Understanding Frailty in Cardiac Rehabilitation: A Scoping Review of Prevalence, Measurement, Sex and Gender Considerations, and Barriers to Completion
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategy
2.3. Selection Process
2.4. Data Extraction and Synthesis
3. Results
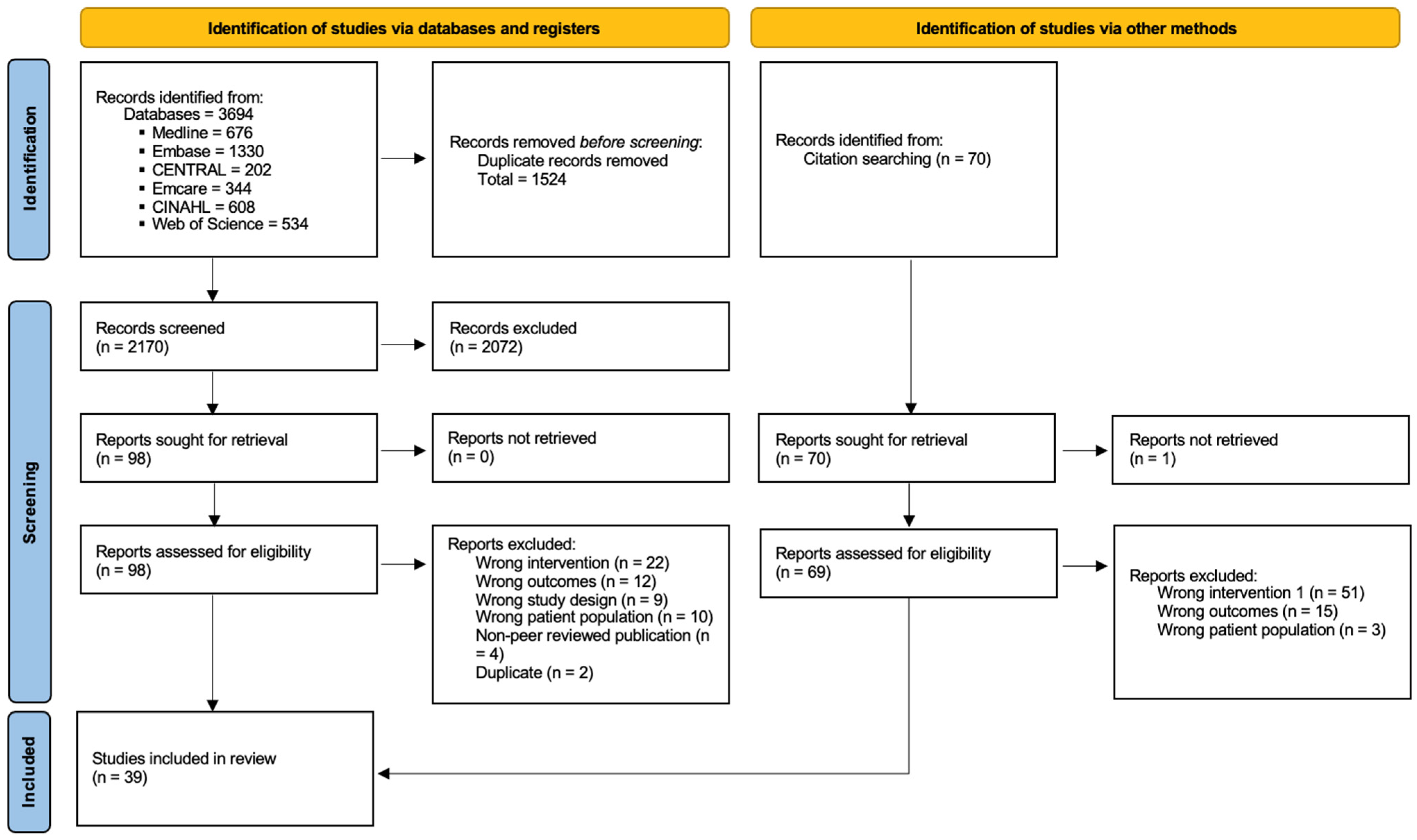
3.1. Selection of Sources of Evidence
3.2. Study Characteristics
3.3. Frailty Assessment
3.4. Sex and Gender Considerations
3.5. Impact of Frailty and Barriers to CR Participation in Patients with Frailty
4. Discussion
4.1. Limitations
4.2. Practice Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Afilalo, J. Evaluating and Treating Frailty in Cardiac Rehabilitation. Clin. Geriatr. Med. 2019, 35, 445–457. [Google Scholar] [CrossRef] [PubMed]
- MacEachern, E.; Quach, J.; Giacomantonio, N.; Theou, O.; Hillier, T.; Abel-Adegbite, I.; Gonzalez-Lara, M.; Kehler, D.S. Cardiac rehabilitation and frailty: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2024, 31, 1960–1976. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, M.; Afilalo, J. Cardiac Rehabilitation: Are We Missing an Important Means to Defrail and Reverse Adverse Consequences of Aging? Can. J. Cardiol. 2020, 36, 457–458. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.-L. The Frailty Syndrome: Definition and Natural History. Clin. Geriatr. Med. 2011, 27, 1–15. [Google Scholar] [CrossRef]
- Grace, S.L.; Turk-Adawi, K.; Contractor, A.; Atrey, A.; Campbell, N.R.; Derman, W.; Lima de Melo Ghisi, G.; Sarkar, B.; Yeo, T.J.; Lopez-Jimenez, F.; et al. Cardiac Rehabilitation Delivery Model for Low-Resource Settings: An International Council of Cardiovascular Prevention and Rehabilitation Consensus Statement. Prog. Cardiovasc. Dis. 2016, 59, 303–322. [Google Scholar] [CrossRef]
- Taylor, R.S.; Dalal, H.M.; McDonagh, S.T.J. The role of cardiac rehabilitation in improving cardiovascular outcomes. Nat. Rev. Cardiol. 2022, 19, 180–194. [Google Scholar] [CrossRef]
- Grace, S.L.; Kotseva, K.; Whooley, M.A. Cardiac Rehabilitation: Under-Utilized Globally. Curr. Cardiol. Rep. 2021, 23, 118. [Google Scholar] [CrossRef]
- MacEachern, E.; Quach, J.; Giacomantonio, N.; Theou, O.; Hillier, T.; Firth, W.; Kehler, D.S. The association of frailty on cardiac rehabilitation goal achievement. Front. Cardiovasc. Med. 2024, 11, 1441336. [Google Scholar] [CrossRef]
- Kaufman, M.; Eschliman, E.; Sanchez Karver, T. Differentiating sex and gender in health research to achieve gender equity. Bull. World Health Organ. 2023, 101, 666–671. [Google Scholar] [CrossRef]
- Norris, C.M.; Mullen, K.-A.; Foulds, H.J.A.; Jaffer, S.; Nerenberg, K.; Gulati, M.; Parast, N.; Tegg, N.; Gonsalves, C.A.; Grewal, J.; et al. The Canadian Women’s Heart Health Alliance ATLAS on the Epidemiology, Diagnosis, and Management of Cardiovascular Disease in Women—Chapter 7: Sex, Gender, and the Social Determinants of Health. CJC Open 2024, 6, 205–219. [Google Scholar] [CrossRef]
- Lima de Melo Ghisi, G.; Kim, W.-S.; Cha, S.; Zhang, L.; Tourkmani, N.; Grace, S.L. Women’s Cardiac Rehabilitation Barriers: Results of the International Council of Cardiovascular Prevention and Rehabilitation’s First Global Assessment. Can. J. Cardiol. 2023, 39, S375–S383. [Google Scholar]
- Samayoa, L.; Grace, S.L.; Gravely, S.; Scott, L.B.; Marzolini, S.; Colella, T.J. Sex differences in cardiac rehabilitation enrollment: A meta-analysis. Can. J. Cardiol. 2014, 30, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Quach, J.; Theou, O.; Godin, J.; Rockwood, K.; Kehler, D.S. The impact of cardiovascular health and frailty on mortality for males and females across the life course. BMC Med. 2022, 20, 394. [Google Scholar] [CrossRef] [PubMed]
- Denfeld, Q.E.; Habecker, B.A.; Camacho, S.A.; Roberts Davis, M.; Gupta, N.; Hiatt, S.O.; Medysky, M.E.; Purnell, J.Q.; Winters-Stone, K.; Lee, C.S. Characterizing Sex Differences in Physical Frailty Phenotypes in Heart Failure. Circ. Heart Fail. 2021, 14, e008076. [Google Scholar] [CrossRef]
- Díez-Villanueva, P.; Jiménez-Méndez, C.; Bonanad, C.; Ortiz-Cortés, C.; Barge-Caballero, E.; Goirigolzarri, J.; Esteban-Fernández, A.; Pérez-Rivera, A.; Cobo, M.; Sanz-García, A.; et al. Sex differences in the impact of frailty in elderly outpatients with heart failure. Front. Cardiovasc. Med. 2022, 9, 1000700. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Francesco Cacciatore, F.; Mazzella, F.; Longobardi, G.; Vitale, D.F.; Furgi, G.; Nicolino, A.; Abete, P. Role of clinical frailty in elderly patients with cardiovascular disease undergoing cardiac rehabilitation. Eur. J. Prev. Cardiol. 2013, 20, S63. [Google Scholar]
- Dinesh, V.; Pierce, R.; Hespe, L.; Thakkar, S.; Wong, M.; El Sabbagh, L.; Honeysett, L.; Brown, P.; Delbaere, K.; Havryk, A.; et al. The Relationship Between Rehabilitation and Frailty in Advanced Heart or Lung Disease. Transplant. Direct. 2024, 10, e1606. [Google Scholar] [CrossRef]
- German-Sallo, M.; Preg, Z.; Balint Szentendrey, D.; Pal, T.; Nagy, Z.; Tatar, M.C. Frailty evaluation a key to tailored cardiovascular rehabilitation in elderly patients. Eur. J. Prev. Cardiol. 2024, 31 (Suppl. S1), zwae175.030. [Google Scholar] [CrossRef]
- Bauer, T.M.; Hou, H.; Likosky, D.S.; Pagani, F.D.; Keteyian, S.J.; Sukul, D.; Thompson, M.P. Abstract 16761: Preprocedural Frailty is Associated With Lower Cardiac Rehabilitation Use Despite Greater Benefit. Circulation 2023, 148 (Suppl. S1), A16761. [Google Scholar] [CrossRef]
- Fonteles Ritt, L.; Matos E Oliveira, F.; Santos Pereira Ramos, J.; Braga Linhares De Albuquerque, R.; Borges De Oliveira, Q.; Amoedo Da Costa Pinto, D.; Costa Claro, T.; Miura Feitosa, C.; Santos Gramacho, M.; Freitas Feitosa, G.; et al. Impact of a cardiovascular rehabilitation program on frailty indicators in elderly patients with heart disease. Eur. J. Prev. Cardiol. 2021, 28 (Suppl. S1), zwab061.037. [Google Scholar] [CrossRef]
- Hillier, T.; MacEachern, E.; Kehler, D.S.; Giacomantonio, N. Contribution of individual and cumulative frailty-related health deficits on cardiac rehabilitation completion. BMC Geriatr. 2023, 23, 34. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Sato, Y.; Takahashi, T.; Tsuchihashi-Makaya, M.; Kotooka, N.; Ikegame, T.; Takura, T.; Yamamoto, T.; Nagayama, M.; Goto, Y.; et al. Multidisciplinary Cardiac Rehabilitation and Long-Term Prognosis in Patients With Heart Failure. Circ. Heart Fail. 2020, 13, e006798. [Google Scholar] [CrossRef] [PubMed]
- Kimber, D.E.; Kehler, D.S.; Lytwyn, J.; Boreskie, K.F.; Jung, P.; Alexander, B.; Hiebert, B.M.; Dubiel, C.; Hamm, N.C.; Stammers, A.N.; et al. Pre-Operative Frailty Status Is Associated with Cardiac Rehabilitation Completion: A Retrospective Cohort Study. J. Clin. Med. 2018, 7, 560. [Google Scholar] [CrossRef]
