Telemedicine-Supported CPAP Therapy in Patients with Obstructive Sleep Apnea: Association with Treatment Adherence and Clinical Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Ethical Approval
2.3. Participants
2.4. Data Collection and Management
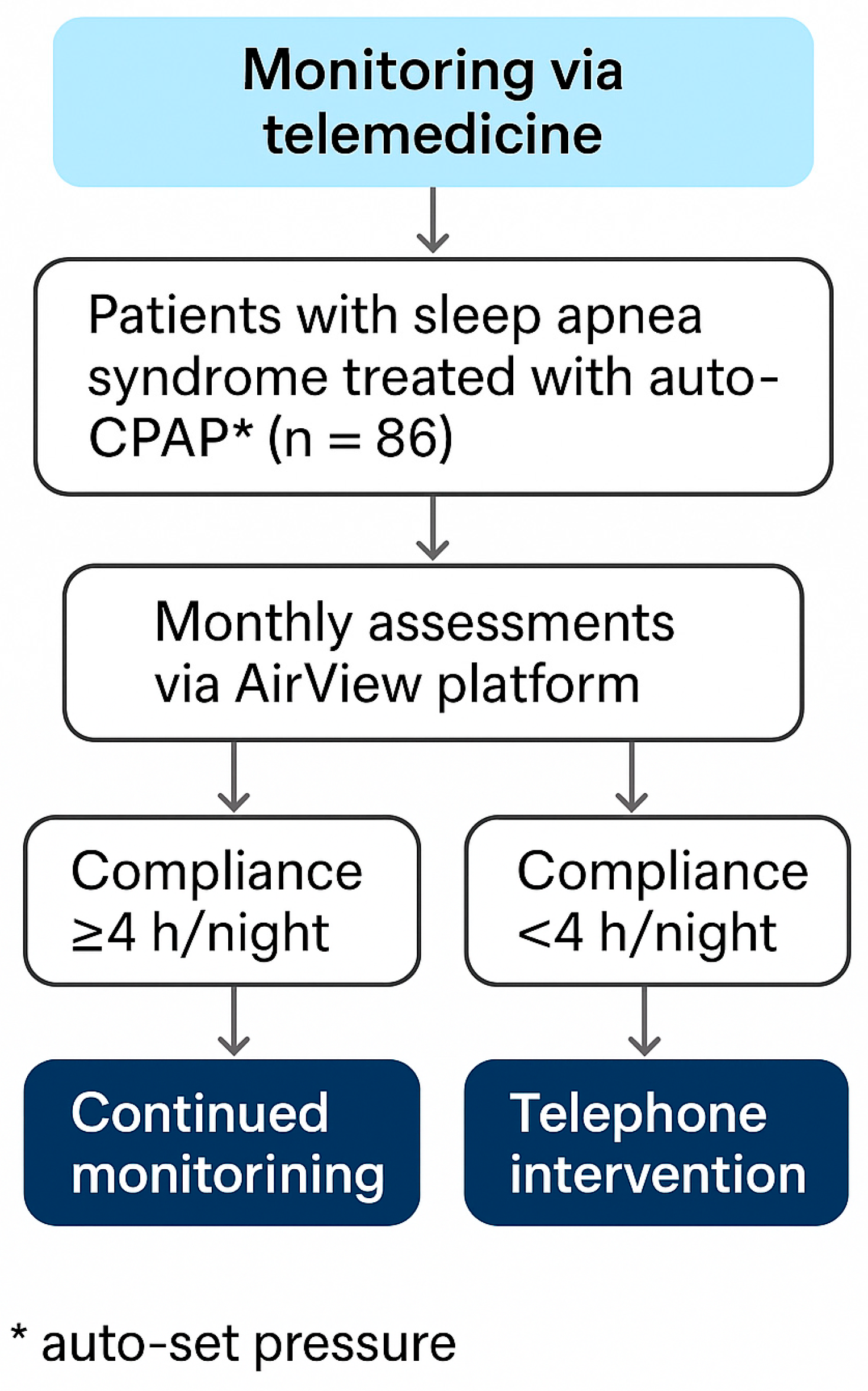
2.5. Remote Monitoring and Adherence Support
Rationale for Human-Delivered Phone Interventions
2.6. Clinical Assessments
2.7. Unsupervised Machine Learning Analysis
2.7.1. Optimal Cluster Number Determination
2.7.2. K-Means Clustering Implementation
2.7.3. Dimensionality Reduction and Visualization
- Perplexity: 30.
- Learning rate: 200.
- Maximum iterations: 1000.
2.7.4. Cluster Quality Assessment
- Silhouette Score: Measure of cohesion within clusters relative to separation between clusters (range of −1 to 1, where higher indicates better clustering). We obtained a maximum value of 0.21 at k = 4.
- Calinski–Harabasz Index: Ratio of between-cluster to within-cluster dispersion (higher values indicate better-defined clusters). This index had a maximum of 33.60 at k = 4.
- Davies–Bouldin Score: Average similarity ratio of each cluster with its most similar cluster (lower values indicate better clustering). This score also indicated k = 4 as optimal with a low value of 1.40.
2.7.5. Feature Categorization
- Demographics: Age and Body Mass Index (BMI).
- Baseline Diagnosis: Initial AHI, minimum oxygen saturation, and Epworth Sleepiness Scale score.
- Treatment Adherence: Compliance > 4 h/night at 6 months and average nightly usage (minutes) at 6 months.
- Clinical Outcomes: AHI at 6 months and WHOQOL-BREF average score at 6 months.
