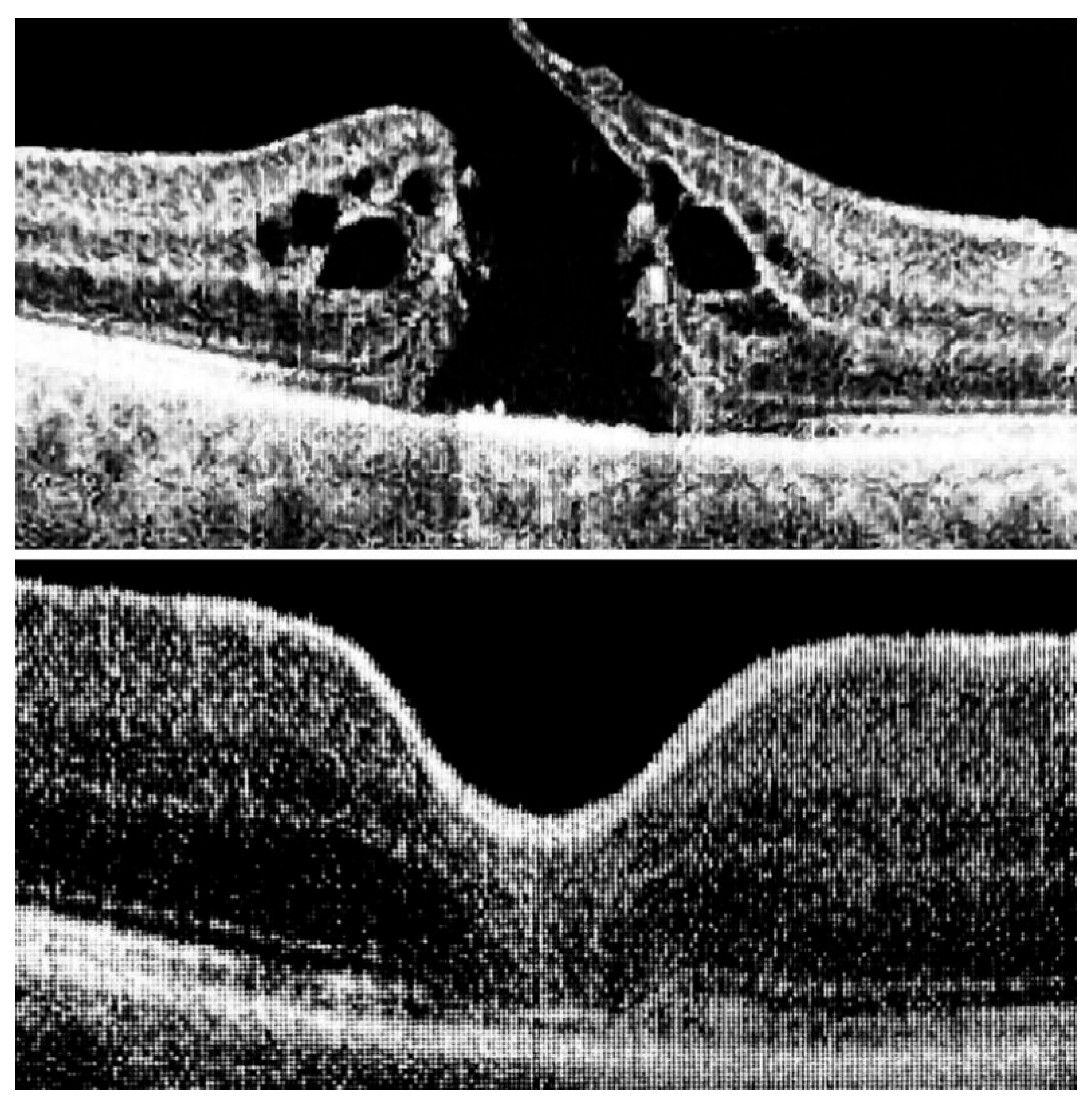
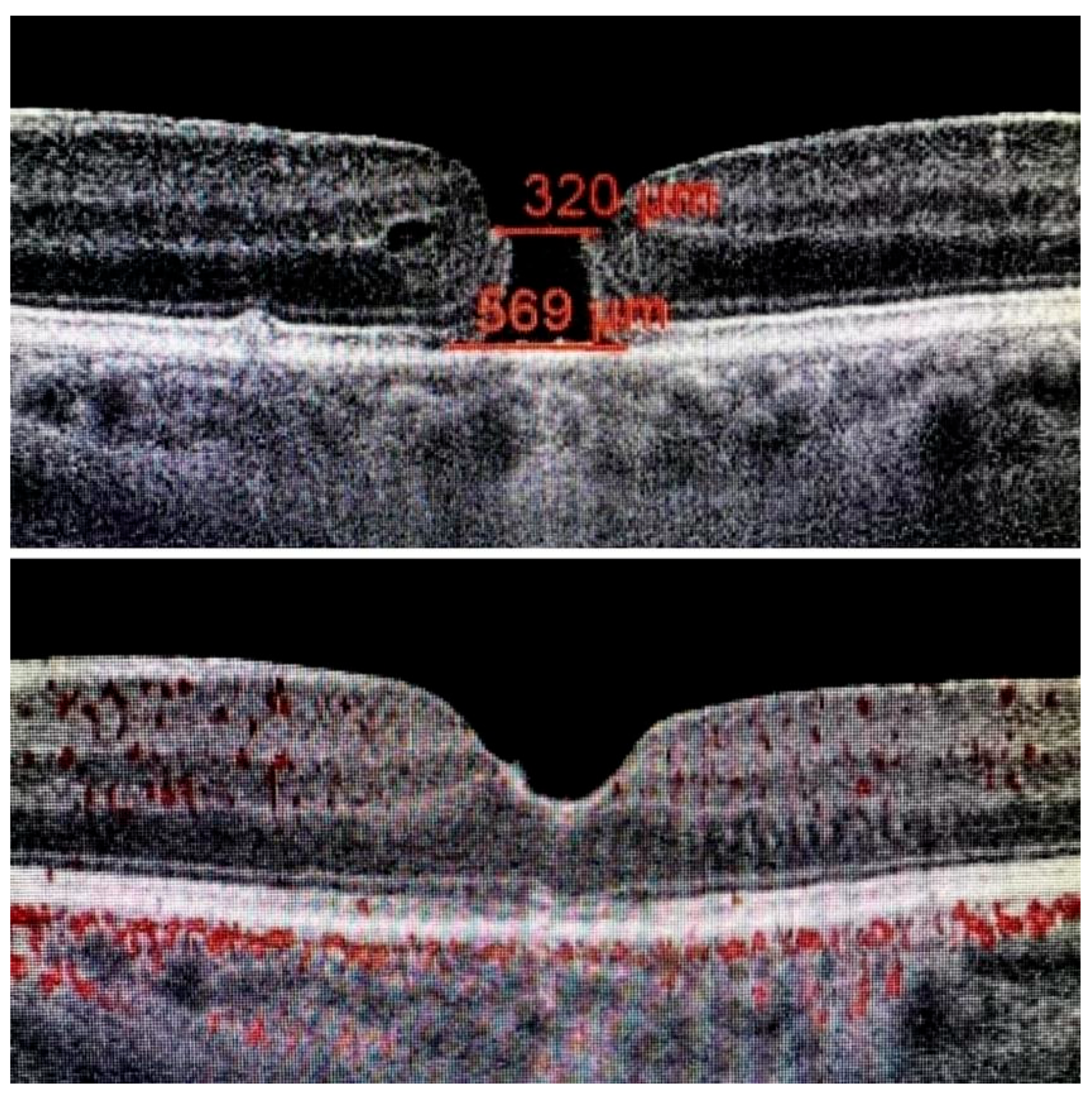
The ILM was peeled without the use of vital dyes, employing visualization techniques for transparent structures. A 25G silicone soft-tip cannula was used to gently approximate the edges of the macular hole under a balanced salt solution. Triamcinolone aceonide (Kenalog) was deposited on the ILM surface to enhance its visibility, and additional guidance was provided by instrument shadows under green-light illumination. Peeling was initiated at the point of weakest adhesion between the ILM and the retina. A pair of vitreoretinal forceps (e.g., Alcon Grieshaber AG, Schaffhausen, Switzerland) was used to gently grasp and lift the ILM to initiate separation. The extent of peeling covered an area of approximately 2–3 optic disc diameters around the hole to relieve tangential traction. Following ILM peeling and centripetal tissue mobilization, the macular hole edges were approximated under balanced salt solution or, in selected cases, perfluorocarbon liquid. Closure was monitored in real time using high-resolution intraoperative OCT (RESCAN™ 700 integrated with OPMI LUMERA
® 700 microscope, Carl Zeiss Meditec AG, Jena, Germany). Representative intraoperative OCT images before and after ACP application are presented in
Figure 1,
Figure 2 and
Figure 3.