- Landry, M.; Delos-Reyes, F.; Childerhose, D.; Fong, M.; Harvey, P.; Price, J. Assessment of Frailty in Women Participating in Cardiac Rehabilitation. J. Cardiopulm. Rehabil. Prev. 2018, 38, E20–E21. [Google Scholar]
- Lutz, A.; Delligatti, A.; Allsup, K.; Forman, D. Can Cardiac Rehabilitation Improve Frailty in Adults with Cardiovascular Disease? JACC 2019, 73 (Suppl. S1), 1730. [Google Scholar] [CrossRef]
- Lutz, A.H.; Delligatti, A.; Allsup, K.; Afilalo, J.; Forman, D.E. Cardiac Rehabilitation Is Associated with Improved Physical Function in Frail Older Adults With Cardiovascular Disease. J. Cardiopulm. Rehabil. Prev. 2020, 40, 310–318. [Google Scholar] [CrossRef]
- Mathew, A.; Youngson, E.; Wirzba, B.; Graham, M. The Trajectory of Frailty Scores Over the Course of Cardiac Rehabilitation. Can. J. Cardiol. 2019, 35, S50. [Google Scholar] [CrossRef]
- Tanaka, T.; Shoichiro, F.; Nishimura, M.; Keiko, T.; Hidenobu, K.; Takayuki, U.; Akira, Y. Effect of outpatient cardiac rehabilitation program on outcome of elderly patients, especially in frailty status. Eur. J. Prev. Cardiol. 2018, 25 (Suppl. S1), S138. [Google Scholar] [CrossRef]
- Yokote, T.; Nishimura, T.; Furukawa, S.; Inoue, S. Association of Frailty and Depressive Symptoms With the Establishment of Exercise Habits in Patients Undergoing Outpatient Cardiac Rehabilitation. Arch. Rehabil. Res. Clin. Transl. 2023, 5, 100290. [Google Scholar] [CrossRef]
- Yu, Z.; Zhao, Q.; Ye, Y.; Wang, M.; Zhou, Z.; Zhang, H.; Zhao, Z.; Liu, Q.; Zhang, Z.; Wu, Y.; et al. Comprehensive Geriatric Assessment and Exercise Capacity in Cardiac Rehabilitation for Patients Referred to Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2021, 158, 98–103. [Google Scholar] [CrossRef]
- Adachi, T.; Iritani, N.; Kamiya, K.; Iwatsu, K.; Kamisaka, K.; Iida, Y.; Yamada, S. Prognostic effects of cardiac rehabilitation in heart failure patients classified according to physical frailty: A propensity score–matched analysis of a nationwide prospective cohort study. Int. J. Cardiol. Cardiovasc. Risk Prev. 2023, 17, 200177. [Google Scholar] [CrossRef]
- Baldasseroni, S.; Silverii, M.V.; Herbst, A.; Orso, F.; Di Bari, M.; Pratesi, A.; Burgisser, C.; Ungar, A.; Marchionni, N.; Fattirolli, F. Predictors of physical frailty improvement in older patients enrolled in a multidisciplinary cardiac rehabilitation program. Heart Vessel. 2023, 38, 1056–1064. [Google Scholar] [CrossRef]
- Bencivenga, L.; Femminella, G.D.; Ambrosino, P.; Bosco, Q.; De Lucia, C.; Perrotta, G.; Formisano, R.; Komici, K.; Vitale, D.F.; Ferrara, N.; et al. Role of frailty on cardiac rehabilitation in hospitalized older patients. Aging Clin. Exp. Res. 2022, 34, 2675–2682. [Google Scholar] [CrossRef]
- Eichler, S.; Hadzic, M.; Völler, H.; Salzwedel, A. Octogenarians in interventional cardiology: Feasibility and safety of functional and nutritional assessments for a new patient group in cardiac rehabilitation. Eur. J. Prev. Cardiol. 2020, 27, 2345–2347. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Hirashiki, A.; Ozaki, K.; Kawamura, K.; Sugioka, J.; Tanioku, S.; Sato, K.; Ueda, I.; Itoh, N.; Nomoto, K.; et al. Benefits of a Balance Exercise Assist Robot in the Cardiac Rehabilitation of Older Adults with Cardiovascular Disease: A Preliminary Study. J. Cardiovasc. Dev. Dis. 2022, 9, 191. [Google Scholar] [CrossRef] [PubMed]
- Henderson, G.; Althouse, A.D.; Allsup, K.; Forman, D.E. Is Cardiac Rehabilitation Useful for Cardiovascular Disease Patients Who are Frail? PM&R 2017, 9 (Suppl. S1), S142–S143. [Google Scholar] [CrossRef]
- Honzawa, A.; Nishitani-Yokoyama, M.; Shimada, K.; Kunimoto, M.; Matsubara, T.; Matsumori, R.; Kasuya, H.; Fujiwara, K.; Doi, M.; Takagi-Kawahara, K.; et al. Effects of Phase II Cardiac Rehabilitation on Physical Function and Anxiety Levels in Frail Patients. Circ. Rep. 2022, 4, 308–314. [Google Scholar] [CrossRef]
- Kehler, D.S.; Giacomantonio, N.; Firth, W.; Blanchard, C.M.; Rockwood, K.; Theou, O. Association Between Cardiac Rehabilitation and Frailty. Can. J. Cardiol. 2020, 36, 482–489. [Google Scholar] [CrossRef]
- MacEachern, E.; Giacomantonio, N.; Firth, W.; Kehler, S. Influence of Frailty on Cardiac Rehabilitation Goal Achievement. J. Cardiopulm. Rehabil. Prev. 2021, 41, E13. [Google Scholar]
- Tarro Genta, F.; Eleuteri, E.; Bertolin, F.; Bouslenko, Z.; Taglieri, C.; Tidu, M.; Giannuzzi, P. Is cardiac rehabilitation (CR) safe and useful in octogenarians after transcatheter aortic valve implantation (TAVI) compared to surgical aortic valve replacement (sAVR) for aortic stenosis? Eur. Heart J. 2015, 36 (Suppl. S1), 515. [Google Scholar]
- Ushijima, A.; Morita, N.; Hama, T.; Yamamoto, A.; Yoshimachi, F.; Ikari, Y.; Kobayashi, Y. Effects of cardiac rehabilitation on physical function and exercise capacity in elderly cardiovascular patients with frailty. J. Cardiol. 2021, 77, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Honzawa, A.; Nishitani-Yokoyama, M.; Shimada, K.; Kunimoto, M.; Yamada, M.; Matsubara, T.; Matsumori, R.; Fujiwara, K.; Abulimiti, A.; Aikawa, T.; et al. Relationship Between Kihon Checklist Score and Anxiety Levels in Elderly Patients Undergoing Early Phase II Cardiac Rehabilitation. Cardiol. Res. 2020, 11, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Iritani, N.; Adachi, T.; Iwatsu, K.; Kamiya, K.; Kamisaka, K.; Yamada, S. Reasons for Nonparticipation in Outpatient Cardiac Rehabilitation Among Older Patients with Heart Failure: A Report of the Flagship Study. J. Cardiopulm. Rehabil. Prev. 2023, 43, 303–305. [Google Scholar] [CrossRef]
- Kunimoto, M.; Shimada, K.; Yokoyama, M.; Matsubara, T.; Aikawa, T.; Ouchi, S.; Shimizu, M.; Fukao, K.; Miyazaki, T.; Kadoguchi, T.; et al. Relationship between the Kihon Checklist and the clinical parameters in patients who participated in cardiac rehabilitation. Geriatr. Gerontol. Int. 2019, 19, 287–292. [Google Scholar] [CrossRef]
- Nishitani-Yokoyama, M.; Shimada, K.; Yamada, M.; Honzawa, A.; Kunimoto, M.; Sugita, Y.; Fujiwara, K.; Matsubara, T.; Matsumori, R.; Abulimiti, A.; et al. Association Between Constipation and Frailty Components in Patients Undergoing Late Phase II Cardiac Rehabilitation. Cardiol. Res. 2021, 12, 169–176. [Google Scholar] [CrossRef]
- Xu, J.; Yokoyama, M.; Shimada, K.; Fujiwara, K.; Abidan, A.; Kunimoto, M.; Nozawa, Y.; Kasuya, H.; Tabata, M.; Daida, H.; et al. The relationship between frailty and HRQoL in elderly cardiovascular patients in Phase II Cardiac Rehabilitation. Eur. J. Prev. Cardiol. 2023, 30 (Suppl. S1), zwad125.101. [Google Scholar] [CrossRef]