2.7.6. Statistical Analysis and Reporting
3. Results
3.1. Comprehensive Baseline Population Characterization
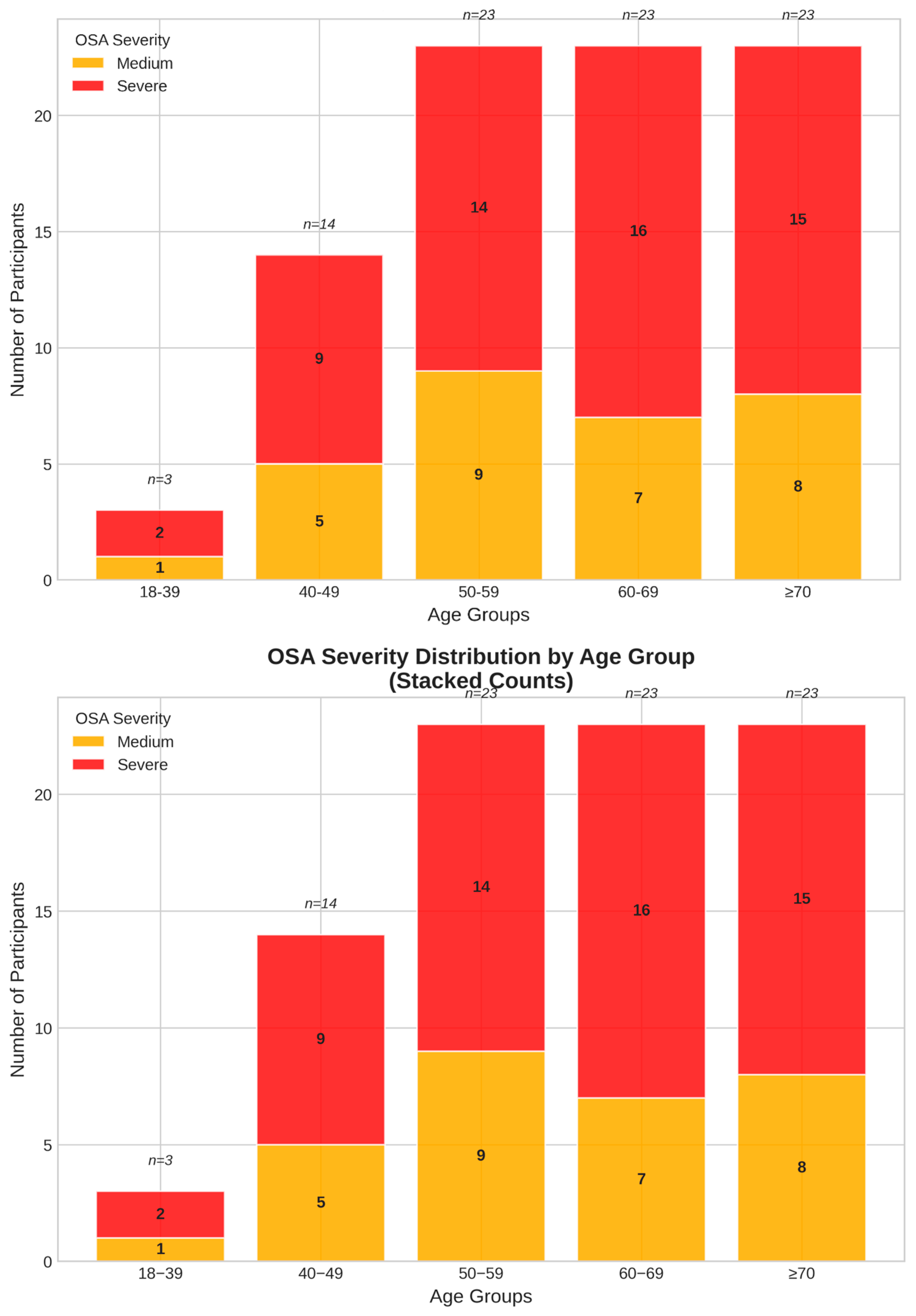
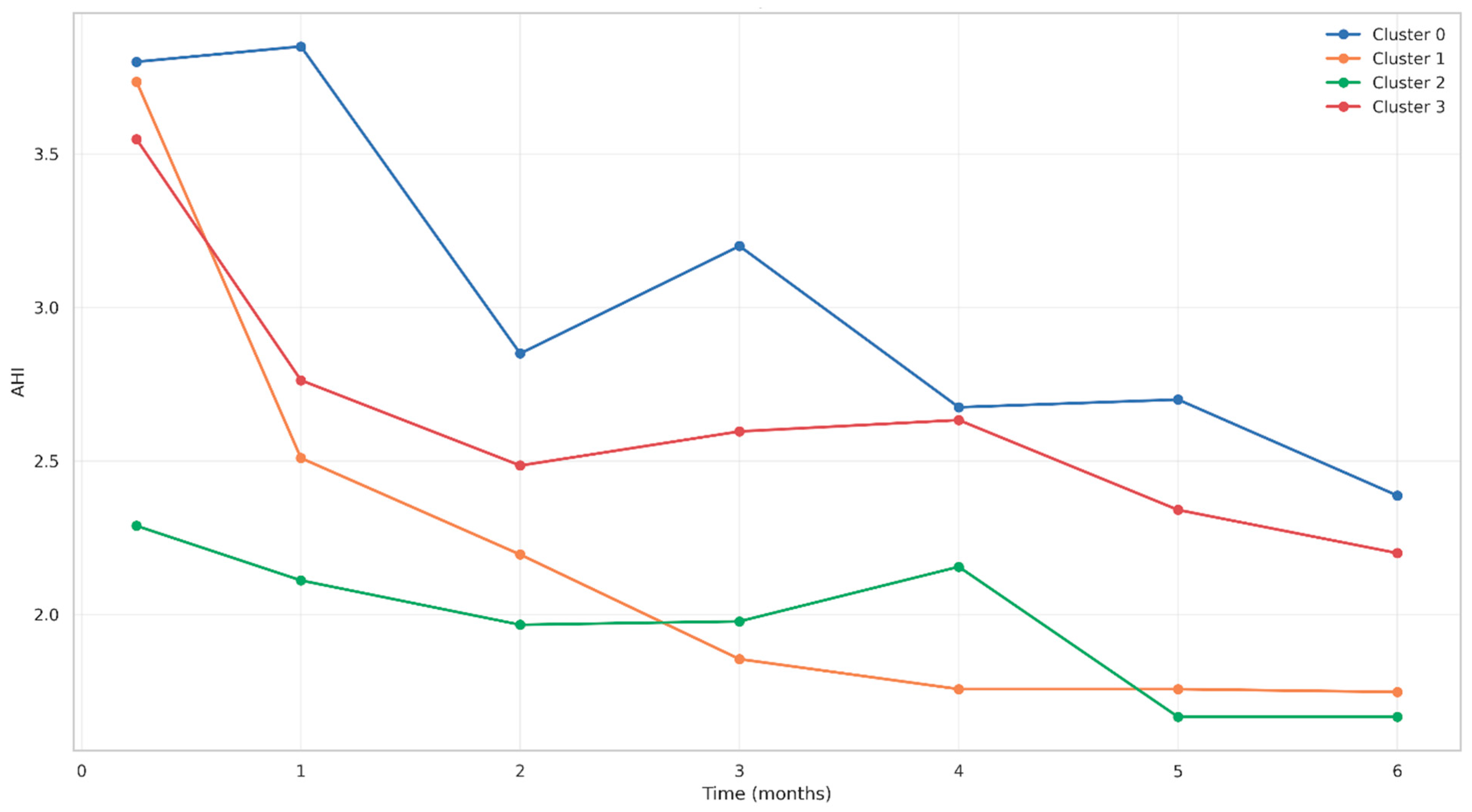
3.2. Treatment Efficacy: Respiratory Parameters
