Effects of a ROCK Inhibitor on Retinal Ganglion Cells In Vivo and In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. ROCK Inhibitor
2.3. Optic NC Injury and Eye Drop Instillation
2.4. IOP Measurements
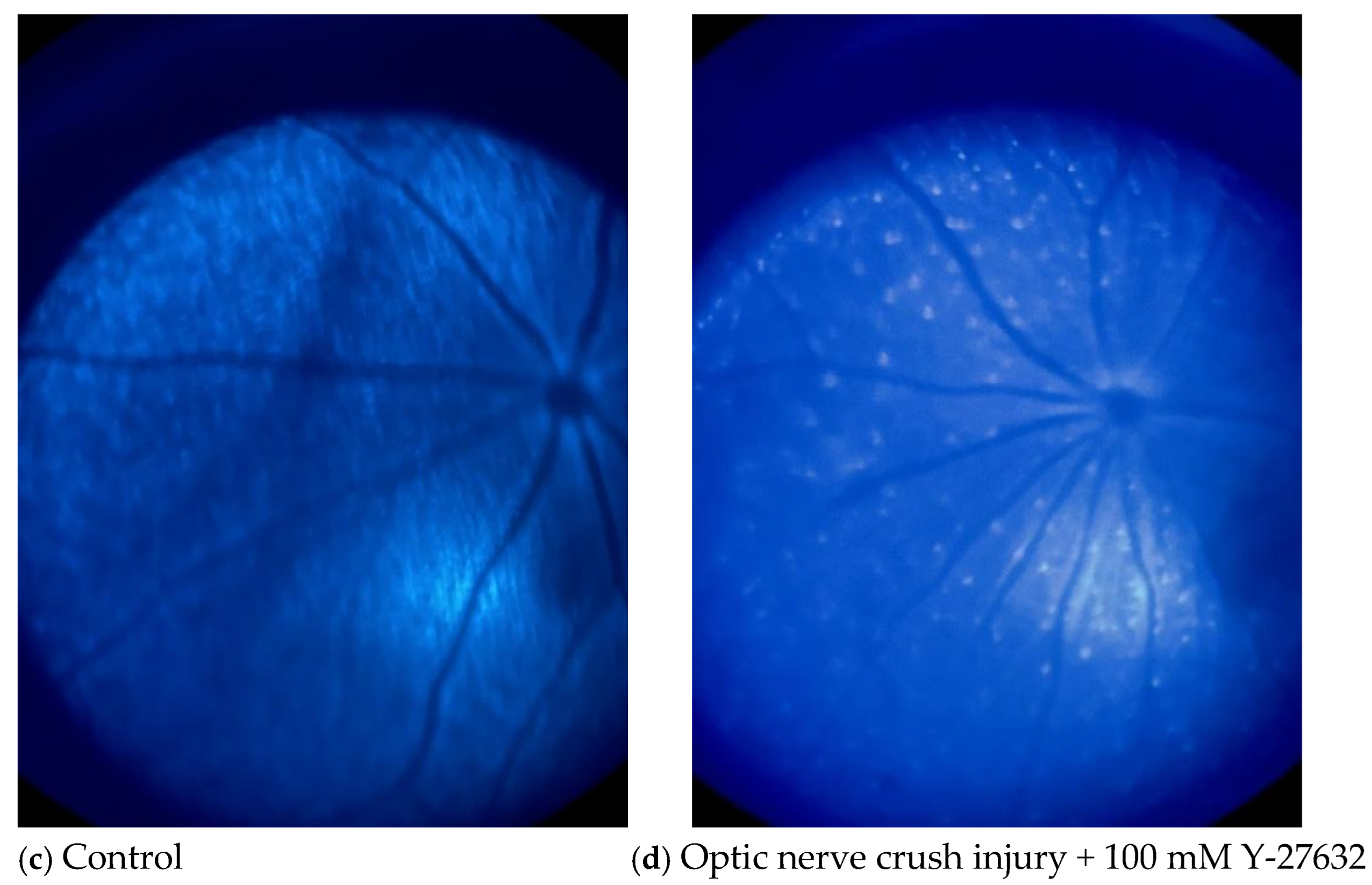
2.5. Optic Fundus Imaging
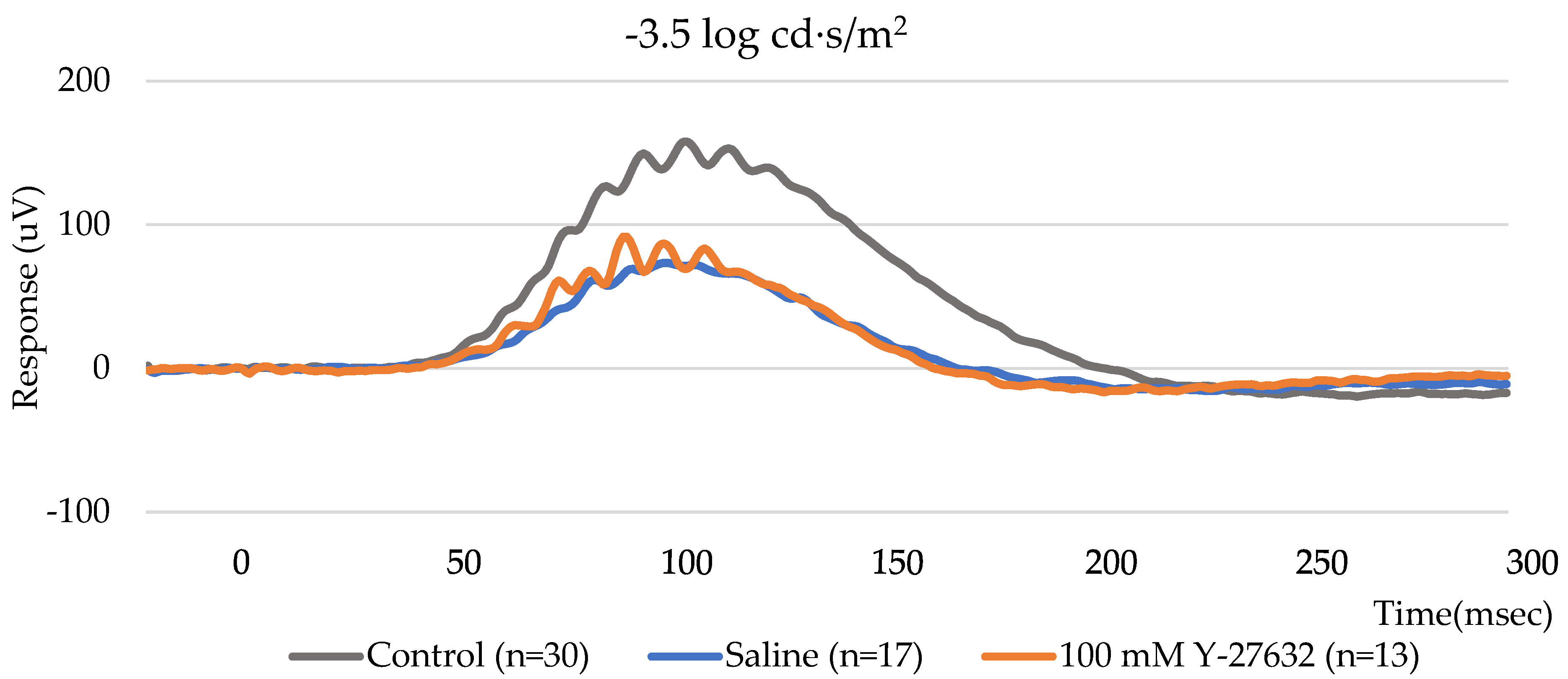
2.6. Retinal Electrophysiology/Electroretinography
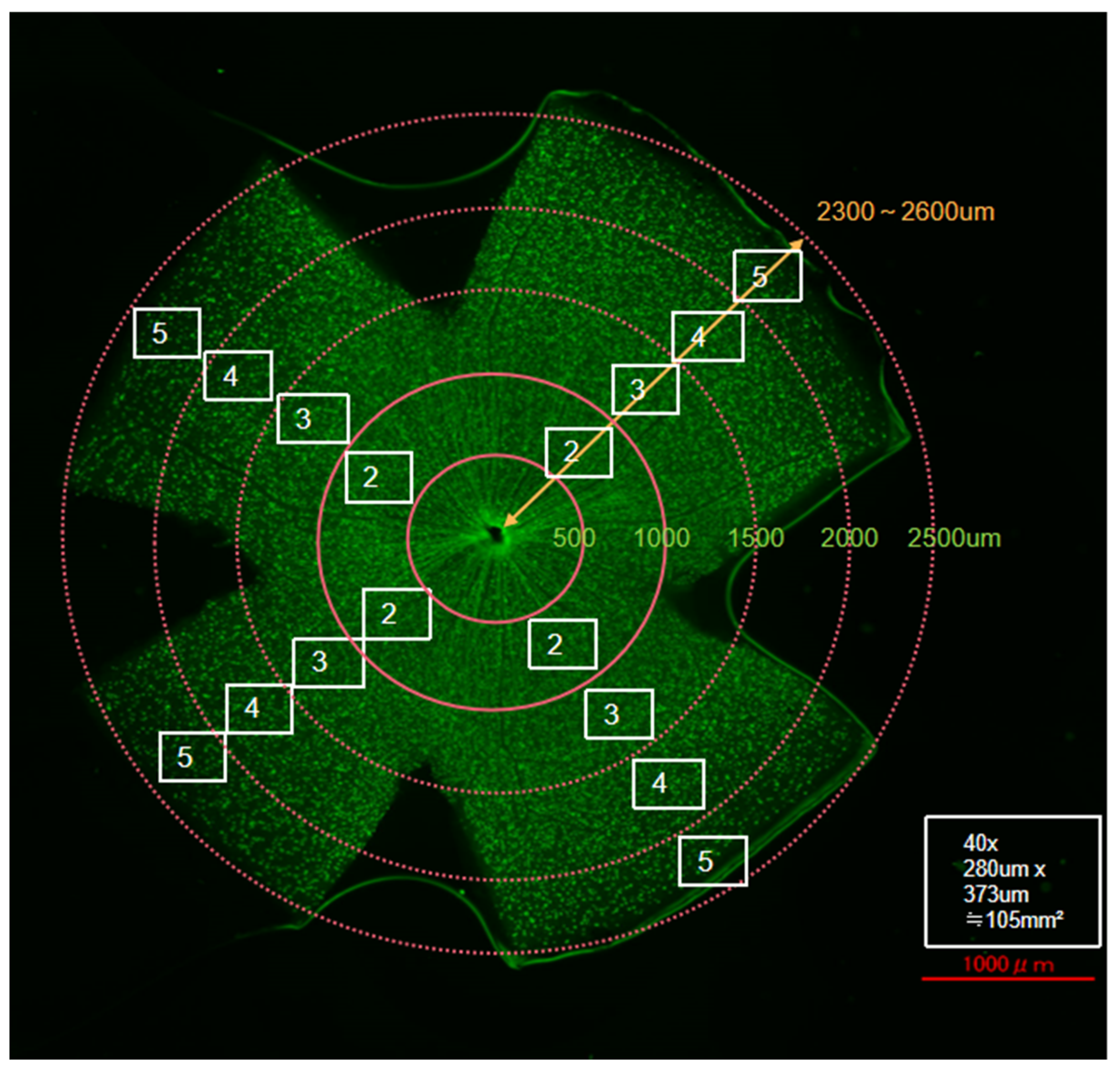
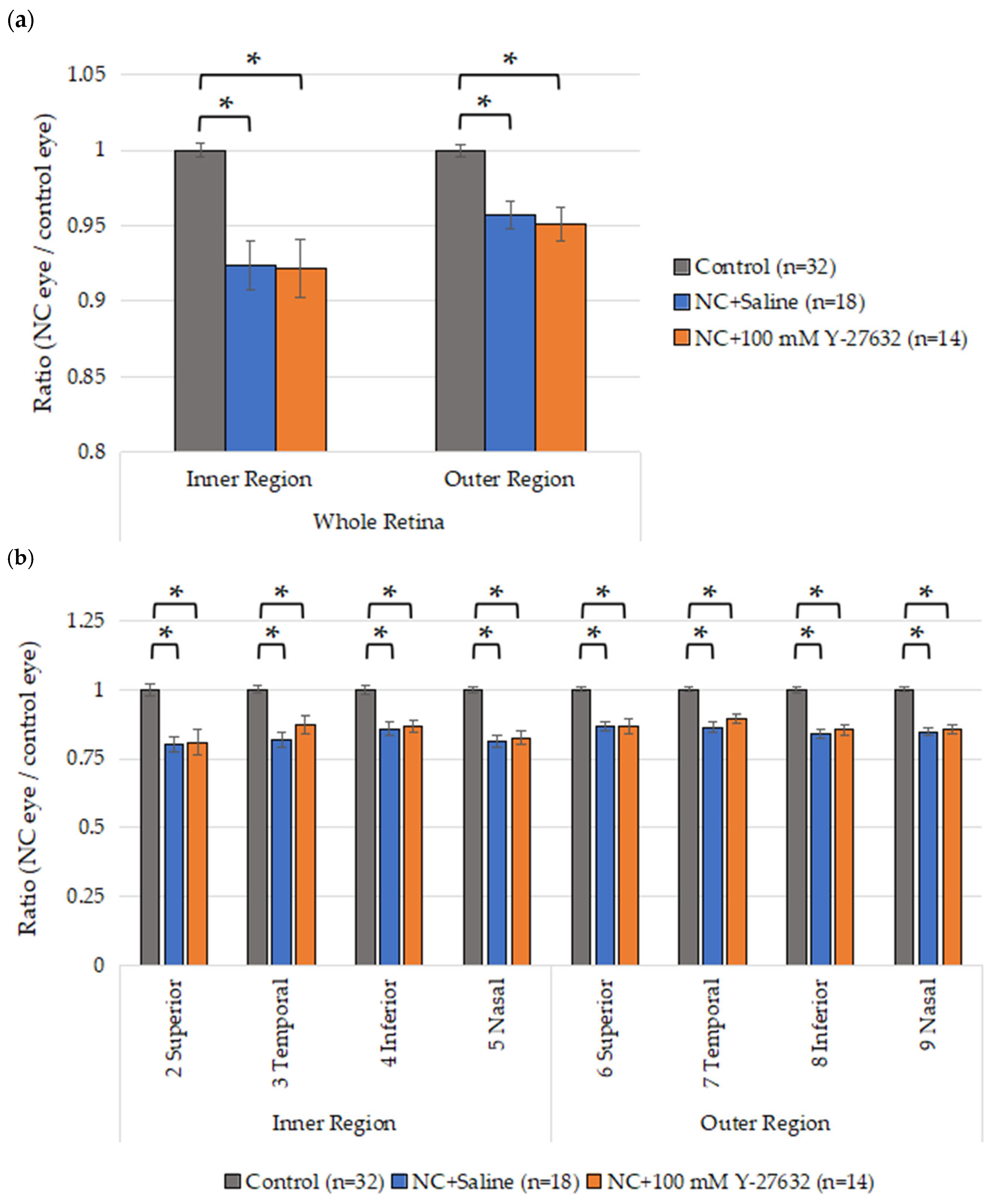
2.7. Flat-Mount Whole Retina Evaluation
2.8. Quantification of the Retinal Nerve Fiber Layer by Optical Coherence Tomography (OCT)
2.9. RGC Isolation
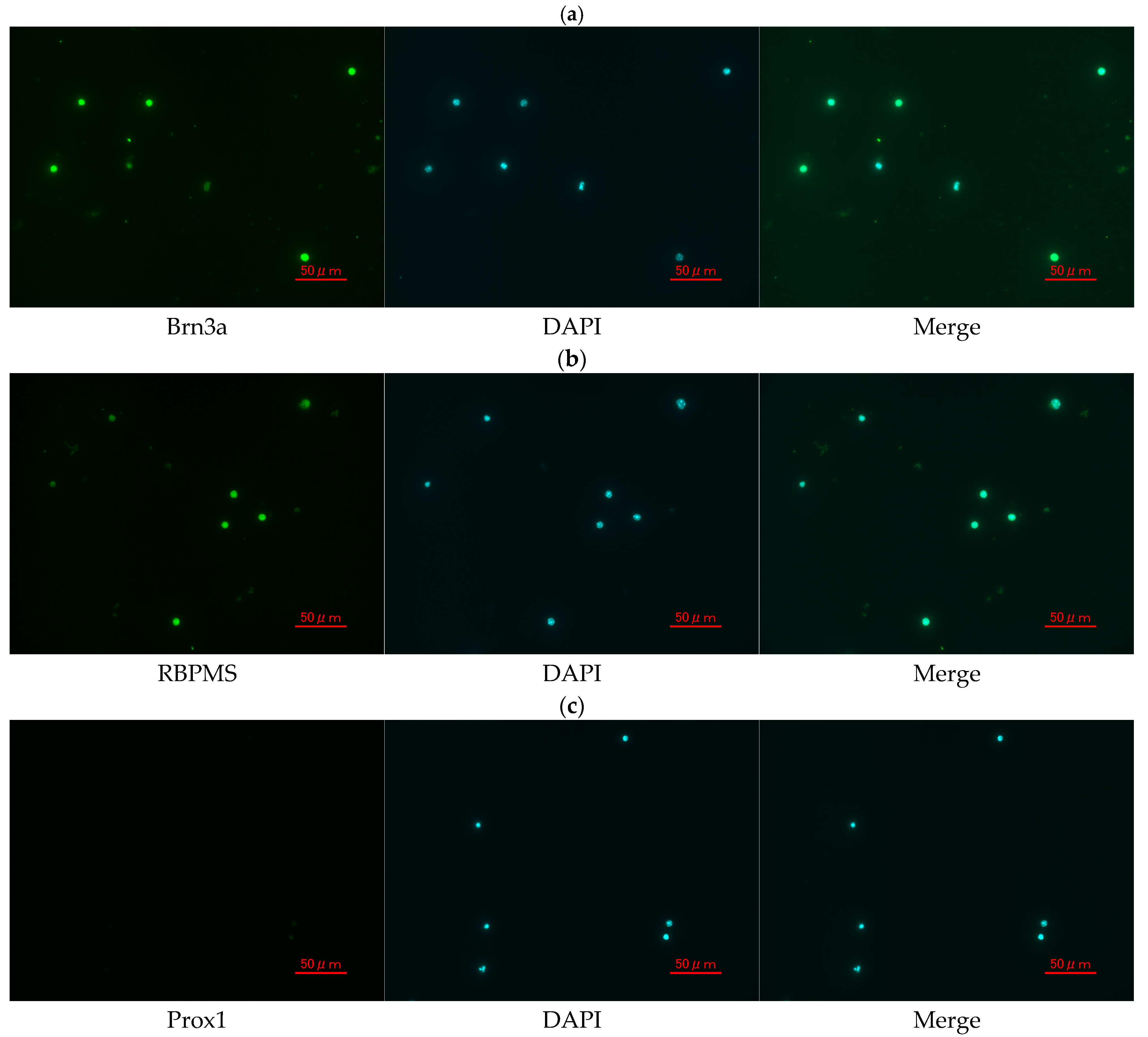
2.10. Immunocytochemistry for RGC Identification and Purity Assessment
2.11. TUNEL Assay
2.12. Statistical Analysis
3. Results
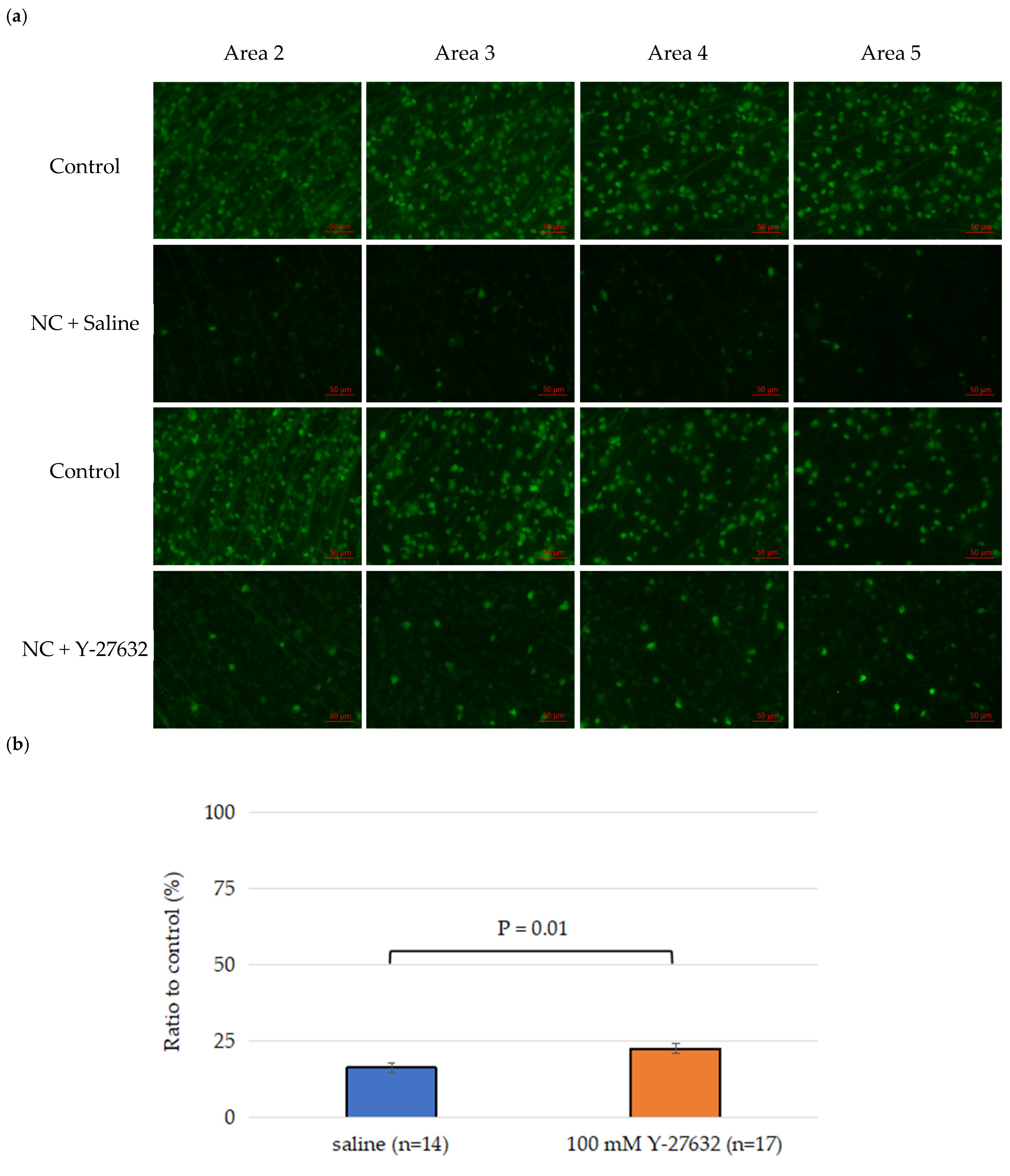
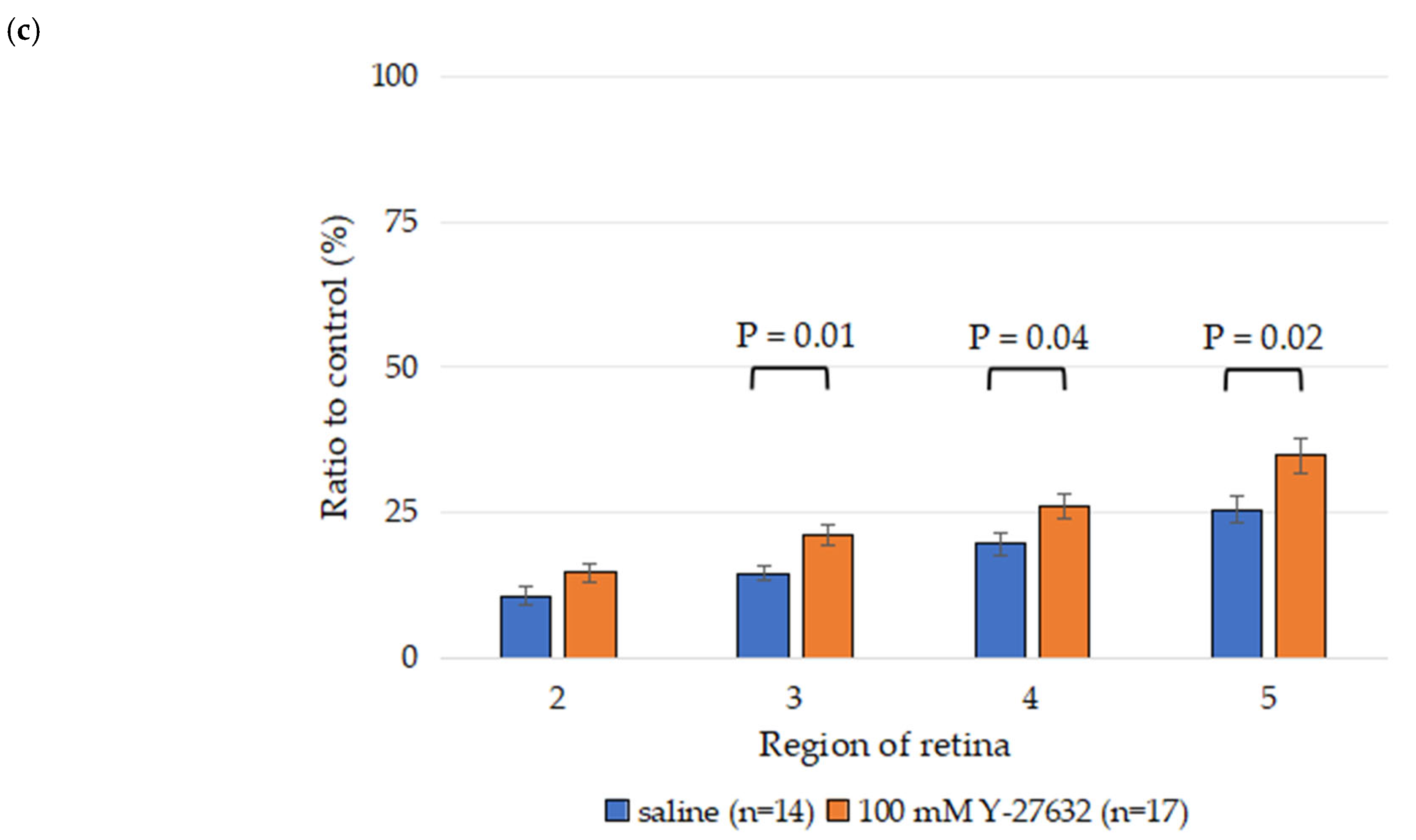
3.1. Effects of Y-27632 on RGC Survival After NC
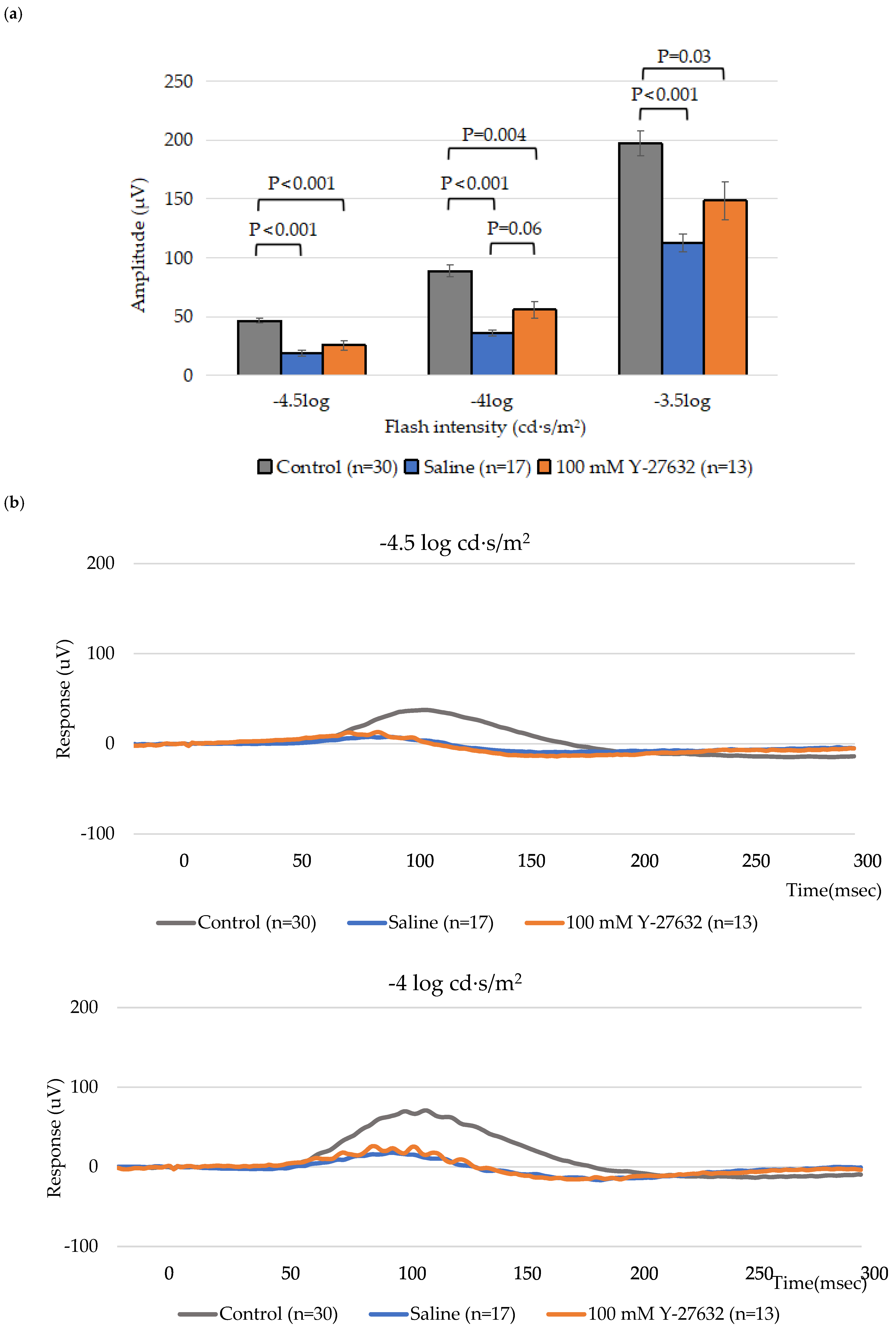
3.2. ERG Assessment of RGC Function
3.3. Effects of NC and Y-27632 on RGCs Determined by Fundus Imaging
3.4. OCT Quantification of the Retinal Layers
3.5. Effects of Y-27632 on IOP
3.6. Confirmation and Purity Assessment of Primary Mouse RGCs
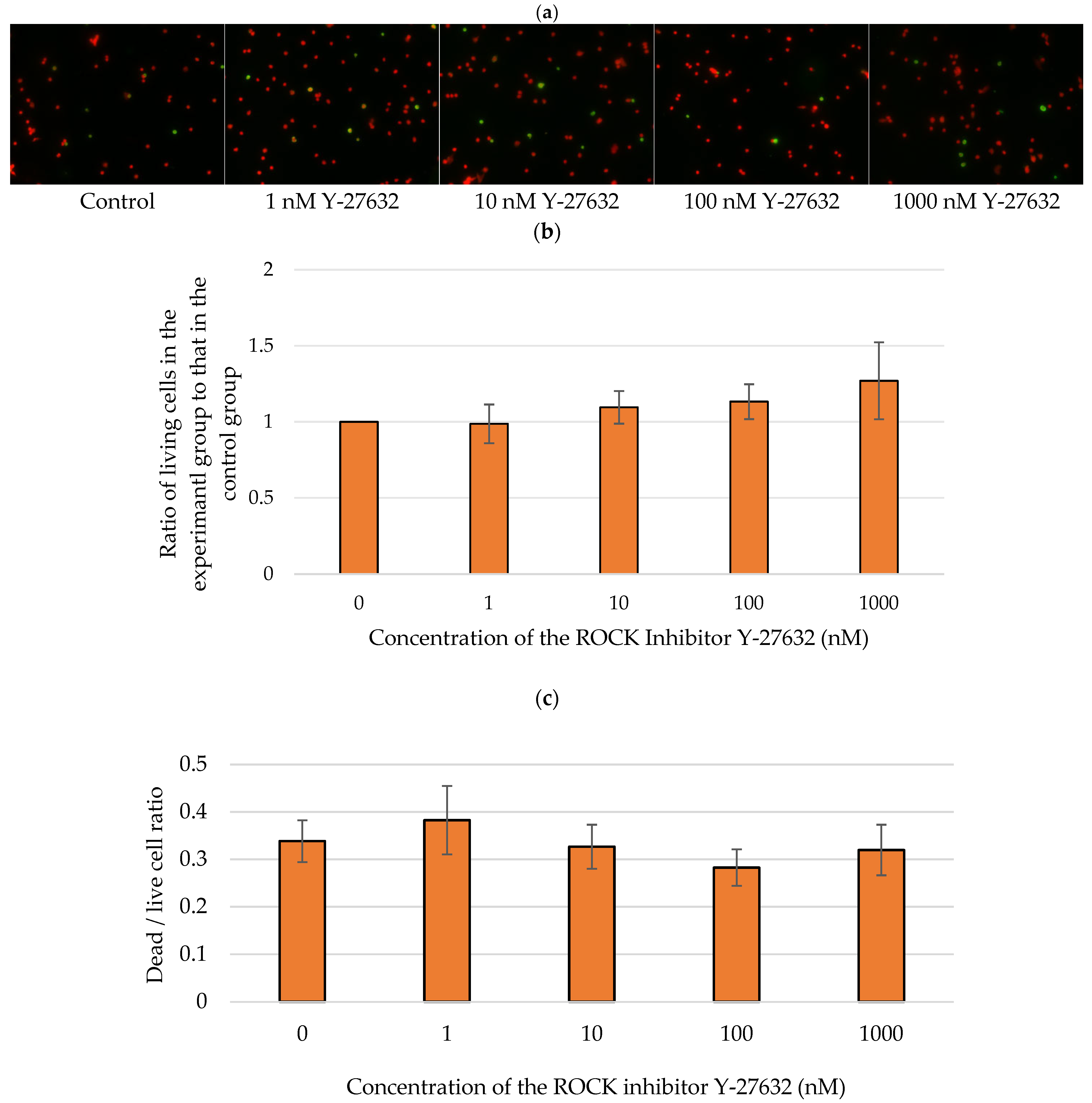