- MacEachern, E.; Giacomantonio, N.; Theou, O.; Quach, J.; Firth, W.; Abel-Adegbite, I.; Kehler, D.S. Comparing Virtual and Center-Based Cardiac Rehabilitation on Changes in Frailty. Int. J. Environ. Res. Public Health 2023, 20, 1554. [Google Scholar] [CrossRef]
- Mudge, A.M.; Pelecanos, A.; Adsett, J.A. Frailty implications for exercise participation and outcomes in patients with heart failure. J. Am. Geriatr. Soc. 2021, 69, 2476–2485. [Google Scholar] [CrossRef]
- Nelson, M.B.; Gilbert, O.N.; Duncan, P.W.; Kitzman, D.W.; Reeves, G.R.; Whellan, D.J.; Mentz, R.J.; Chen, H.; Hewston, L.A.; Taylor, K.M.; et al. Intervention Adherence in REHAB-HF: Predictors and Relationship with Physical Function, Quality of Life, and Clinical Events. J. Am. Heart Assoc. 2022, 11, e024246. [Google Scholar] [CrossRef]
- Quach, J.; Kehler, D.S.; Giacomantonio, N.; McArthur, C.; Blanchard, C.; Firth, W.; Rockwood, K.; Theou, O. Association of admission frailty and frailty changes during cardiac rehabilitation with 5-year outcomes. Eur. J. Prev. Cardiol. 2023, 30, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, O.N.; Mentz, R.J.; Bertoni, A.G.; Kitzman, D.W.; Whellan, D.J.; Reeves, G.R.; Duncan, P.W.; Nelson, M.B.; Blumer, V.; Chen, H.; et al. Relationship of Race with Functional and Clinical Outcomes with the REHAB-HF Multidomain Physical Rehabilitation Intervention for Older Patients With Acute Heart Failure. J. Am. Heart Assoc. 2023, 12, e030588. [Google Scholar] [CrossRef] [PubMed]
- Nagatomi, Y.; Ide, T.; Higuchi, T.; Nezu, T.; Fujino, T.; Tohyama, T.; Nagata, T.; Higo, T.; Hashimoto, T.; Matsushima, S.; et al. Home-based cardiac rehabilitation using information and communication technology for heart failure patients with frailty. ESC Heart Fail. 2022, 9, 2407–2418. [Google Scholar] [CrossRef] [PubMed]
- Rogers, P.; Al-Aidrous, S.; Banya, W.; Haley, S.R.; Mittal, T.; Kabir, T.; Panoulas, V.; Raja, S.; Bhudia, S.; Probert, H.; et al. Cardiac rehabilitation to improve health-related quality of life following trans-catheter aortic valve implantation: A randomised controlled feasibility study. Pilot Feasibility Stud. 2018, 4, 185. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Available online: https://www.who.int/countries (accessed on 9 June 2025).[Green Version]
- Searle, S.D.; Mitnitski, A.; Gahbauer, E.A.; Gill, T.M.; Rockwood, K. A standard procedure for creating a frailty index. BMC Geriatr. 2008, 8, 24. [Google Scholar] [CrossRef]
- Heidari, S.; Babor, T.F.; De Castro, P.; Tort, S.; Curno, M. Sex and Gender Equity in Research: Rationale for the SAGER guidelines and recommended use. Res. Integr. Peer Rev. 2016, 1, 2. [Google Scholar] [CrossRef]
- Alfaraidhy, M.A.; Regan, C.; Forman, D.E. Cardiac rehabilitation for older adults: Current evidence and future potential. Expert Rev. Cardiovasc. Ther. 2022, 20, 13–34. [Google Scholar] [CrossRef]
- Markle-Reid, M.; Browne, G. Conceptualizations of frailty in relation to older adults. J. Adv. Nurs. 2003, 44, 58–68. [Google Scholar] [CrossRef]
- Richter, D.; Guasti, L.; Walker, D.; Lambrinou, E.; Lionis, C.; Abreu, A.; Savelieva, I.; Fumagalli, S.; Bo, M.; Rocca, B.; et al. Frailty in cardiology: Definition, assessment and clinical implications for general cardiology. A consensus document of the Council for Cardiology Practice (CCP), Association for Acute Cardio Vascular Care (ACVC), Association of Cardiovascular Nursing and Allied Professions (ACNAP), European Association of Preventive Cardiology (EAPC), European Heart Rhythm Association (EHRA), Council on Valvular Heart Diseases (VHD), Council on Hypertension (CHT), Council of Cardio-Oncology (CCO), Working Group (WG) Aorta and Peripheral Vascular Diseases, WG e-Cardiology, WG Thrombosis, of the European Society of Cardiology, European Primary Care Cardiology Society (EPCCS). Eur. J. Prev. Cardiol. 2022, 29, 216–227. [Google Scholar]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- Rockwood, K.; Theou, O. Using the Clinical Frailty Scale in Allocating Scarce Health Care Resources. Can. Geriatr. J. 2020, 23, 254–259. [Google Scholar] [CrossRef]
- Linn, N.; Goetzinger, C.; Regnaux, J.-P.; Schmitz, S.; Dessenne, C.; Fagherazzi, G.; Aguayo, G.A. Digital Health Interventions among People Living with Frailty: A Scoping Review. J. Am. Med. Dir. Assoc. 2021, 22, 1802–1812.e21. [Google Scholar] [CrossRef] [PubMed]
- Flint, K.M.; Stevens-Lapsley, J.; Forman, D.E. Cardiac Rehabilitation in Frail Older Adults With Cardiovascular Disease: A New Diagnostic and Treatment Paradigm. J. Cardiopulm. Rehabil. Prev. 2020, 40, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Pilote, L.; Humphries, K.H. Incorporating Sex and Gender in Cardiovascular Research: The Time Has Come. Can. J. Cardiol. 2014, 30, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Santos, A.-E.; Facal, D.; Vicho de la Fuente, N.; Vilanova-Trillo, L.; Gandoy-Crego, M.; Rodríguez-González, R. Gender impact of caring on the health of caregivers of persons with dementia. Patient Educ. Couns. 2021, 104, 2165–2169. [Google Scholar] [CrossRef]
- Donato, K.M.; León-Pérez, G.; Wallston, K.A.; Kripalani, S. Something Old, Something New: When Gender Matters in the Relationship between Social Support and Health. J. Health Soc. Behav. 2018, 59, 352–370. [Google Scholar] [CrossRef]
- Daher, M.; Al Rifai, M.; Kherallah, R.Y.; Rodriguez, F.; Mahtta, D.; Michos, E.D.; Khan, S.U.; Petersen, L.A.; Virani, S.S. Gender disparities in difficulty accessing healthcare and cost-related medication non-adherence: The CDC behavioral risk factor surveillance system (BRFSS) survey. Prev. Med. 2021, 153, 106779. [Google Scholar] [CrossRef]
- Sérvio, T.C.; Britto, R.R.; de Melo Ghisi, G.L.; da Silva, L.P.; Silva, L.D.N.; Lima, M.M.O.; Pereira, D.A.G.; Grace, S.L. Barriers to cardiac rehabilitation delivery in a low-resource setting from the perspective of healthcare administrators, rehabilitation providers, and cardiac patients. BMC Health Serv. Res. 2019, 19, 615. [Google Scholar] [CrossRef]
- Redfern, J.; Gallagher, R.; Maiorana, A.; Candelaria, D.; Hollings, M.; Gauci, S.; O’Neil, A.; Chaseling, G.K.; Zhang, L.; Thomas, E.E.; et al. Cardiac rehabilitation and secondary prevention of CVD: Time to think about cardiovascular health rather than rehabilitation. NPJ Cardiovasc. Health 2024, 1, 22. [Google Scholar] [CrossRef]
- Prommaban, A.; Moonkayaow, S.; Phinyo, P.; Siviroj, P.; Sirikul, W.; Lerttrakarnnon, P. The Effect of Exercise Program Interventions on Frailty, Clinical Outcomes, and Biomarkers in Older Adults: A Systematic Review. J. Clin. Med. 2024, 13, 6570. [Google Scholar] [CrossRef]
- Liu, C.K.; Fielding, R.A. Exercise as an Intervention for Frailty. Clin. Geriatr. Med. 2011, 27, 101–110. [Google Scholar] [CrossRef]