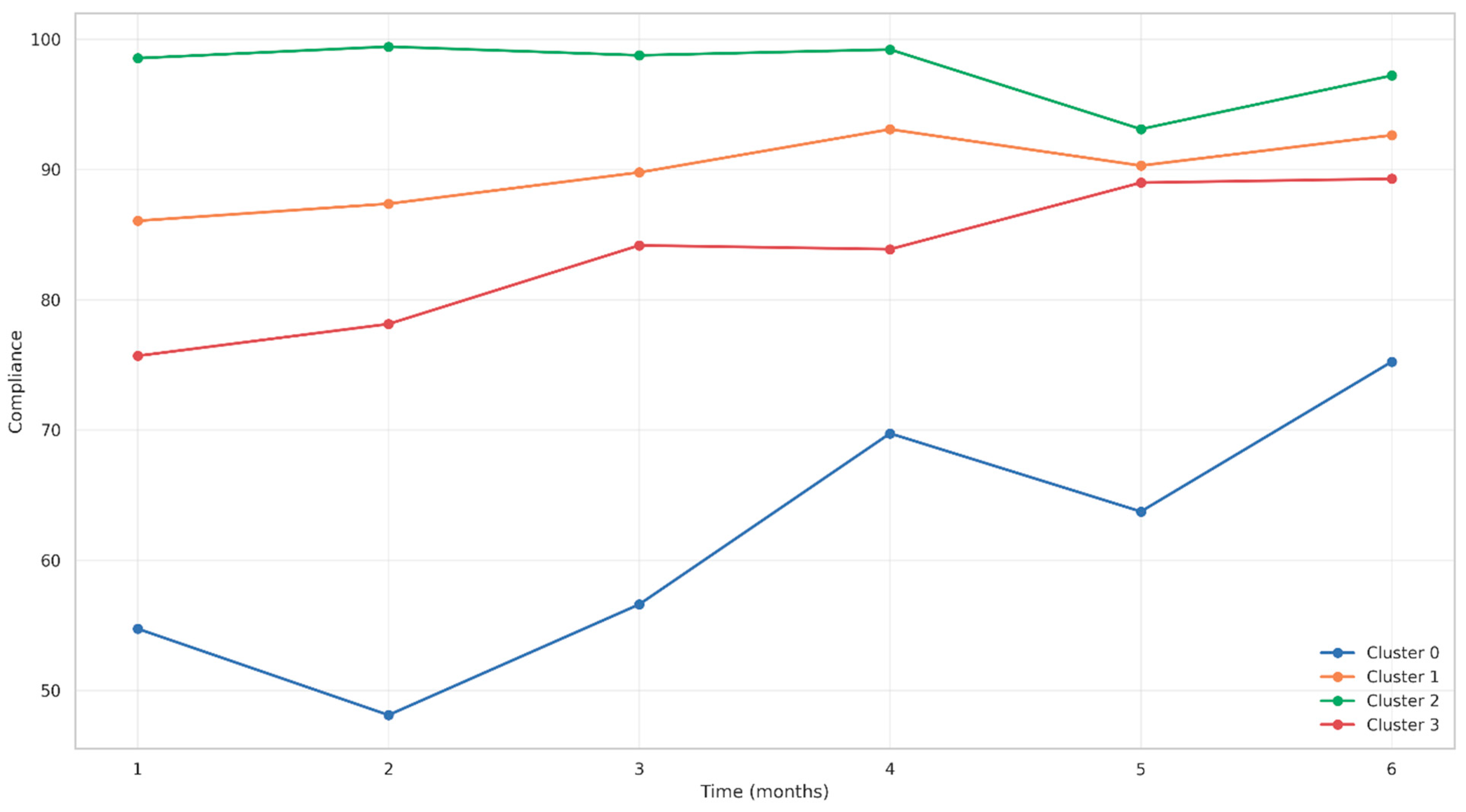
3.3. Compliance Rates
3.4. Daily Usage
Impact of Telemonitoring Support
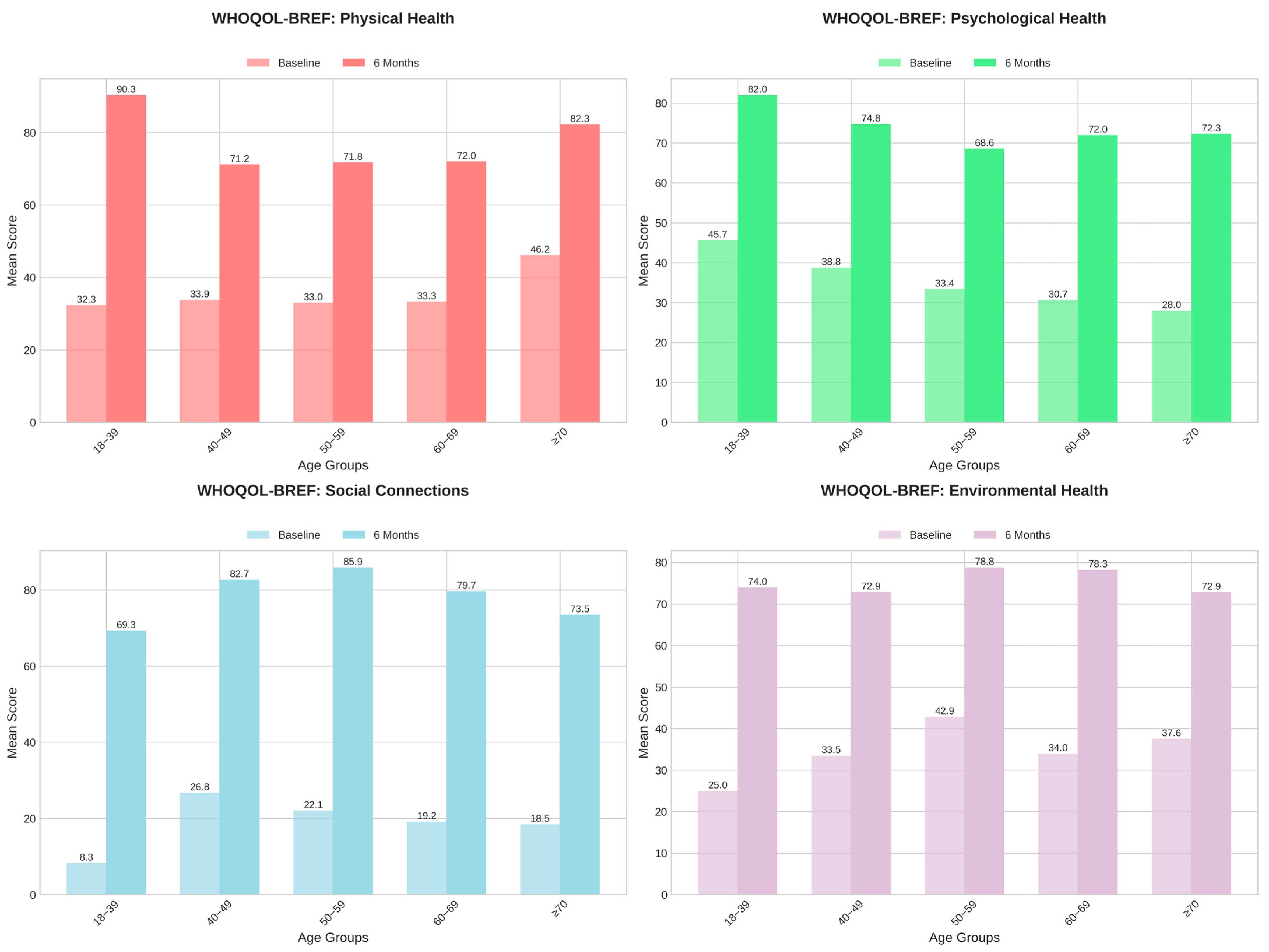
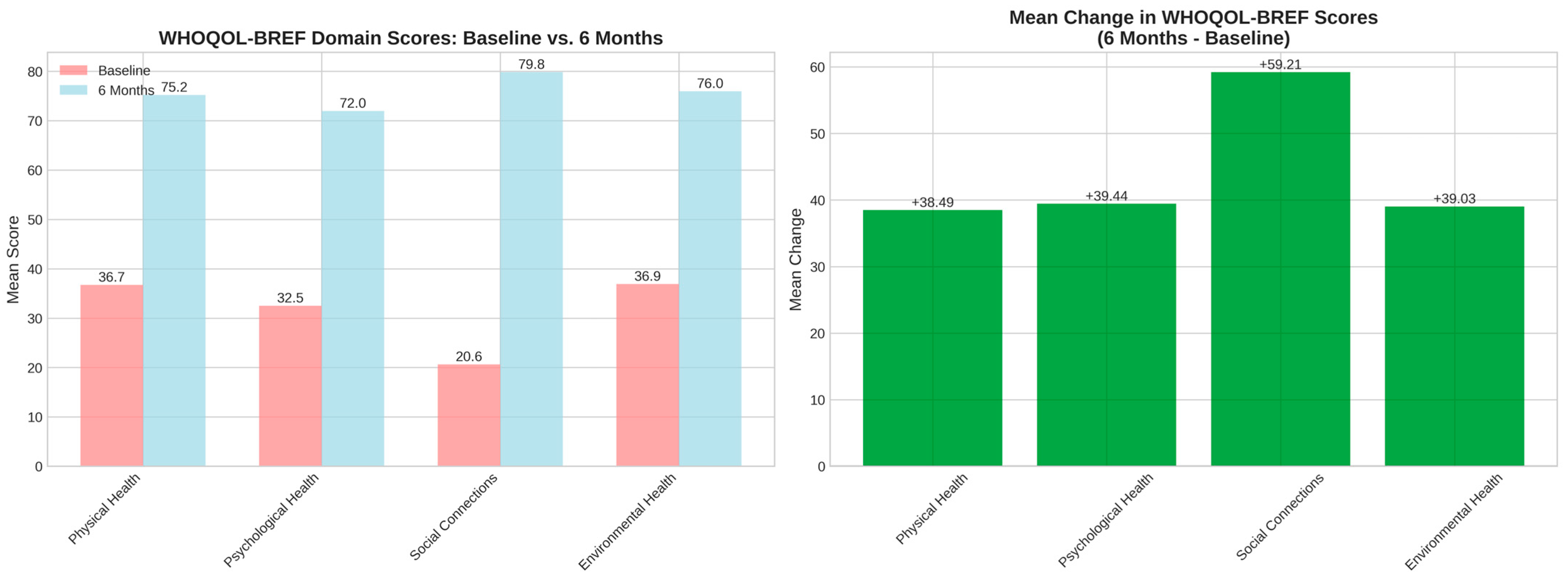