3.7. Effects of Y-27632 on Isolated RGCs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vision Loss Expert Group of the Global Burden of Disease Study; GBD 2019 Blindness and Vision Impairment Collaborators. Global Estimates on the Number of People Blind or Visually Impaired by Glaucoma: A Meta-Analysis from 2000 to 2020. Eye (Lond) 2024, 38, 2036–2046. [Google Scholar] [CrossRef] [PubMed]
- Cen, L.-P.; Park, K.K.; So, K.-F. Optic Nerve Diseases and Regeneration: How Far Are We from the Promised Land? Clin. Exp. Ophthalmol. 2023, 51, 627–641. [Google Scholar] [CrossRef]
- Casson, R.J. Medical Therapy for Glaucoma: A Review. Clin. Exp. Ophthalmol. 2022, 50, 198–212. [Google Scholar] [CrossRef]
- Inoue, T.; Tanihara, H. Ripasudil Hydrochloride Hydrate: Targeting Rho Kinase in the Treatment of Glaucoma. Expert Opin. Pharmacother. 2017, 18, 1669–1673. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-C.; Chen, Y.-H.; Lu, D.-W. The Application of Rho Kinase Inhibitors in the Management of Glaucoma. Int. J. Mol. Sci. 2024, 25, 5576. [Google Scholar] [CrossRef]
- Pagano, L.; Lee, J.W.; Posarelli, M.; Giannaccare, G.; Kaye, S.; Borgia, A. ROCK Inhibitors in Corneal Diseases and Glaucoma-A Comprehensive Review of These Emerging Drugs. J. Clin. Med. 2023, 12, 6736. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Patel, B.C. Netarsudil Ophthalmic Solution. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Amano, M.; Nakayama, M.; Kaibuchi, K. Rho-Kinase/ROCK: A Key Regulator of the Cytoskeleton and Cell Polarity. Cytoskeleton 2010, 67, 545–554. [Google Scholar] [CrossRef]