ACP was applied over the retinal surface in the area of ILM removal. To ensure safe and controlled delivery, ACP was aspirated into two sterile 1 mL syringes. Although the total volume prepared was 1–2 mL, only a single drop (approximately 0.02 mL) was applied over the macular hole under i-OCT guidance.
Within 2–3 min, i-OCT confirmed the formation of a transparent fibrin membrane tightly adherent to the exposed retina, effectively sealing the macular hole and preventing fluid ingress. At the end of surgery, the intraocular fluid was exchanged with air. Patients were instructed to maintain a face-down position for 10 h postoperatively.
After this initial period, patients were allowed to assume any comfortable posture, such as sitting or lying on the side, while strictly avoiding the supine position. This recommendation was intended to prolong macular exposure to the air tamponade and to minimize the risk of lens opacification in phakic eyes. The recommendation for early face-down positioning following surgery is supported by findings from a Cochrane systematic review, which evaluated the impact of postoperative posturing on macular hole closure rates [
13].
All procedures were performed by a single experienced vitreoretinal surgeon under general anesthesia (TIVA).
2.6. ACP Preparation
ACP is an autologous, leukocyte-poor platelet concentrate, commonly used in regenerative medicine and recently adapted for intraocular applications. The details of ACP preparation using the Arthrex system are presented in
Table 1. In this study, ACP was prepared intraoperatively using the Arthrex ACP Double Syringe System. A total of 15 mL of autologous venous blood were collected during surgery, followed by centrifugation at 1600 rpm for 5 min. This process yielded 5 mL of leukocyte-poor plasma. No additional processing or exogenous activation was applied prior to intraocular use.
Routine sterility testing was not performed, as the autologous origin of ACP, the preoperative hematologic screening of all patients, and the low leukocyte content significantly minimized the risk of contamination or immune response. Furthermore, no cases of intraocular infection or inflammation were observed in the postoperative period.
The resulting ACP contains a moderate concentration of platelets and a significantly reduced leukocyte and erythrocyte content compared to classical PRP preparations. This profile minimizes the risk of pro-inflammatory reactions and supports tissue regeneration.
Representative values based on intraoperative ACP preparation using the Arthrex system, consistent with findings reported by Fitzpatrick et al. [
9].
3. Results
Visual acuity improved significantly in the majority of cases. The mean preoperati-ve BCVA was 0.25 ± 0.21. Baseline demographic and clinical characteristics of the study group are summarized in
Table 2. On postoperative day 7, the mean BCVA improved to 0.69 ± 0.20 (
p < 0.001; paired Student’s
t-test). Notably, 55 eyes (78.6%) achieved an improvement of at least three lines on the Snellen chart by day 7 postoperatively. A detailed summary of visual acuity outcomes is presented in
Table 3. Macular hole size ranged from 400 to 800 µm. The mean diameter was 448 µm (SD: 52 µm). Only two cases exceeded 700 µm. The duration of disease ranged from 0.5 to 14 months, with a mean of 4.2 months. All patients completed the 12-month postoperative follow-up.