3.5. Self-Reported Outcome Measures: Quality of Life and Psychological Outcomes
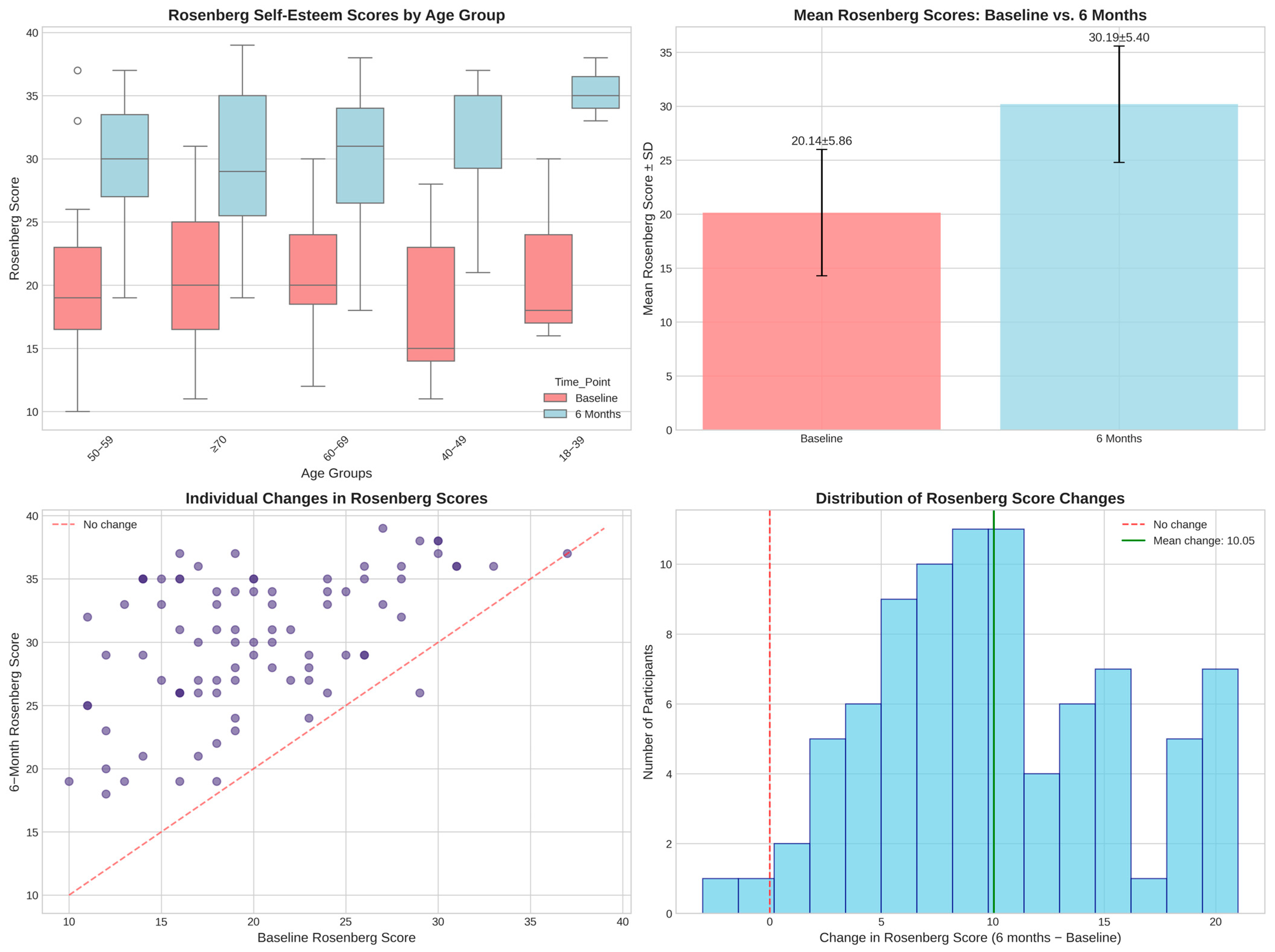
3.5.1. Self-Esteem Assessment and Validation Analysis
- Self-esteem changes vs. AHI reduction: r = 0.164 (p = 0.132).