- Frost, R.; Nair, P.; Aw, S.; Gould, R.L.; Kharicha, K.; Buszewicz, M.; Walters, K. Supporting frail older people with depression and anxiety: A qualitative study. Aging Ment. Health 2020, 24, 1977–1984. [Google Scholar] [CrossRef]
- Broome, K.; Worrall, L.; Fleming, J.; Boldy, D. Evaluation of flexible route bus transport for older people. Transp. Policy 2012, 21, 85–91. [Google Scholar] [CrossRef]
- Gaalema, D.E.; Mahoney, K.; Ballon, J.S. Cognition and Exercise: General Overview and Implications for Cardiac Rehabilitation. J. Cardiopulm. Rehabil. Prev. 2021, 41, 400–406. [Google Scholar] [CrossRef]
| First Author Year Country | Aim | Study Design Setting | Participants | Total N, % Women Age: Mean ± SD (Range) or Median (Range) | Characteristics of Participants ‡ |
|---|---|---|---|---|---|
| Adachi [32] 2023 Japan | To examine the effects of CR on the 2-year prognosis of patients with HF, according to their frailty status. | Prospective cohort study Multicentre (number of centres NR) | Patients hospitalized for HF and capable of walking at discharge | N = 2697, 39.6% women Overall: 76.0 (67–83) years Frailty CR group: 78.0 ± NR (74–83) years | Atrial fibrillation (35.1%), diabetes mellitus (34.8%) at admission |
| Baldasseroni [33] 2023 Italy | To examine the effect of standardized CR after ACS on physical frailty. | Prospective cohort study 1 centre | CR participants with ACS | N = 100, 20.0% women 80.8 ± 0.5 (75–94) years | CABG (17.0%), current smoking (14.0%), diabetes mellitus (20.0%), dyslipidemia (56.0%), hypertension (78.0%), NSTEMI (36.0%), STEMI (31.0%), valvular surgery (16.0%) at admission |
| Bauer [20] 2023 USA | To evaluate the relationship between preprocedural frailty, CR use, and one-year mortality. | Retrospective cohort study Multicentre (number of centres NR) | Patients who underwent inpatient percutaneous or surgical revascularization or aortic valve replacement | N = 570,851, % women: NR Age: NR | NR |
| Bencivenga [34] 2022 Italy | To determine the relationship between frailty and CR outcomes in hospitalized older adults. | Prospective cohort study 1 centre | Patients referring to CR after HF exacerbation, IHD, VHD, cardio-aortic surgery, and other CVD | N = 559, 30.8% women Overall: 72.0 (69–76) years Frailty group: 73.0 (69–77) years | HF (24.7%), IHD (41.5%), VHD (29.7%), Other CVD (4.1%) at admission |
| Dinesh [18] 2024 Australia | To identify whether patients with advanced heart disease participating in a hospital-based rehabilitation programme or a home-based structured exercise program had lower frailty scores compared with non-rehabilitation participants. | Retrospective cohort study 1 centre | Advanced heart disease (DCM, IHD, valvular, congenital, HCM RCM, other) | N = 124, 18% women Overall for heart disease group: 53.0 ± 12.0 (range NR) years Frailty group: 55.0 ± 10.0 (range NR) years Pre-frail group: 53.0 ± 13.0 (range NR) years Robust group: 54.0 ± 12.0 (range NR) years | CHD (5.6%), DCM (52.4%), HCM (4.0%), IHD (25.0%), RCM (3.2%), VHD (6.5%), other CVD (3.2%) at admission |
| Eichler [35] 2020 Germany | To investigate the feasibility and safety of functional and nutritional assessments in patients after PCI in CR. | Prospective cohort study 1 centre | Patients over or equal to 75 years of age after TAVI, AVI or PCI | N = 124, 47.6% women 81.8 ± 3.5 (range NR) years | Arrhythmia (10.5%), diabetes mellitus (32.3%), dyslipidemia (39.5%), hypertension (82.3%), infections (12.1%) musculoskeletal diseases (20.2%), renal insufficiency (21.8%) at admission |
| Fonteles Ritt [21] 2021 Brazil | To evaluate the association of the CR programme with frailty indicators in elderly patients with heart disease referred to a CR program. | Retrospective cohort study 1 centre | Patients over 65 years old referred to CR | N = 51, 35.0% women 75.0 ± 6.0 (range NR) years | CAD (77.0%), HF (50.0%), diabetes mellitus (41.0%), hypertension (67.0%), dyslipidemia (80.0%) at admission |
| Francesco-Cacciatore [17] 2013 Italy | To verify the prevalence of frailty and its predictive role on functional recovery after CR in elderly patients with CVD. | Prospective cohort study NR | Patients older than 65 years with CVD after a major cardiovascular acute event | N = 350, % women NR Age: NR | NR |
| German-Sallo [19] 2024 Hungary | To assess frailty in elderly patients, as part of a comprehensive CR programme and to identify the main components contributing to loss of self–care. | Cross-sectional study 1 centre | Patients aged 60 years or older admitted to CR | N = 92, 53.3% women 72.4 ± 7.4 (range NR) years | Atrial fibrillation (57.1%), CKD (46.5%), osteoarticular diseases (39%), stroke (26%) at admission |
| Gilbert [52] 2023 USA | To evaluate whether the effects of physical rehabilitation interventions vary across racial groups in ADHF. | Randomized control trial Multicentre (7 hospitals; 4 academic, 3 community-based) | Patients aged 60 years or older hospitalized for ≥24 h for ADHF, including both HF with preserved EF and HF with reduced EF | N = 349, 51.3% women 72.5 ± 3.0 (range NR) years | Hypertension (92.0%), atrial fibrillation (50.4%), hyperlipidemia (66.2%) at admission |
| Hashimoto [36] 2022 Japan | To examine whether adding robotic balance exercises to CR improved the balance ability of older adults with CVD. | Prospective cohort study 1 centre | Older adults who had been hospitalized for worsening CVD | N = 52, 46.2% women 76.9 ± 6.8 (65–95) years | Diabetes mellitus (15.4%), dyslipidemia (32.4%), and tobacco users (1.9%) at admission |
| Henderson [37] 2017 USA | To evaluate if CR may benefit frail patients by enabling functional gains that may even exceed relative improvements among non-frail patients. | Prospective cohort study 1 centre | Patients with CVD of diverse etiologies (CAD, HF, VHD) | N = 60, % women NR 68.0 (45–81) years | NR |
| Hillier [22] 2023 Canada | To determine which patient characteristics and age-related health deficits are important in patients completing CR. | Retrospective cohort study 1 centre | Patients who experienced acute CVD, including CAD, MI, PCI, CABG, valve surgery, HF, or a combination of other diagnoses, with few referrals (e.g., arrhythmia, heart transplant) | N = 4004, 25.5% women CR group: 62.4 ± 10.7 (range NR) years | CAD (27.0%), PCI (15.0%), cardiac surgery (20.0%), HF (7.0%), MI (28.0%), other (4.0%) at admission |
| Honzawa [38] 2022 Japan | To investigate the effects of phase II CR on physical function and anxiety levels based on the prevalence of frailty. | Prospective cohort study 1 centre | Patients who participated in early-phase II CR | N = 137, 29.2% women Overall: 66.4 ± 2.3 (range NR) years Frail: 69.0 ± 13.2 (range NR) years | Hypertension (67.9%), dyslipidemia (52.6%), diabetes mellitus (21.9%), current smoker (11.7%), open heart surgery (47.4%), HF (24.8%) at admission |