- Karri, R.; Chong, E.W. ROCK Inhibitors in Ophthalmology: A Critical Review of the Existing Clinical Evidence. Clin. Exp. Ophthalmol. 2023, 51, 472–483. [Google Scholar] [CrossRef]
- Abbhi, V.; Piplani, P. Rho-Kinase (ROCK) Inhibitors—A Neuroprotective Therapeutic Paradigm with a Focus on Ocular Utility. Curr. Med. Chem. 2020, 27, 2222–2256. [Google Scholar] [CrossRef]
- Saha, B.C.; Kumari, R.; Kushumesh, R.; Ambasta, A.; Sinha, B.P. Status of Rho Kinase Inhibitors in Glaucoma Therapeutics—An Overview. Int. Ophthalmol. 2022, 42, 281–294. [Google Scholar] [CrossRef]
- Watabe, H.; Abe, S.; Yoshitomi, T. Effects of Rho-Associated Protein Kinase Inhibitors Y-27632 and Y-39983 on Isolated Rabbit Ciliary Arteries. Jpn. J. Ophthalmol. 2011, 55, 411–417. [Google Scholar] [CrossRef]
- Hein, T.W.; Rosa, R.H.; Yuan, Z.; Roberts, E.; Kuo, L. Divergent Roles of Nitric Oxide and Rho Kinase in Vasomotor Regulation of Human Retinal Arterioles. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1583–1590. [Google Scholar] [CrossRef]
- Sugiyama, T.; Shibata, M.; Kajiura, S.; Okuno, T.; Tonari, M.; Oku, H.; Ikeda, T. Effects of Fasudil, a Rho-Associated Protein Kinase Inhibitor, on Optic Nerve Head Blood Flow in Rabbits. Investig. Ophthalmol. Vis. Sci. 2011, 52, 64–69. [Google Scholar] [CrossRef]