- Self-esteem changes vs. CPAP compliance: r = −0.081 (p = 0.460).
- Self-esteem changes vs. daily usage minutes: r = −0.051 (p = 0.642).
- Self-esteem changes vs. WHOQOL improvement: r = 0.153 (p = 0.159).
- Low compliance (<70%, n = 2): 13.0 ± 7.1-point improvement.
- Medium compliance (70–89%, n = 30): 10.0 ± 5.5-point improvement.
- High compliance (≥90%, n = 54): 10.0 ± 5.7-point improvement.
3.5.2. Cluster-Based Validation
- Cluster 1 (best clinical outcomes: 95.9% AHI reduction): 9.8 ± 5.9-point SE improvement.
- Cluster 0 (94.1% AHI reduction): 9.1 ± 6.2-point SE improvement.
- Cluster 2 (94.8% AHI reduction): 8.9 ± 6.9-point SE improvement.
- Cluster 3 (worst clinical outcomes: 92.5% AHI reduction): 11.1 ± 4.5-point SE improvement.
3.5.3. Quality-of-Life Measurements
3.5.4. Methodological Implications
- -
- Hawthorne effects from intensive monitoring.
- -
- Social desirability bias in patient–provider interactions.
- -
- Response shift bias following treatment initiation.
- -
- Placebo responses to perceived “high-tech” interventions.
3.6. Patient Phenotyping and Clustering Analysis
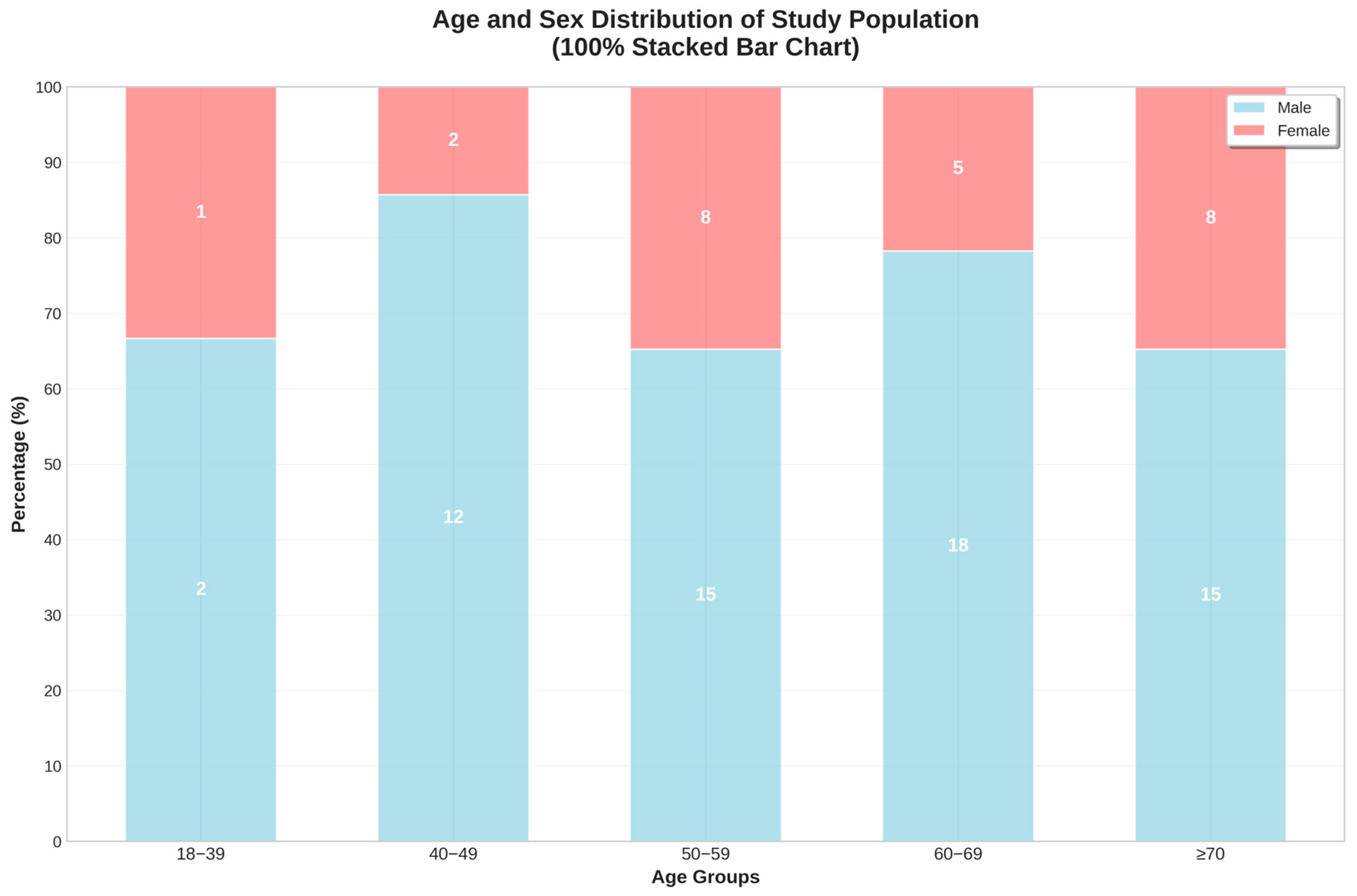
3.6.1. Demographic Patterns
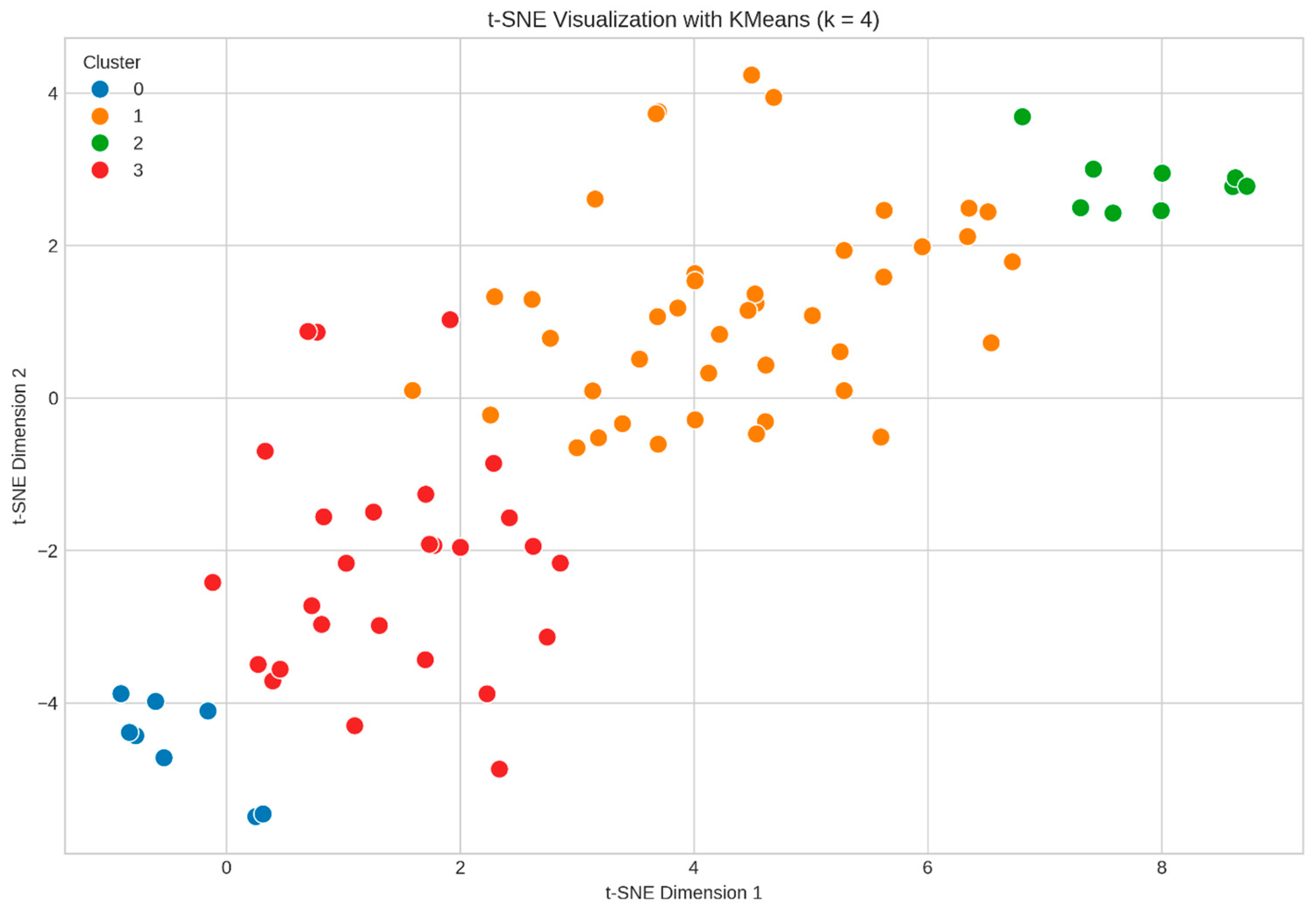
3.6.2. Machine Learning Clustering
- Cluster 0 (Poor Responders): 8 patients (9.30%)—the lowest CPAP adherence, the poorest quality-of-life outcomes, and the highest residual AHI.
- Cluster 1 (Good Responders): 42 patients (48.84%)—intermediate compliance with gradual improvement over time.
- Cluster 2 (Optimal Responders): 9 patients (10.47%)—the highest CPAP adherence, the lowest residual AHI, and consistently high WHOQOL scores.
- Cluster 3 (Moderate Responders): 27 patients (31.40%)—delayed but steady improvements in adherence and outcomes.
3.6.3. Longitudinal Adherence Patterns by Cluster
3.6.4. AHI Evolution by Cluster
3.6.5. CPAP Usage Patterns by Cluster
4. Discussion
4.1. Clinical Effectiveness of CPAP with Telemonitoring
4.2. Population Representativeness and Clinical Context
4.3. Adherence Patterns Through Structured Support
Human-Centered Care in Telemonitoring
4.4. Comprehensive Quality-of-Life Improvements
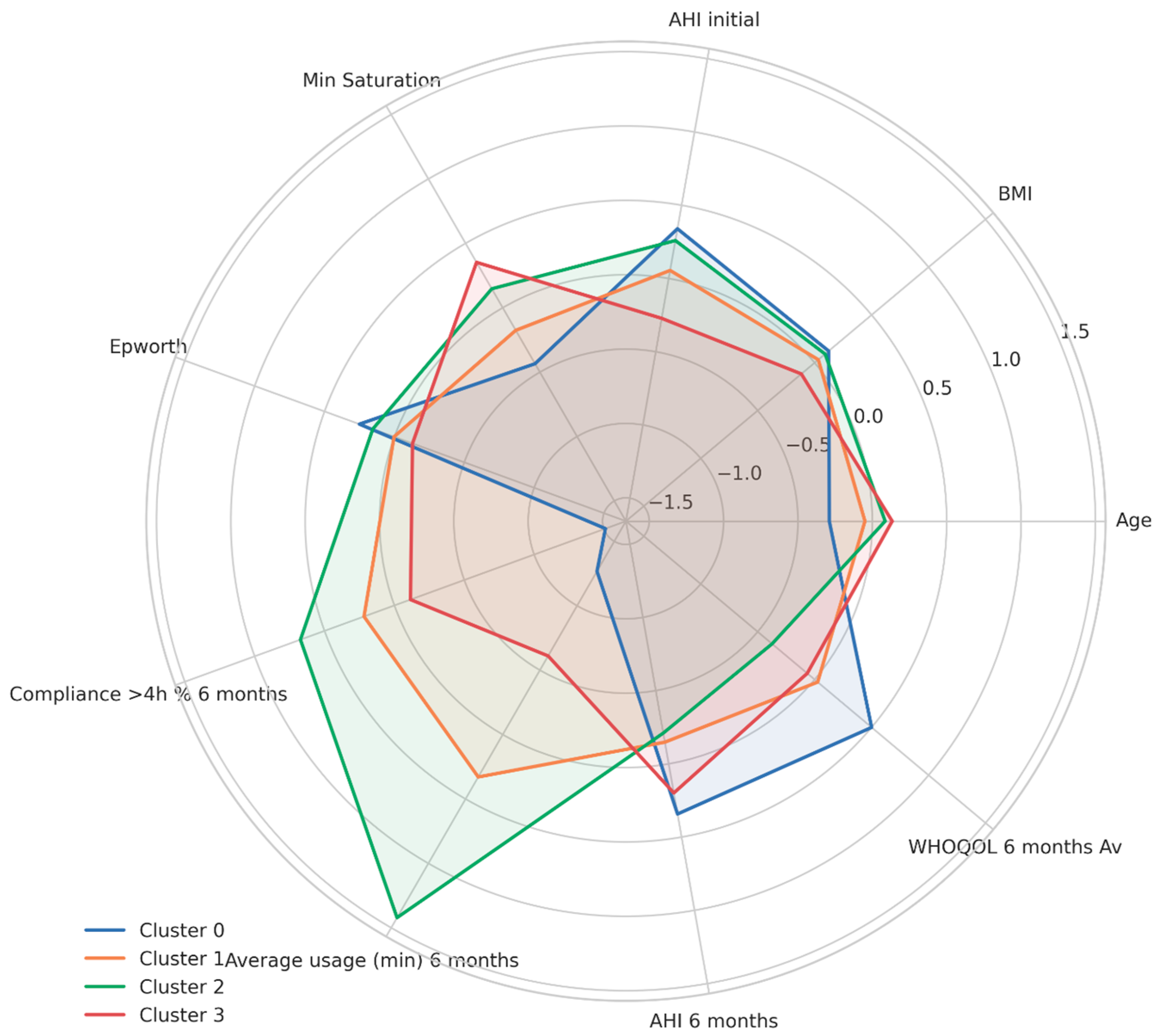
4.5. Clinical Phenotyping and Personalized Medicine
- Optimal responders (Cluster 2—10.47%) may require minimal intervention beyond standard care.
- Poor responders (Cluster 0—9.30%) need intensive, tailored interventions to improve outcomes.
- Intermediate responders (Clusters 1 and 3—80.24%) may benefit from targeted support strategies.
4.6. Subjective vs. Objective Outcomes
4.7. Critical Evaluation of Psychological Outcome Measures
4.8. Gender and Demographic Considerations
4.9. Study Limitations
4.10. Clinical Implications
- Early identification of non-adherent patients.
- Targeted interventions based on patient phenotypes.
- Comprehensive outcome assessment beyond traditional respiratory parameters.