| Honzawa [43] 2020 Japan | To retrospectively examine the relationship between KCL score and anxiety levels in elderly patients undergoing early phase II CR. | Cross-sectional study 1 centre | Patients who participated in early-phase II CR | N = 255, 33.3% women Overall: 74.9 ± 5.8 (range NR) years Frail: 75.5 ± 5.8 (range NR) years | Hypertension (69.8%), dyslipidemia (47.1%), diabetes mellitus (31.4%), current smoker (14.1%) CVD diagnoses: IHD (52.9%), open heart surgery (60.4%), HF (22.0%) at admission |
| Iritani [44] 2023 Japan | To determine the reasons for non-participation of HF patients in CR and whether frailty impacted these reasons. | Cross-sectional study NR | Patients aged 65 years or older who were hospitalized for HF and were ambulatory at the time of discharge | N = 1993, 43.0% women 78.0 (73–84) years | NR |
| Kamiya [23] 2020 Japan | To estimate the impact of CR on prognosis in patients with HF. | Retrospective cohort study Multicentre (15 hospitals) | Patients hospitalized for HF | N = 3277, 41.1% women 74.9 ± 14.9 (range NR) years | Atrial fibrillation (30.0%), diabetes mellitus (40.0%), hypertension (69.0%) at admission |
| Kehler [39] 2020 Canada | To provide a comprehensive evaluation of frailty changes at CR completion in relation to admission frailty levels. | Prospective cohort study 1 centre | Patients enrolled in CR with CAD, MI, PCI, CABG, HF, or a combination of “other” diagnoses with low referral rates (eg, arrhythmia, heart transplant) | N = 3756, 15.3% women Overall: 62.6 ± 10.7 (range NR) years >0.50 FI (most frail): 62.0 ± (9.5) (range NR) years | CAD (27.5%), PCI (14.9%), MI (27.8%), cardiac surgery (19.3%), HF (6.5%), other (3.9%) at admission |
| Kimber [24] 2018 Canada | To determine the impact of pre-operative frailty on CR completion rates. | Retrospective cohort study 1 centre | Cardiac surgery patients undergoing either elective or urgent CABG and/or valve procedures | N = 114, 36.8% women Overall: 71.0 (66–78) years CR non-completers: 71.5 (66.3–78) years CR completers: 70.5 (66–72) years | MI (29.8%), HF (49.1%), diabetes mellitus (25.4%), CRF (3.5%), COPD (11.4%), depression (11.4%) at admission |
| Kunimoto [45] 2019 Japan | To evaluate the relationship between the Kihon Checklist and the clinical parameters in patients who participated in CR. | Cross-sectional study 1 centre | Patients with CVD (ACS, after open heart surgery or TAVI, HF, major vessel disease and PAD) who participated in phase II CR | N = 845, 30.8% women Overall: 70.6 ± 2.1 years Frailty: 73.0 ± 8.5 (range NR) years | MI (11.7%), PCI (16.7%), HF (24.1%), diabetes mellitus (34.6%), hypertension (64.1%), dyslipidemia (49.8%), current smoker (8.9%) at admission |
| Landry [25] 2018 Canada | To describe the prevalence of functional exercise testing modality in patients previously classified as “moderately frail” using the Frailty Index Scale in CR patients. | Retrospective cohort study 1 centre | Women living with CVD and participating in CR | N = 800, 100% women Moderate frailty: 65.8 ± 10.2 (44–92) years | NR |
| Lutz [26] 2019 USA | To examine if CR benefits frail adults as much as pre-frail and non-frail. | Retrospective cohort study NR | CVD patients who completed a phase II CR programme | N = 163, % women NR Age: NR | NR |
| Lutz [27] 2020 USA | To study changes in physical function among frail, intermediate-frail, and nonfrail older adults CR patients enrolled in a phase II CR programme. | Retrospective cohort study 1 centre | Patients with a diagnosis of HF, cardiac surgery, implantable cardiac defibrillator or pacemaker, cardiac ablation, TAVI MI, or PCI within the past year | N= 243, 0.8% women Overall: 68.0 ± NR (45–92) years Frail: 71 ± NR (49–92) years | Hypertension (80.7%), hyperlipidemia (77.0%), CAD (81.5%), PCI (39.5%), MI (30%), PAD (15.2%), depression (23.0%), anxiety (2.1%), and tobacco use (22.6%) at admission |
| MacEachern [40] 2021 Canada | To examine whether patient frailty levels affect their goal attainment in CR. | Prospective cohort study 1 centre | Patients with heart disease | N = 759, 27.0% women 59.5 ± 9.8 (range NR) years | NR |
| MacEachern [48] 2023 Canada | To compare the changes in frailty levels from CR admission to completion in patients who enrolled in either centre-based CR or virtual-based CR, and to determine if admission frailty affects frailty changes and cardiovascular risk factors in both programme models. | Retrospective observational study 1 centre | Patients referred to CR following an acute cardiovascular event by an automated referral system or a healthcare professional (e.g., a cardiologist) | N = 132, 36.4% women 64.5 ± 10.5 (40–90) years | Dyslipidemia (89.4%), hypertension (77.3%), diabetes mellitus (28.8%), current smoker (14.4%), CAD (18.9%), ACS (10.6%), MI (47.0%), CABG (15.9%), cardiomyopathy (3.8%), PCI (38.6%) at admission |
| MacEachern [8] 2024 Canada | To examine if frailty influences achieving goals in CR. | Retrospective observational study 1 centre | Patients referred to CR | N = 759, 23.6% women 59.5 ± 9.8 (range NR) years | CAD (30.6%), MI (33.2%), PCI (7.8%), surgery (19.2%), HF (7.5%), other (1.7%) at admission |
| Mathew [28] 2019 Canada | To determine if frailty improves with CR and to assess the impact of frailty on CR completion rates. | Retrospective cohort study 1 centre | Patients referred to CR | N = 764, 26.8% women Overall: 64.5 ± 11.9 (range NR) years | Dyslipidemia (0.0%), diabetes mellitus (30.0%), hypertension (70.8%), atrial fibrillation (18.6%) at admission |
| Mudge [49] 2021 Australia | To describe the characteristics, exercise participation, and outcomes of frail and non-frail participants enrolled in a randomized trial of exercise training within a CR programme. | Retrospective observational study Multicentre (5 centres) | Adults hospitalized with clinical evidence of acute HF | N = 256, 24.2% women NR | Race: 218 (85.2%) Caucasian, 38 (14.8%) other |
| Nagatomi [53] 2022 Japan | To investigate the efficacy and safety of a comprehensive home-based CR programme using information and communication technology. | Randomized control trial 1 centre | Outpatients with chronic HF and physical frailty | N = 30, 46.7% women 63.7 ± 10.1(range NR) years | Hypertension (20.0%), diabetes mellitus (17.0%), hyperlipidemia (20.0%), CKD (23.0%), PCI (10.0%), valvular surgery (10.0%), PMI (10.0%), ICD (20.0%) at admission |