Cataract progression was observed as a common postoperative event. In the studied cohort, 59 out of 70 eyes (84.3%) developed cataracts within six months following vitrectomy.
Table 4 presents postoperative outcomes at 12-month follow-up, including cataract progression and anatomical closure status.
4. Discussion
The surgical management of large full-thickness MHs remains a significant challenge in vitreoretinal surgery. This study introduces a conceptually novel approach, viewing MHs as mechanical ruptures of retinal tissue rather than empty defects. This paradigm shift has implications for surgical technique, emphasizing precise edge approximation and biologically integrated closure.
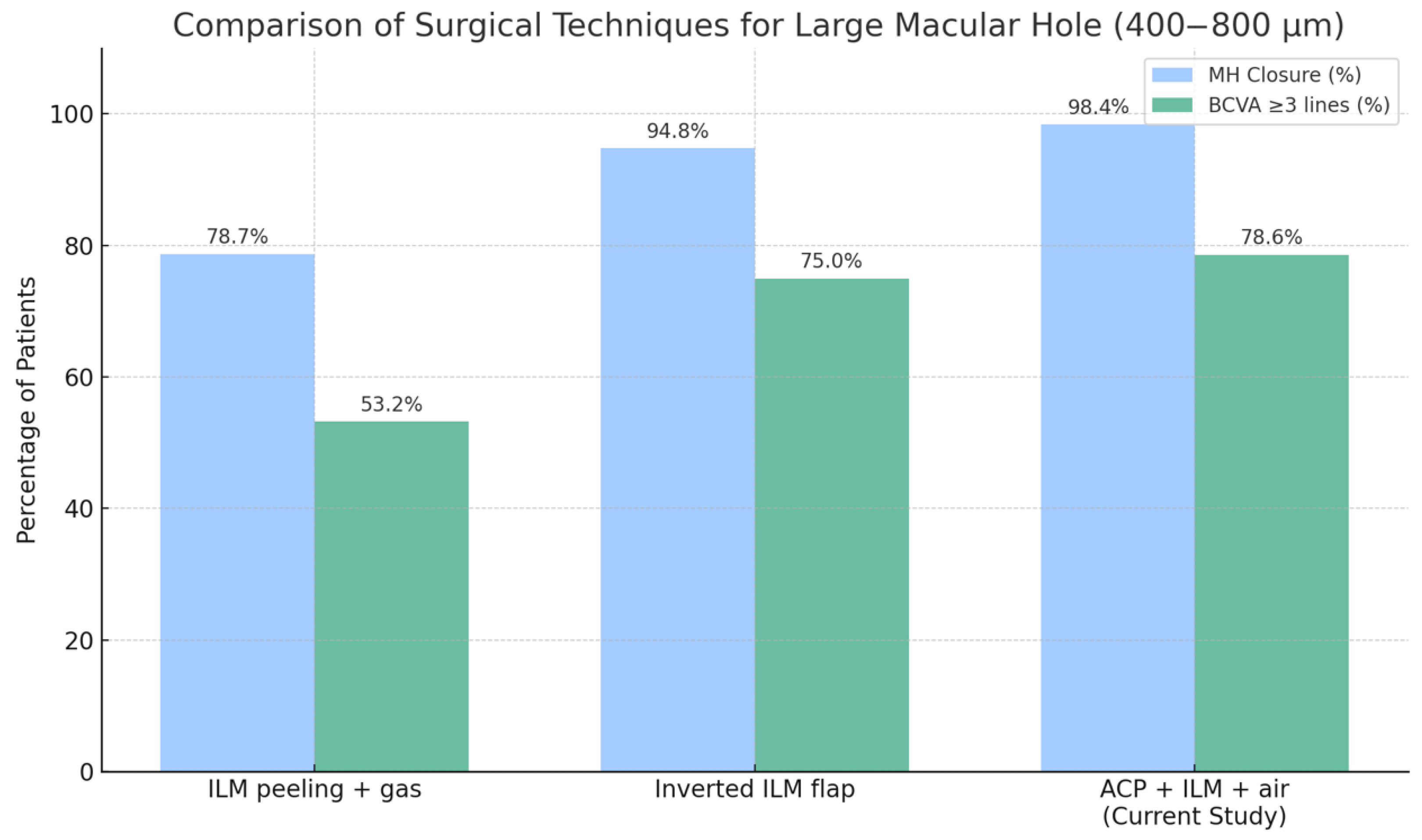
Our results demonstrate that the use of ACP, combined with intraoperative optical coherence tomography (i-OCT), is associated with a high closure rate of 98.6% and early functional improvement, with 78.6% of patients gaining three or more Snellen lines of BCVA by day 7. These outcomes compare favorably to those achieved using techniques such as internal limiting membrane (ILM) peeling with gas tamponade or inverted ILM flap procedures.