4.11. Future Research Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sleep Disordered Breathing. Available online: https://qol.thoracic.org/sections/specific-diseases/sleep-disordered-breathing.html (accessed on 27 March 2024).
- Munteanu, I.; Mahler, B.; Parliteanu, O.A. Ghid privind managementul pacientului cu tulburari respiratorii in timpul somnului. Unpublished work. 2023. [Google Scholar] [CrossRef]
- Sateia, M.J. International Classification of Sleep Disorders—Third Edition. Chest 2014, 146, 1387–1394. [Google Scholar] [CrossRef]
- Leger, D.; Guilleminault, C.; Dreyfus, J.P.; Delahaye, C.; Paillard, M. Prevalence of insomnia in a survey of 12 778 adults in France. J. Sleep Res. 2000, 9, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Ahn, E.; Baek, Y.; Park, J.-E.; Lee, S.; Jin, H.-J. Elevated prevalence and treatment of sleep disorders from 2011 to 2020: A nationwide population-based retrospective cohort study in Korea. BMJ Open 2024, 14, e075809. [Google Scholar] [CrossRef] [PubMed]
- Yazdanirad, S.; Khoshakhlagh, A.H.; Al Sulaie, S.; Drake, C.L.; Wickwire, E.M. The effects of occupational noise on sleep: A systematic review. Sleep Med. Rev. 2023, 72, 101846. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.G.; Perlis, M.L.; Neale, L.F.; Espie, C.A.; Bastien, C.H. The natural history of insomnia: Focus on prevalence and incidence of acute insomnia. J. Psychiatr. Res. 2012, 46, 1278–1285. [Google Scholar] [CrossRef]
- Pallesen, S.; Sivertsen, B.; Nordhus, I.H.; Bjorvatn, B. A 10-year trend of insomnia prevalence in the adult Norwegian population. Sleep Med. 2014, 15, 173–179. [Google Scholar] [CrossRef]
- Schlack, R.; Hapke, U.; Maske, U.; Busch, M.; Cohrs, S. Häufigkeit und Verteilung von Schlafproblemen und Insomnie in der deutschen Erwachsenenbevölkerung: Ergebnisse der Studie zur Gesundheit Erwachsener in Deutschland (DEGS1). Bundesgesundheitsblatt—Gesundheitsforschung—Gesundheitsschutz 2013, 56, 740–748. [Google Scholar] [CrossRef]
- Fauroux, B.; Bonsignore, M.R.; Verbraecken, J. Remote consultations in sleep disorders. In Digital Respiratory Healthcare; Pinnock, H., Poberezhets, V., Drummond, D., Eds.; European Respiratory Society: Sheffield, UK, 2023; pp. 236–249. ISBN 978-1-84984-172-6. [Google Scholar]
- Swanson Kazley, A.; McLeod, A.C.; Wager, K.A. Telemedicine in an International Context: Definition, Use, and Future. In Advances in Health Care Management; Menachemi, N., Singh, S., Eds.; Emerald Group Publishing Limited: Leeds, UK, 2012; Volume 12, pp. 143–169. ISBN 978-1-78052-858-8. [Google Scholar]
- Pinnock, H.; Poberezhets, V.; Drummond, D.; Calverley, P.M.A. ERS Monograph—Digital Respiratory Healthcare; European Respiratory Society: Lausanne, Switzerland, 2023; ISBN 978-1-84984-172-6. [Google Scholar]