| Nelson [50] 2022 USA | To examine the relationship between adherence to the REHAB-HF intervention and trial outcomes, as well as to identify baseline factors associated with adherence. | Retrospective observational study Multicentre (7 clinical sites) | Older acute HF patients | N = 175, 49% women 73.1 ± 8.5 (range NR)years | Hypertension (91.0%), atrial fibrillation (51.0%), hyperlipidemia (63.0%), diabetes mellitus (59.0%), smoking (10.0%), more than high school education (80.0%) at admission |
| Nishitani-Yokoyama [46] 2021 Japan | To investigate the complaints and prevalence of constipation in patients undergoing CR and the association between constipation and frailty components. | Cross-sectional study 1 centre | Patients with CVD (CAD, open-heart surgery, HF, aorta disease, macrovascular surgery, stenting, PAD and TAVI. | N = 102, 33.3% women 62.7 ± 13.4 (range NR) years | Hypertension (58.0%), diabetes mellitus (12.0%), dyslipidemia (29.0%), current smoker (18.0%), HF (63.0%), aortic disease (21.0%), MI (16.0%), atrial fibrillation (25.0%) at admission |
| Quach [51] 2023 Canada | To [1] examine the association between frailty and long-term outcomes and to [2] Investigate the association between frailty changes during CR and long-term outcomes. | Retrospective observational study 1 centre | CR participants | N = 3371, 25.7% women 61.9 ± 10.7 (21–94) years | CAD (26.7%), MI (28.3%), HF (6.6%), current smoker (10.9%), education: (31.8%) technical college, marital status: (76.6%) married/living with a partner at admission |
| Rogers [54] 2018 UK | To inform the feasibility and design of future randomized control trials. | Randomized control trial (pilot) 1 centre | Patients scheduled for TAVI | N = 27, 55.6% women 82.04 ± 4.8 (range NR) years | Diabetes mellitus (14.8%), smoking (48.1%) at admission |
| Tarro Genta [41] 2015 Italy | To compare the safety and outcome of residential CR in octogenarians after TAVI or AVR. | Prospective cohort study Multicentre [2] | TAVI and sAVR patients, aged ≥80 years participating in CR | N = 110, 64.5% women Overall: 84.0 ± 2.0 (range NR) years | NR |
| Toshie Tanaka [29] 2018 Japan | To assess the effects of outpatient CR on elderly patients, especially in frailty status. | Retrospective cohort study NR | Patients hospitalized with CVD | N = 47, % women NR Age: NR | NR |
| Ushijima [42] 2021 Japan | To investigate the effect of CR on the physical function as well as exercise capacity in elderly CVD patients with frailty. | Prospective cohort study 1 centre | CVD patients | N = 89, 23.6% women 75.0 ± 6.0 (range NR) years | Hypertension (62.9%), diabetes mellitus (29.2%), dyslipidemia (66.3%), smoking (13.5%), MI (16.9%), HF (9.0%), aortic disease (11.1%) at admission |
| Xu [47] 2023 Japan | To investigate the relationship between frailty and health-related quality of life in elderly patients undergoing CR. | Cross-sectional study 1 centre | Elderly patients undergoing CR | N = 217, 33.0% women 74.6 ± 5.8 (range NR) years | NR |
| Yokote [30] 2023 Japan | To assess whether patients undergoing outpatient CR who have frailty and depressive symptoms at discharge are less likely than those without these condition to establish positive exercise habits. | Retrospective cohort study 1 centre | CR participants | N = 242, 28.7% women 68.2 ± 11.1 (range NR) years | HF (23.4%), IHD (46.7%), depressive symptoms only (8.7%), frailty and depressive symptoms (4.1%) at admission |
| Yu [31] 2021 China | To assess the changes In CGA, including self-care ability, cognitive function, nutritional status, anxiety, depression and frailty index, and exercise capacity in such CR strategy; to explore the associated factors of the change in exercise capacity. | Retrospective cohort study 1 centre | Patients scheduled for TAVI | N = 90, 40.0% women 74.7 ± 8.1 (range NR) years | Hypertension (71.0%), hyperlipidemia (78.0%), diabetes mellitus (30.0%), smoking (33.0%), CHD (51.0%), PCI (20.0%), CABG (4.0%), MI (6.0%), PAD (26.0%) at admission |
| First Author | Location | Mode | Duration | Frequency | Components |
|---|---|---|---|---|---|
| Adachi [32] | Hospital and Rehab Centre | Centre-based | 150 days | 3×/week | Exercise training, patient education |
| Baldasseroni [33] | Rehab Centre | Centre-based | 4 weeks | 5×/week | Exercise training (biking or callisthenics, 30 min/session) |
| Bauer [20] | NR | NR | NR | NR | NR |
| Bencivenga [34] | Rehab Centre | Centre-based | NR | NR | Exercise training, lifestyle modification, psychological support |
| Dinesh [18] | NR | Hybrid | NR | NR | Exercise training |
| Eichler [35] | NR | NR | NR | NR | NR |
| Fonteles Ritt [21] | NR | NR | NR | NR | NR |
| Francesco Cacciatore [17] | NR | NR | NR | NR | NR |
| German-Sallo [19] | Rehab Centre | NR | NR | NR | NR |
| Gilbert [52] | Hospital and Rehab Centre | Centre-based | 12 weeks | 3×/week | Exercise training |
| Hashimoto [36] | Rehab Centre | Centre-based | 4 months | 1×/week | Exercise training |
| Henderson [37] | NR | Centre-based | ≥24 sessions | NR | Exercise training, risk factor education |
| Hillier [22] | Community centre | Centre-based | 12 weeks | 2×/week exercise, 1×/week education | Exercise training, patient education |
| Honzawa [38] | Hospital | Centre-based | 150 days | 1–2×/week | Risk stratification, exercise training, patient education, psychosocial support |
| Honzawa [43] | Hospital | Centre-based | NR | NR | Medical evaluation, exercise training, patient education, psychosocial support |
| Iritani [44] | Hospital | NR | NR | NR | NR |
| Kamiya [23] | Hospital | Centre-based | 5 months | 3–5×/week | Exercise training, patient education |
| Kehler [39] | Community centre | Centre-based | 12 weeks | 2×/week exercise, 1×/week education | Exercise training, patient education |
| Kimber [24] | NR | Centre-based | 16 weeks | NR | Exercise training, patient education |
| Kunimoto [45] | Hospital | NR | NR | NR | Medical evaluation, exercise training, patient education, psychosocial support |
| Landry [25] | NR | NR | 24 weeks | NR | Women’s only programme |
| Lutz [26] | NR | Centre-based | NR | NR | NR |
| Lutz [27] | Rehab Centre | Centre-based | 2–6 weeks | 2–3×/week | Exercise training, medication reconciliation, patient education |
| MacEachern [40] | NR | Centre-based | 12 weeks | 2×/week exercise, 1×/week education | Exercise training, patient education |
| MacEachern [48] | NR | Hybrid | Supervised: 6 weeks; Non-supervised: 10 weeks | Supervised: 1×/week exercise, up to 3×/week education; Non-supervised: 150 min/week exercise, up to 4 group sessions/week + up to 6 individualized sessions/week education | Exercise training, patient education |
| MacEachern [8] | Hospital | Centre-based | 12 weeks | Up to 2×/week exercise, 1×/week education | Exercise training, patient education |
| Mathew [28] | NR | NR | 12 weeks | NR | NR |