The in situ formation of a transparent fibrin membrane following ACP application provides temporary stabilization of the macular rupture and serves as a scaffold for neuroepithelial regeneration. The membrane is fully reabsorbed, leaving no residual material. I-OCT allowed real-time visualization and control of macular hole edge approximation and membrane application, which likely contributed to the favorable anatomical and functional results.
In two cases, a reduction in best corrected visual acuity of three or more Snellen lines was observed at both day 7 and 12-month follow-up. In both instances, retinal pigment epithelium (RPE) dysfunction was identified as the primary limiting factor. No intraoperative complications or reopenings were reported. A comparison of surgical outcomes and complications using different techniques is shown in
Figure 4 and
Figure 5.
4.1. Comparison with Platelet-Rich Plasma (PRP)
Several studies have investigated the use of autologous blood derivatives such as PRP in macular hole surgery. PRP contains a high concentration of leukocytes, erythrocytes, and inflammatory mediators, which may promote gliosis and fibrotic healing, potentially limiting functional outcomes [
9,
11,
12]. ACP, in contrast, is a leukocyte-poor autologous plasma concentrate designed to minimize the risk of postoperative inflammation. It forms a transparent fibrin membrane that supports mechanical closure without inducing secondary tissue proliferation. The present study found that ACP reduced leukocyte content approximately ninefold compared to standard PRP preparations, which may contribute to its favorable clinical profile [
9,
11]. Earlier reports, including those by Liggett et al. [
15] and Gotzaridis et al. [
16], described anatomical closure with PRP or autologous serum, but without consistent visual improvement. The reduced inflammatory profile of ACP may address this limitation, suggesting a more suitable biological environment for macular healing [
9,
11,
12].
4.2. Comparison with ILM Grafts
ILM grafting is a widely used technique for treating large MHs, providing mechanical coverage of the defect and improving anatomical closure rates. However, this approach often results in the formation of gliotic scar tissue within the foveal region, which may impair functional outcomes due to architectural distortion and limited neuroretinal recovery [
17]. Morizane et al. reported anatomical closure rates of 85–90% using autologous ILM transplantation, yet emphasized that visual gains were frequently suboptimal, primarily due to glial proliferation within the grafted area [
17].
In contrast, the ACP-based technique evaluated in the present study facilitates closure without introducing permanent or fibrotic material into the foveal space. The fibrin membrane formed by ACP is fully resorbable and supports tension-free sealing, minimizing the risk of gliosis and promoting more physiological neuroepithelial regeneration [
10,
11,
18]. Additionally, i-OCT guidance ensures real-time monitoring of edge approximation and precise placement of the fibrin matrix, further enhancing surgical predictability and minimizing postoperative distortion [
3,
4].
These findings suggest that ACP combined with i-OCT may offer a superior alternative to ILM grafting for large MHs, particularly in cases where preservation of the foveal microarchitecture and early functional recovery are primary goals. Other biological adjuvants, such as human amniotic membrane plugs, have also been investigated in the treatment of complex or recurrent macular holes, offering an alternative scaffold for retinal repair [
19].