- Di Pumpo, M.; Nurchis, M.C.; Moffa, A.; Giorgi, L.; Sabatino, L.; Baptista, P.; Sommella, L.; Casale, M.; Damiani, G. Multiple-access versus telemedicine home-based sleep apnea testing for obstructive sleep apnea (OSA) diagnosis: A cost-minimization study. Sleep Breath. 2022, 26, 1641–1647. [Google Scholar] [CrossRef]
- Fields, B.G.; Behari, P.P.; McCloskey, S.; True, G.; Richardson, D.; Thomasson, A.; Korom-Djakovic, D.; Davies, K.; Kuna, S.T. Remote Ambulatory Management of Veterans with Obstructive Sleep Apnea. Sleep 2016, 39, 501–509. [Google Scholar] [CrossRef]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef]
- Verbraecken, J. Telemedicine in Sleep-Disordered Breathing. Sleep Med. Clin. 2021, 16, 417–445. [Google Scholar] [CrossRef]
- Pontier-Marchandise, S.; Texereau, J.; Prigent, A.; Gonzalez-Bermejo, J.; Rabec, C.; Gagnadoux, F.; Letierce, A.; Winck, J.C. Home NIV treatment quality in patients with chronic respiratory failure having participated to the French nationwide telemonitoring experimental program (The TELVENT study). Respir. Med. Res. 2023, 84, 101028. [Google Scholar] [CrossRef]
- Niu, Y.; Xi, H.; Zhu, R.; Guo, Y.; Wang, S.; Xiong, X.; Wang, S.; Guo, L. Effects of telemedicine-based follow-up management on adults with obstructive sleep apnea: A systematic review and meta-analysis. Int. J. Med. Inf. 2023, 176, 105108. [Google Scholar] [CrossRef]
- Schoch, O.D.; Baty, F.; Boesch, M.; Benz, G.; Niedermann, J.; Brutsche, M.H. Telemedicine for Continuous Positive Airway Pressure in Sleep Apnea. A Randomized, Controlled Study. Ann. Am. Thorac. Soc. 2019, 16, 1550–1557. [Google Scholar] [CrossRef]
- Chung, F.; Yegneswaran, B.; Liao, P.; Chung, S.A.; Vairavanathan, S.; Islam, S.; Khajehdehi, A.; Shapiro, C.M. STOP Questionnaire. Anesthesiology 2008, 108, 812–821. [Google Scholar] [CrossRef]
- Pivetta, B.; Chen, L.; Nagappa, M.; Saripella, A.; Waseem, R.; Englesakis, M.; Chung, F. Use and Performance of the STOP-Bang Questionnaire for Obstructive Sleep Apnea Screening Across Geographic Regions: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2021, 4, e211009. [Google Scholar] [CrossRef]
- Chung, F.; Subramanyam, R.; Liao, P.; Sasaki, E.; Shapiro, C.; Sun, Y. High STOP-Bang score indicates a high probability of obstructive sleep apnoea. Br. J. Anaesth. 2012, 108, 768–775. [Google Scholar] [CrossRef]
- Velescu, D.R.; Marc, M.S.; Traila, D.; Pescaru, C.C.; Hogea, P.; Suppini, N.; Crisan, A.F.; Wellmann, N.; Oancea, C. A Narrative Review of Self-Reported Scales to Evaluate Depression and Anxiety Symptoms in Adult Obstructive Sleep Apnea Patients. Medicina 2024, 60, 261. [Google Scholar] [CrossRef] [PubMed]
- Chung, F.; Abdullah, H.R.; Liao, P. STOP-Bang Questionnaire. Chest 2016, 149, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Johns, M.W. A New Method for Measuring Daytime Sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.T.; Malafaia, S.; Moutinho Dos Santos, J.; Roth, T.; Marques, D.R. Epworth sleepiness scale: A meta-analytic study on the internal consistency. Sleep Med. 2023, 109, 261–269. [Google Scholar] [CrossRef]