- Kitaoka, Y.; Kitaoka, Y.; Kumai, T.; Lam, T.T.; Kuribayashi, K.; Isenoumi, K.; Munemasa, Y.; Motoki, M.; Kobayashi, S.; Ueno, S. Involvement of RhoA and Possible Neuroprotective Effect of Fasudil, a Rho Kinase Inhibitor, in NMDA-Induced Neurotoxicity in the Rat Retina. Brain Res. 2004, 1018, 111–118. [Google Scholar] [CrossRef]
- Shaw, P.X.; Sang, A.; Wang, Y.; Ho, D.; Douglas, C.; Dia, L.; Goldberg, J.L. Topical Administration of a Rock/Net Inhibitor Promotes Retinal Ganglion Cell Survival and Axon Regeneration after Optic Nerve Injury. Exp. Eye Res. 2017, 158, 33–42. [Google Scholar] [CrossRef]
- Okumura, N.; Koizumi, N.; Kay, E.P.; Ueno, M.; Sakamoto, Y.; Nakamura, S.; Hamuro, J.; Kinoshita, S. The ROCK Inhibitor Eye Drop Accelerates Corneal Endothelium Wound Healing. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2493–2502. [Google Scholar] [CrossRef] [PubMed]
- Okumura, N.; Kinoshita, S.; Koizumi, N. Application of Rho Kinase Inhibitors for the Treatment of Corneal Endothelial Diseases. J. Ophthalmol. 2017, 2017, 2646904. [Google Scholar] [CrossRef] [PubMed]
- Honjo, M.; Tanihara, H.; Kameda, T.; Kawaji, T.; Yoshimura, N.; Araie, M. Potential Role of Rho-Associated Protein Kinase Inhibitor Y-27632 in Glaucoma Filtration Surgery. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5549–5557. [Google Scholar] [CrossRef]