4.3. Comparison with Gas Tamponade
Gas tamponade with face-down positioning is considered a standard approach for small and medium-sized MHs. However, its efficacy is reduced in large defects (>400 µm), where closure often relies on secondary connective tissue formation [
20]. Altaweel et al. (2003) reported limited functional improvement when gas tamponade was used in large MHs [
21]. In contrast, ACP promotes fibrin membrane formation, facilitating immediate macular hole edge approximation in a fluid-filled eye. The combination of ACP with i-OCT enables direct visualization and real-time adjustment of the MH edges, improving closure rates and limiting reliance on postoperative positioning or scar tissue formation [
4,
14,
18].
4.4. Advantages of the ACP + Intra-OCT Approach
The combination of ACP and i-OCT offers several advantages over traditional methods. It is important to note that ACP was used exclusively as an intraoperative coadjuvant. All patients underwent standard surgical procedures including ILM peeling, mechanical edge approximation, and air tamponade. ACP enables rapid fibrin membrane formation with no inflammatory response due to its leukocyte-poor composition [
4,
11,
18]. The i-OCT guidance ensures safe and precise edge approximation without the use of vital dyes, reducing the risk of phototoxicity and mechanical damage [
3,
10]. This strategy results in both a high closure rate and rapid functional recovery. The early BCVA improvement observed in this study, with stabilization over 12 months, supports the regenerative potential of this technique and its clinical applicability, as demonstrated by comparison with outcomes from recent meta-analyses, including Mihalache et al. (2024) [
20]. A comparative summary of published outcomes is presented in
Table 5.
4.5. Mechanism of Action of Activated Conditioned Plasma (ACP)
Unlike silicone oil or inverted ILM flaps, ACP forms a fully resorbable, in situ polymerized fibrin membrane that provides effective sealing without requiring removal or manipulation. It eliminates the need for vital dyes and prolonged tamponade, reducing risks of toxicity and mechanical trauma. The use of green-filtered illumination during surgery further protects the retinal pigment epithelium and ganglion cells, contributing to overall surgical safety. Activated conditioned plasma (ACP) is an autologous, leukocyte-poor platelet concentrate used in ophthalmology to stabilize macular hole edges and promote retinal tissue regeneration. Its mechanism of action involves three key biological processes:
Following intraocular application, especially in areas where the internal limiting membrane (ILM) has been removed, ACP undergoes rapid fibrin polymerization. Within approximately 5 min, fibrin monomers are converted into a fibrin polymer, resulting in the formation of a thin, transparent membrane firmly adherent to the retinal surface. This fibrin membrane acts as a biological scaffold that supports macular hole edge approximation and facilitates neuroepithelial regeneration.
Intraoperative OCT imaging confirmed the presence and stable adhesion of the fibrin membrane throughout the surgical procedure.
Prevention of subretinal fluid ingress.
Due to its favorable surface tension properties, the fibrin membrane effectively seals the macular hole, preventing subretinal fluid ingress. This mechanical barrier plays a crucial role in stabilizing the postoperative architecture and reducing the risk of hole reopening.
ACP promotes tissue regeneration through the localized release of platelet-derived growth factors, which are essential for wound healing and cellular proliferation. The reduced leukocyte concentration (WBC = 1.3 × 109/L) minimizes postoperative inflammation, contributing to favorable anatomical and functional outcomes.
4.6. Study Limitations and Future Directions
Despite the encouraging results, this study has certain limitations. It was a retrospective, single-center investigation without a randomized control group, which may limit the strength of statistical inference. The sample size (70 eyes, 50 patients) was moderate, and the inclusion of both eyes from some individuals may have introduced intra-subject correlation that was not accounted for in the analysis. Although the follow-up period extended to 12 months, further studies are required to fully assess the durability of the structural and functional outcomes. It is worth emphasizing that the surgical procedure was highly standardized and guided by i-OCT, and the results were contextualized with data from recent meta-analyses on the treatment of large macular holes [
14,
20]. This comparative approach allows the findings to be positioned within a broader clinical and scientific framework. Given the very high anatomical closure rate and the early, sustained improvement in visual acuity, the presented technique merits further investigation. Future prospective, multicenter studies involving larger patient populations and direct comparison with established methods such as the inverted ILM flap or autologous PRP are warranted to validate both its anatomical and functional efficacy and define its role in routine clinical practice. Although Kenalog
® (triamcinolone acetonide Bristol-Myers Squibb, New York, NY, USA) was used intraoperatively to assist visualization of the posterior hyaloid and ILM, it should be noted that this formulation contains preservatives and is not specifically approved for intraocular use. While previous reports have highlighted potential concerns regarding off-label intravitreal administration, no adverse reactions or inflammatory complications were observed in our study cohort.