| Mudge [49] | Hospital and Home | Hybrid | 6 months | 2×/week first 3 months, 1×/week subsequent 3 months exercise, 1×/week education | Exercise training, patient education, telephone and clinic follow-up, and medication titration |
| Nagatomi [53] | NR | Home-based | 3 months | Up to 3–5×/week aerobic exercise + 2–3×/week resistance training, education NR | Exercise training, patient education, self-management, nutrition guidance |
| Nelson [50] | Rehab Centre and Home | Hybrid | 12 weeks | 3×/week | Exercise training |
| Nishitani-Yokoyama [46] | NR | NR | NR | NR | NR |
| Quach [51] | NR | Centre-based | 12 weeks | 2×/week exercise, 1×/week education | Exercise training, patient education, risk stratification, nutrition guidance, psychosocial support |
| Rogers [54] | NR | Centre-based | 6 weeks | 1×/week | Exercise training, patient education |
| Tarro Genta [41] | NR | NR | 3 weeks | 2×/day | Exercise training |
| Toshie Tanaka [29] | Rehab Centre | Centre-based | NR | NR | NR |
| Ushijima [42] | Rehab Centre | Centre-based | 3 months | 3–5×/week | Exercise training, patient education, nutrition guidance, medication guidance. |
| Xu [47] | Hospital | Centre-based | NR | NR | NR |
| Yokote [30] | Hospital | Centre-based | 3 months | 1×/week | Exercise training, patient education |
| Yu [31] | NR | Home-based | NR | 5–6×/week | Exercise training |
| Frailty Assessment Tool(s) Used | Tool Description | First Author Year Country | Time of Assessment | Pre-CR Frailty Prevalence | Post-CR Frailty Prevalence |
|---|---|---|---|---|---|
| Kihon Checklist (KCL) | 25 yes/no items across 7 domains (activities of daily living, physical function, nutritional status, oral function, social activities of daily living, cognitive function, depressive mood of participants). ≥8 = frail, 4–7 = prefrail, ≤3 = non-frail. | Honzawa [38] 2022 Japan | Pre-CR | Frail: n = 34 (24.8%) | NR |
| Honzawa [43] 2020 Japan | Pre-CR | Frail: n = 99 (38.8%) | NR | ||
| Kunimoto [45] 2019 Japan | Pre-CR | Frail: n = 288 (34.1%) | NR | ||
| Nagatomi [53] 2022 Japan | Pre-CR | Frail: n = 10 (33.0%) | NR | ||
| Nishitani-Yokoyama [46] 2021 Japan | Pre-CR | Frail: n = 35 (34.0%) | NR | ||
| Xu [47] 2023 Japan | Pre-CR | Frail: n = 81 (37.0%) | NR | ||
| Fried’s Frailty Criteria | Assesses weight loss, exhaustion, grip strength, walking speed, and physical activity. Frailty if ≥3 criteria met. | Francesco-Cacciatore [17] 2013 Italy | Pre-CR | Frail: n = 109 (31.1%) | NR |
| Gilbert [52] 2023 USA | Post-CR | NR | NR | ||
| Nelson [50] 2022 USA | Pre-CR | Frail: n = 92 (53.0%) | NR | ||
| Rogers [54] 2018 UK | Pre-CR and Post-CR | Frailty by FRIED scale: 3, n = 5/25 (20.0%), 2, n = 8/25 (32.0%), 1, n = 9/25 (36.0%) | Frailty by FRIED scale: 3, n = 1/14 (7.1%), 2, n = 6/14 (42.9%), 1, n = 6/14 (42.9%) | ||
| Toshie Tanaka [29] 2018 Japan | Pre-CR and Post-CR | Frail: n = 8 (25.8%) | Frail: n = 2 (6.5%) | ||
| Yu [31] 2021 China | Pre-CR and Post-CR | Frail: n = 75 (83.0%) | Frail: n = 23 (26.0%) | ||
| 25-Item Frailty Index (FI) | Domains: (1) CV risk factors, (2) CV symptoms (NYHA class), (3) stress test, (4) Quality of life (SF-36), (5) body composition, (6) diet. Score: 0–1. Categorized by 0.1 increments. | Hillier [22] 2023 Canada | Pre-CR | Frailty by FI categories: >0.5 FI: n = 227 (9.0%), 0.4–0.5 FI: n = 462 (17.0%), 0.3–0.4 FI: n = 791 (30.0%), 0.2–0.3 FI: n = 715 (27.0%), <0.2 FI: n = 448 (17.0%) | NR |
| Kehler [39] 2020 Canada | Pre-CR and Post-CR | Frailty by FI categories: >0.50 FI: 175 (7.5%), 0.4–0.5 FI: n = 401 (17.3%), 0.3–0.4 FI: n = 690 (29.7%), 0.2–0.3 FI: n = 642 (27.6%), <0.2 FI: n = 414 (17.8%) | Frailty by FI categories: >0.50 FI: 95 (4.1%), 0.4–0.5 FI: n = 209 (9.0%), 0.3–0.4 FI: n = 447 (19.3%), 0.2–0.3 FI: n = 659 (28.4%), <0.2 FI: n = 912 (39.3%) | ||
| MacEachern [40] 2021 Canada | Pre-CR and Post-CR | NR | NR | ||
| MacEachern [8] 2024 Canada | Pre-CR | Frailty by FI categories: >0.40 FI: n = 204 (26.9%), 0.30–0.39 FI: n = 219 (28.9%), 0.20–0.29 FI: n = 207 (27.3%), <0.2 FI: n = 129 (17.0%) | NR | ||
| Quach [51] 2023 Canada | Pre-CR | Frailty by FI categories: 0.5 > FI: n = 356 (10.6%), 0.4–0.5 FI: n = 650 (19.3%), 0.3–0.4 FI: n = 964 (28.6%), 0.2–0.3 FI: n = 872 (25.9%), <0.2 FI: n = 529 (15.7%) | NR | ||
| 6-Minute Walk Distance (6MWD) | <300 m = frailty | Henderson [37] 2017 USA | Pre-CR and Post-CR | Frail: n = 24 (40.0%) | Frail: n = 11 (18.3%) |
| Lutz [26] 2019 USA | Pre-CR | Frail: n = 49 (30.1%) | NR | ||
| Lutz [27] 2020 USA | Pre-CR | Frail: n = 75 (30.9%) | NR | ||
| Tarro Genta [41] 2015 Italy | Post-CR | NR | Frail: n = 22 (20.0%) | ||
| Clinical Frailty Scale (CFS)—also known as Rockwood Frailty Scale | 1–9 scale; score ≥ 4 indicates frailty. | Francesco-Cacciatore [17] 2013 Italy | Pre-CR | Frail: n = 90 (25.6%) | NR |
| German-Sallo [19] 2024 Hungary | Pre-CR | Frail: n = 30 (32.6%) | NR | ||
| Kimber [24] 2018 Canada | Pre-CR and Post-CR | NR | NR | ||
| Edmonton Frail Scale (EFS) | Assesses 10 domains including cognition, nutrition, mood, and function. Score range: 0–17 (higher = more frail). | Fonteles Ritt [21] 2021 Brazil | Pre-CR and Post-CR (≥3 months after start) | Mean EFS: 5.4 ± 2.0 (frailty level not reported as n/%) | Mean EFS score 4.8 ± 1.9 (frailty level not reported as n/%) |
| Mathew [28] 2019 Canada | Pre-CR and Post-CR | NR | No improvement in EFS score: n = 489, mean EFS score: 3.2, Any improvement: n = 275, mean EFS score: 5.0, Total: n = 764 (completers), mean EFS score: 3.8 | ||
| Rogers [54] 2018 UK | Pre-CR and Post-CR | EFS: 5.08 (2.2) | EFS: 4.4 (1.7) | ||
| Gait Speed (GS) | GS < 1 m/s = frailty | Henderson [37] 2017 USA | Pre-CR and Post-CR | Frail: n = 24 (40.0%) | Frail: n = 11 (18.3%) |
| Lutz [26] 2019 USA | Pre-CR | Frail: n = 49 (30.1%) | NR | ||
| Lutz [27] 2020 USA | Pre-CR | Frail: n = 75 (30.9%) | NR | ||
| Japanese Version of the Cardiovascular Health Study Standard (J-CHS)—A revised version of Fried’s Frailty Criteria | Assesses 5 domains: weight loss, low activity, fatigue, weakness, gait speed. Frailty if ≥3 criteria present. | Hashimoto [36] 2022 Japan | Pre-CR | Frail: n = 15 (28.8%) | NR |
| Ushijima [42] 2021 Japan | Pre-CR and Post-CR | Frail: n = 23 (25.8%) | Frail: n = 3 (3.4%) | ||
| Yokote [30] 2023 Japan | Pre-CR | Frail: n = 48 (19.8%) | NR | ||
| Tandem Stand (TS) | TS < 10 s = frailty | Henderson [37] 2017 USA | Pre-CR and Post-CR | Frail: n = 24 (40.0%) | Frail: n = 11 (18.3%) |
| Lutz [26] 2019 USA | Pre-CR | Frail: n = 49 (30.1%) | NR | ||
| Lutz [27] 2020 USA | Pre-CR | Frail: n = 75 (30.9%) | NR | ||
| Timed Up and Go (TUG) | TUG > 15 s = frailty | Henderson [37] 2017 USA | Pre-CR and Post-CR | Frail: n = 24 (40.0%) | Frail: n = 11 (18.3%) |