- Okumura, N.; Inoue, R.; Okazaki, Y.; Nakano, S.; Nakagawa, H.; Kinoshita, S.; Koizumi, N. Effect of the Rho Kinase Inhibitor Y-27632 on Corneal Endothelial Wound Healing. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6067–6074. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-C.; Chiu, H.-T.; Lin, Y.-F.; Lee, K.-Y.; Pang, J.-H.S. Y-27632, a ROCK Inhibitor, Promoted Limbal Epithelial Cell Proliferation and Corneal Wound Healing. PLoS ONE 2015, 10, e0144571. [Google Scholar] [CrossRef]
- Zhang, W.; Shao, C.; Yu, F.; Chen, J.; Fu, Y.; Fan, X. Y-27632 Promotes the Repair Effect of Umbilical Cord Blood-Derived Endothelial Progenitor Cells on Corneal Endothelial Wound Healing. Cornea 2021, 40, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-H.; Feng, Z.-H.; Wang, X.-Y. The ROCK Pathway Inhibitor Y-27632 Mitigates Hypoxia and Oxidative Stress-Induced Injury to Retinal Müller Cells. Neural Regen. Res. 2018, 13, 549–555. [Google Scholar] [CrossRef]
- Inoue, T.; Tanihara, H. Rho-Associated Kinase Inhibitors: A Novel Glaucoma Therapy. Prog. Retin. Eye Res. 2013, 37, 1–12. [Google Scholar] [CrossRef]
- Buffault, J.; Brignole-Baudouin, F.; Reboussin, É.; Kessal, K.; Labbé, A.; Mélik Parsadaniantz, S.; Baudouin, C. The Dual Effect of Rho-Kinase Inhibition on Trabecular Meshwork Cells Cytoskeleton and Extracellular Matrix in an In Vitro Model of Glaucoma. J. Clin. Med. 2022, 11, 1001. [Google Scholar] [CrossRef]
- Hirata, A.; Inatani, M.; Inomata, Y.; Yonemura, N.; Kawaji, T.; Honjo, M.; Tanihara, H. Y-27632, a Rho-Associated Protein Kinase Inhibitor, Attenuates Neuronal Cell Death after Transient Retinal Ischemia. Graefes Arch. Clin. Exp. Ophthalmol. 2008, 246, 51–59. [Google Scholar] [CrossRef]