5. Conclusions
Activated conditioned plasma (ACP), applied under intraoperative OCT guidance, appears to be a safe and effective technique for managing large full-thickness macular holes (400–800 µm) [
3,
7,
10]. The method yielded a 98.6% closure rate and early visual improvement in most patients without requiring gas tamponade, dyes, or grafts [
9,
11,
14]. The fibrin membrane formed in situ promoted neuroretinal healing without adverse inflammatory responses [
10,
11,
12]. These results support ACP as a promising alternative to conventional approaches. Further prospective studies are needed to validate and refine this technique [
14,
20].
Author Contributions
Conceptualization: L.I.B.; methodology: L.I.B.; software: M.P. and L.P.; validation: M.P.; formal analysis: M.P. and L.P. investigation: L.P. and J.P.S. data curation: M.P., writing—original draft preparation: M.P.; writing—review and editing: J.P.S. and M.Ł.-G.; visualization: M.P.; supervision: J.P.S. and M.Ł.-G.; project administration: M.P.; funding acquisition: M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Local Bioethics Committee of the Medical University of Warsaw (approval number: AKBE/27/2025, date of approval 24 September 2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent was also obtained from the patients for the publication of this paper.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author. Restrictions apply to the availability of these data due to patient privacy protection.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jenisch, T.M.; Zeman, F.; Koller, M.; Märker, D.; Helbig, H.; Herrmann, W. Macular hole surgery: An analysis of risk factors for the anatomical and functional outcomes with a special emphasis on the experience of the surgeon. Clin. Ophthalmol. 2017, 11, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Alpatov, S.A. Surgical treatment of full-thickness macular holes of large diameter. Ophthalmic Surg. 2005, 1, 8–12. [Google Scholar]
- Baiborodov, Y.V.; Balashevich, L.I. The concept of the foveola anatomical reconstruction in the surgical treatment of full-thickness macular tears using intraoperative OCT control. In Makula-2016; Sv. Fyodorov Eye Microsurgery Institute and North-Western State Medical University: Saint Petersburg, Russia, 2016; pp. 283–294. Available online: https://www.researchgate.net/publication/321091320 (accessed on 23 July 2025).
- Machida, S.; Nishimura, T.; Ohzeki, T.; Murai, K.I.; Kurosaka, D. Comparisons of focal macular electroretinograms after indocyanine green-, brilliant blue G-, or triamcinolone acetonide-assisted macular hole surgery. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 485–492. [Google Scholar] [CrossRef] [PubMed]
- von Jagow, B.; Höing, A.; Gandorfer, A.; Rudolph, G.; Kohnen, T.; Kampik, A.; Haritoglou, C. Functional outcome of indocyanine green-assisted macular surgery: 7-year follow-up. Retina 2009, 29, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Shrvorchenko, D.O.; Kolesnik, S.V.; Kislitsina, N.M.; Kakunina, S.A.; Norman, K.S.; Kolesnik, A.I.; Krupina, E.A.; Ivanova, T.V.; Petrov, M.I.; Fedorov, B.E.; et al. Vital dyes for chromovitrectomy. Ophthalmosurgery (Fyodorov J. Ophthalmic Surg.) 2016, 2, 70–77. [Google Scholar] [CrossRef]
- Balashevich, L.I.; Baiborodov, Y.V.; Zhogolev, K.S. Surgical treatment of the vitreo-macular interface pathology. Ophthalmic Surg. 2015, 2, 80–85. [Google Scholar]