| Lutz [26] 2019 USA | Pre-CR | Frail: n = 49 (30.1%) | NR | ||
| Lutz [27] 2020 USA | Pre-CR | Frail: n = 75 (30.9%) | NR | ||
| FLAGSHIP Frailty Score | Assesses physical frailty in heart failure prognosis. 4 domains: slowness, weakness, inactivity, exhaustion. Score 0–14; higher = worse frailty | Adachi [32] 2023 Japan | Pre-CR | Frail: n = 1062 (39.4%) | NR |
| Iritani [44] 2023 Japan | Pre-CR | Frail: n = 1993 (100%) | NR | ||
| Hand Grip Strength (HGS) | HGS based on Fried | Lutz [26] 2019 USA | Pre-CR | Frail: n = 49 (30.1%) | NR |
| Lutz [27] 2020 USA | Pre-CR | Frail: n = 75 (30.9%) | NR | ||
| Modified Fried Criteria (MFC)—A revised version of Fried’s Frailty Criteria | Assesses 5 domains: exhaustion, grip strength, mobility, unintentional weight loss, and physical activity. Score 0: robust; 1–2: prefrail; 3–5: frail. | Dinesh [18] 2024 Australia | Pre-CR and Post-CR (cross-sectional) | Frail: n = 61 (21.3%) | Lower frailty scores in CR groups compared to non-CR groups, n (%) NR |
| Kimber [24] 2018 Canada | Pre-CR and Post-CR | NR | NR | ||
| Short Physical Performance Battery (SPPB) | Gait speed, balance, chair stand; total score 0–12; <7 = frailty | Baldasseroni [33] 2023 Italy | Pre-CR and Post-CR | Mild frailty: n = 27 (27.0%), moderate frailty: n = 14 (14.0%), severe frailty: n = 0 (0.0%) | Mild frailty: n = 22 (22.0%), moderate frailty: n = 3 (3.0%), severe frailty: n = 0 (0.0%) |
| Kimber [24] 2018 Canada | Pre-CR and Post-CR | NR | NR | ||
| Barthel Index (BI) | BI < 75 | Tarro Genta [41] 2015 Italy | Post-CR | NR | Frail: n = 22 (20.0%) |
| Claims-Based Frailty Index (Quartiles) | Patients were stratified into quartiles: Q1 = least frail) to Q4 = most frail. | Bauer [20] 2023 USA | Pre-CR | Most frail (Q4 quartile): n = 117,595 (20.6%) | NR |
| Comprehensive Geriatric Assessment based frailty index (CGA-based FI) | Assesses 40 multidimensional health deficits [56]. Each deficit scored 1 if present, 0 if absence. FI = total score/number of items. Cut-off ≥ 0.25 defines frailty. | Bencivenga [34] 2022 Italy | Pre-CR | Frail: n = 293 (52.4%) | NR |
| FRAIL Scale (FS) | Assesses fatigue, resistance, ambulation, illnesses, weight loss; score 0–5. Frail if FS ≥ 3 or CFS ≥ 5. | German-Sallo [19] 2024 Hungary | Pre-CR | Frail: n = 30 (32.6%) | NR |
| Functional Frailty Index (FFI) | FFI based on 25 items across domains; score ≥ 0.25 = frailty. | Kimber [24] 2018 Canada | Pre-CR and Post-CR | NR | NR |
| Lachs Frailty Scale | Based on absence of comorbidity. | Francesco-Cacciatore [17] 2013 Italy | Pre-CR | Frail: n = 138 (39.3%) | NR |
| Morse Fall Scale (MFS) | MFS ≥ 50 | Tarro Genta [41] 2015 Italy | Post-CR | NR | Frail: n = 22 (20.0%) |
| Multidimensional Geriatric Assessment (MGA) | Scored 0–7; score ≥ 3 indicates probable frailty. | Eichler [35] 2020 Germany | Pre-CR | Frail: n = 76 (61.3%) | NR |
| Validated Frailty Index Scale | Derived from medical intake history. Classification not described in detail. | Landry [25] 2018 Canada | Pre-CR | Mild frailty: n = 367 (46.0%), moderate frailty: n = 33 (4.1%) | NR |
| 19-Item Frailty Index (FI) | Items scored 0–1. Score = positive items/19. Quartiles define frailty: 0–0.12 = fit, >0.12–0.24 = mild, >0.24–0.36 = moderate, >0.36 = severe. | Kamiya [23] 2020 Japan | Pre-CR | FI score: 0.28 ± 0.13 (frailty level NR as n/%) | NR |
| 41-Item Frailty Index (FI) | Assesses function, mood, comorbidity, self-rated health, nutrition, and cognition. Items scored from 0 (non-frail) to 1 (most frail). FI = sum of scores/number of items. Frailty categorized as: <0.20 = non-frail, 0.20–0.39 = frail, ≥0.40 = very frail. | Mudge [49] 2021 Australia | Pre-CR and Post-CR | Frail: n = 119 (46.0%), very frail: n = 27 (11.0%) | Frail/very frail with improvements at 6 months: n = 87 (34.0%) |
| 65-Item Frailty Index (FI) | Includes symptoms, diseases, disability, and signs. Items scored 0 (non-frail) to 1 (most frail). FI = number of deficits/65. Categories: <0.10 (least frail), 0.11–0.19, 0.20–0.29, >0.30 (most frail). | MacEachern [48] 2023 Canada | Pre-CR | Frailty by FI categories: Centre based; >0.30 FI: n = 2 (2.7%), 0.20–0.29 FI: n = 5 (6.7%), 0.11–0.19 FI: n = 32 (43.2%), <0.10 FI: n = 35 (47.2%) Virtual; >0.30 FI: n = 1 (1.7%), 0.20–0.29 FI: n = 4 (6.8%), 0.11–0.19 FI: n = 24 (41.3%), <0.10 FI: n = 29 (50%) | NR |
| Barrier | Description | Study |
|---|---|---|
| Physical Limitations | Declined physical capacity; need for modifications addressing strength, balance, and frailty-specific hazards | Iritani [44], Henderson [37] |
| Mental Health and Emotional Factors | Anxiety, depression, emotional distress, and role-emotional limitations related to frailty | Hillier [22], Honzawa [38], Honzawa [43] |
| Motivational Barriers | Lack of motivation | Iritani [44] |
| Transportation and Access | No transportation | Iritani [44], Rogers [54] |
| Healthcare System-Related Factors | Medical appointments and coordination issues with multiple healthcare providers | Nelson [50] |
| Personal and Social Factors | Conflicting personal commitments (e.g., work, childcare, travel), lack of social support, or caregiver responsibilities. | Nelson [50] |
| Cognitive Impairment | Need for CR modifications to support cognition | Henderson [37] |
| Low Baseline Physical Activity | Frailer participants are significantly less likely to meet PA guidelines | Mudge [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carson, R.P.; Schneider, V.L.P.; Main, E.; Carvalho, C.G.; Ghisi, G.L.M. Understanding Frailty in Cardiac Rehabilitation: A Scoping Review of Prevalence, Measurement, Sex and Gender Considerations, and Barriers to Completion. J. Clin. Med. 2025, 14, 5354. https://doi.org/10.3390/jcm14155354
Carson RP, Schneider VLP, Main E, Carvalho CG, Ghisi GLM. Understanding Frailty in Cardiac Rehabilitation: A Scoping Review of Prevalence, Measurement, Sex and Gender Considerations, and Barriers to Completion. Journal of Clinical Medicine. 2025; 14(15):5354. https://doi.org/10.3390/jcm14155354
Chicago/Turabian StyleCarson, Rachael P., Voldiana Lúcia Pozzebon Schneider, Emilia Main, Carolina Gonzaga Carvalho, and Gabriela L. Melo Ghisi. 2025. "Understanding Frailty in Cardiac Rehabilitation: A Scoping Review of Prevalence, Measurement, Sex and Gender Considerations, and Barriers to Completion" Journal of Clinical Medicine 14, no. 15: 5354. https://doi.org/10.3390/jcm14155354
APA StyleCarson, R. P., Schneider, V. L. P., Main, E., Carvalho, C. G., & Ghisi, G. L. M. (2025). Understanding Frailty in Cardiac Rehabilitation: A Scoping Review of Prevalence, Measurement, Sex and Gender Considerations, and Barriers to Completion. Journal of Clinical Medicine, 14(15), 5354. https://doi.org/10.3390/jcm14155354