- Cameron, E.G.; Xia, X.; Galvao, J.; Ashouri, M.; Kapiloff, M.S.; Goldberg, J.L. Optic Nerve Crush in Mice to Study Retinal Ganglion Cell Survival and Regeneration. Bio-Protocol 2020, 10, e3559. [Google Scholar] [CrossRef]
- Rojas, B.; Gallego, B.I.; Ramírez, A.I.; Salazar, J.J.; de Hoz, R.; Valiente-Soriano, F.J.; Avilés-Trigueros, M.; Villegas-Perez, M.P.; Vidal-Sanz, M.; Triviño, A.; et al. Microglia in Mouse Retina Contralateral to Experimental Glaucoma Exhibit Multiple Signs of Activation in All Retinal Layers. J. Neuroinflamm. 2014, 11, 133. [Google Scholar] [CrossRef]
- Sabbah, S.; Berg, D.; Papendorp, C.; Briggman, K.L.; Berson, D.M. A Cre Mouse Line for Probing Irradiance- and Direction-Encoding Retinal Networks. eNeuro 2017, 4, ENEURO.0065-17.2017. [Google Scholar] [CrossRef]
- Sterratt, D.C.; Lyngholm, D.; Willshaw, D.J.; Thompson, I.D. Standard Anatomical and Visual Space for the Mouse Retina: Computational Reconstruction and Transformation of Flattened Retinae with the Retistruct Package. PLoS Comput. Biol. 2013, 9, e1002921. [Google Scholar] [CrossRef]
- Barres, B.A.; Silverstein, B.E.; Corey, D.P.; Chun, L.L. Immunological, Morphological, and Electrophysiological Variation among Retinal Ganglion Cells Purified by Panning. Neuron 1988, 1, 791–803. [Google Scholar] [CrossRef]