- Kunikata, H.; Abe, T.; Nakazawa, T. Heads-up macular surgery with a 27-gauge microincision vitrectomy system and minimal illumination. Case Rep. Ophthalmol. 2016, 7, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, J.; Bulsara, M.; Olynyk, J.K.; Vasudevan, A. Analysis of platelet-rich plasma extraction: Variations in platelet and blood components between 4 common commercial kits. Orthop. J. Sports Med. 2017, 5, 2325967116675272. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Kadir, M.A.; Lim, L.T. Update on Surgical Management of Complex Macular Holes: A Review. Int. J. Retin. Vitreous. 2021, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- McCannel, C.A.; Ensminger, J.L.; Diehl, N.N.; Hodge, D.O. The Effect of Macular Hole Duration on Surgical Outcomes. Ophthalmology 2022, 129, 841–848. [Google Scholar] [CrossRef]
- Ch’ng, S.W.; Patton, N.; Ahmed, M.; Ivanova, T.; Baumann, C.; Charles, S.; Ezra, E.; Steel, D.H.W.; Chew, C.K.; Ehlers, J.P.; et al. The Manchester Large Macular Hole Study: Is it time to reclassify large macular holes? Am. J. Ophthalmol. 2018, 195, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Cundy, O.; Lange, C.A.K.; Bunce, C.; Bainbridge, J.W.; Solebo, A.L.; Bastawrous, A.; Wilkinson, M.; Hawkins, C.; Kendall, E.; Lucas, J.; et al. Face-down positioning or posturing after macular hole surgery. Cochrane Database Syst. Rev. 2023, 11, CD008228. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Tzekov, R.; Jiang, F.; Mao, S.; Tong, Y.; Li, W. Inverted ILM flap technique versus conventional ILM peeling for idiopathic large macular holes: A meta-analysis of randomized controlled trials. PLoS ONE 2020, 15, e0236431. [Google Scholar] [CrossRef] [PubMed]
- Liggett, P.E.; Skolik, D.S.; Hoflo, B.; Saito, Y.; Mieler, W.F. Human autologous serum for the treatment of full-thickness macular holes. Ophthalmology 1995, 102, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Gotzaridis, S.V.; Liazos, E.; Petrou, P. The effect of autologous serum on vitrectomy with internal limiting membrane peeling for macular hole surgery: A prospective randomized study. Retina 2017, 37, 137–143. [Google Scholar] [CrossRef]
- Morizane, Y.; Shiraga, F.; Kimura, S.; Hosokawa, M.; Shiode, Y.; Kawata, T.; Hosogi, M.; Shirakata, Y.; Okanouchi, T. Autologous transplantation of the internal limiting membrane for refractory macular holes. Am. J. Ophthalmol. 2014, 157, 861–869.e1. [Google Scholar] [CrossRef] [PubMed]
- Bikbov, M.M.; Altynbayev, U.R.; Gilmanshin, T.R. Selecting the method of intraoperative closing of large idiopathic macular holes. Ophthalmic Surg. 2010, 1, 25–28. [Google Scholar]
- Rizzo, S.; Caporossi, T.; Tartaro, R.; Finocchio, L.; Franco, F.; Barca, F.; Giansanti, F.; Genovesi-Ebert, F.; Vento, A.; Martini, R.; et al. A human amniotic membrane plug to promote retinal breaks repair and recurrent macular hole closure. Retina 2019, 39 (Suppl. S1), S95–S103. [Google Scholar] [CrossRef] [PubMed]
- Mihalache, A.; Huang, R.S.; Patil, N.S.; Ahmed, H.; Popovic, M.M.; Kertes, P.J.; Muni, R.H. Pars plana vitrectomy with or without internal limiting membrane peel for macular hole: A systematic review and meta-analysis. Retina 2024, 44, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.C.; Stinnett, S.S.; McCuen, B.W., II. Comparison of silicone oil versus gas tamponade in the treatment of idiopathic full-thickness macular hole. Ophthalmology 2003, 110, 1170–1174. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).