- Kashiwagi, K.; Iizuka, Y.; Araie, M.; Suzuki, Y.; Tsukahara, S. Effects of Retinal Glial Cells on Isolated Rat Retinal Ganglion Cells. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2686–2694. [Google Scholar]
- Hong, S.; Iizuka, Y.; Kim, C.Y.; Seong, G.J. Isolation of Primary Mouse Retinal Ganglion Cells Using Immunopanning-Magnetic Separation. Mol. Vis. 2012, 18, 2922–2930. [Google Scholar]
- Feng, P.-L.; Wang, J.; Yang, Z.-J.; Liu, X.-H.; Zhong, Y.-S. Effect of Y-27632 on the Cultured Retinal Neurocytes of Rats. Int. J. Ophthalmol. 2013, 6, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Quillen, S.E.; Kimball, E.C.; Ritter-Gordy, K.A.; Du, L.; Yuan, Z.; Pease, M.E.; Madhoun, S.; Nguyen, T.D.; Johnson, T.V.; Quigley, H.A.; et al. The Mechanisms of Neuroprotection by Topical Rho Kinase Inhibition in Experimental Mouse Glaucoma and Optic Neuropathy. Investig. Ophthalmol. Vis. Sci. 2024, 65, 43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wei, Y.; Jiang, X.; Li, J.; Qiu, S.; Zhang, S. Protection of Photoreceptors by Intravitreal Injection of the Y-27632 Rho-Associated Protein Kinase Inhibitor in Royal College of Surgeons Rats. Mol. Med. Rep. 2015, 12, 3655–3661. [Google Scholar] [CrossRef]
- Honjo, M.; Tanihara, H.; Inatani, M.; Kido, N.; Sawamura, T.; Yue, B.Y.; Narumiya, S.; Honda, Y. Effects of Rho-Associated Protein Kinase Inhibitor Y-27632 on Intraocular Pressure and Outflow Facility. Investig. Ophthalmol. Vis. Sci. 2001, 42, 137–144. [Google Scholar]
- Koch, J.C.; Tönges, L.; Barski, E.; Michel, U.; Bähr, M.; Lingor, P. ROCK2 Is a Major Regulator of Axonal Degeneration, Neuronal Death and Axonal Regeneration in the CNS. Cell Death Dis. 2014, 5, e1225. [Google Scholar] [CrossRef] [PubMed]
- Templeton, J.P.; Geisert, E.E. A Practical Approach to Optic Nerve Crush in the Mouse. Mol. Vis. 2012, 18, 2147–2152. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Iizuka, Y.; Mabuchi, F.; Kashiwagi, K. Effects of a ROCK Inhibitor on Retinal Ganglion Cells In Vivo and In Vitro. J. Clin. Med. 2025, 14, 5344. https://doi.org/10.3390/jcm14155344
Chen W, Iizuka Y, Mabuchi F, Kashiwagi K. Effects of a ROCK Inhibitor on Retinal Ganglion Cells In Vivo and In Vitro. Journal of Clinical Medicine. 2025; 14(15):5344. https://doi.org/10.3390/jcm14155344
Chicago/Turabian StyleChen, Wanjing, Yoko Iizuka, Fumihiko Mabuchi, and Kenji Kashiwagi. 2025. "Effects of a ROCK Inhibitor on Retinal Ganglion Cells In Vivo and In Vitro" Journal of Clinical Medicine 14, no. 15: 5344. https://doi.org/10.3390/jcm14155344
APA StyleChen, W., Iizuka, Y., Mabuchi, F., & Kashiwagi, K. (2025). Effects of a ROCK Inhibitor on Retinal Ganglion Cells In Vivo and In Vitro. Journal of Clinical Medicine, 14(15), 5344. https://doi.org/10.3390/jcm14155344