- Study protocol for the World Health Organization project to develop a Quality of Life assessment instrument (WHOQOL). Qual. Life Res. 1993, 2, 153–159. [CrossRef]
- Vahedi, S. World Health Organization Quality-of-Life Scale (WHOQOL-BREF): Analyses of Their Item Response Theory Properties Based on the Graded Responses Model. Iran. J. Psychiatry 2010, 5, 140–153. [Google Scholar] [PubMed]
- Whoqol Group. Development of the WHOQOL: Rationale and Current Status. Int. J. Ment. Health 1994, 23, 24–56. [Google Scholar] [CrossRef]
- Rosenberg, M. Rosenberg Self-Esteem Scale; APA: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Murase, K.; Tanizawa, K.; Minami, T.; Matsumoto, T.; Tachikawa, R.; Takahashi, N.; Tsuda, T.; Toyama, Y.; Ohi, M.; Akahoshi, T.; et al. A Randomized Controlled Trial of Telemedicine for Long-Term Sleep Apnea Continuous Positive Airway Pressure Management. Ann. Am. Thorac. Soc. 2020, 17, 329–337. [Google Scholar] [CrossRef]
- Cooper, J.R.; Acker, K.A.; Geyer, J.D.; Henderson, M.M.; Henderson-Mitchell, R.; Higginbotham, J.C. Optimizing Positive Airway Pressure Compliance and Outcomes in Rural Patients with Obstructive Sleep Apnea Through Telehealth. Int. J. Environ. Res. Public Health 2025, 22, 522. [Google Scholar] [CrossRef]
- Dallari, V.; Sacchetto, A.; Saetti, R.; Calabrese, L.; Vittadello, F.; Gazzini, L. Is artificial intelligence ready to replace specialist doctors entirely? ENT specialists vs ChatGPT: 1-0, ball at the center. Eur. Arch. Otorhinolaryngol. 2024, 281, 995–1023. [Google Scholar] [CrossRef]
- Patel, H.; Hussain, F. Do AI Chatbots Incite Harmful Behaviours in Mental Health Patients? BJPsych Open 2024, 10, S70–S71. [Google Scholar] [CrossRef]
- Dergaa, I.; Ben Saad, H.; Glenn, J.M.; Amamou, B.; Ben Aissa, M.; Guelmami, N.; Fekih-Romdhane, F.; Chamari, K. From tools to threats: A reflection on the impact of artificial-intelligence chatbots on cognitive health. Front. Psychol. 2024, 15, 1259845. [Google Scholar] [CrossRef]
- Grabb, D.; Lamparth, M.; Vasan, N. Risks from Language Models for Automated Mental Healthcare: Ethics and Structure for Implementation. arXiv 2024, arXiv:2406.11852. [Google Scholar] [CrossRef]
- Trecca, E.M.C.; Caponio, V.C.A.; Turri-Zanoni, M.; Di Lullo, A.M.; Gaffuri, M.; Lechien, J.R.; Maniaci, A.; Maruccio, G.; Reale, M.; Visconti, I.C.; et al. Comparative Analysis of Information Quality in Pediatric Otorhinolaryngology: Clinicians, Residents, and Large Language Models. Otolaryngol. Neck Surg. 2025, 173, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Chavouzis, N.; Fekete-Passa, K.; Pataka, A.; Kalamaras, G.; Mpagkalas, V.; Pitsiou, G.; Stanopoulos, I.; Kontakiotis, T.; Argyropoulou, P. Rosenberg self-esteem evaluation is correlated with insomnia, not with sleep apnea. In Proceedings of the 4.2 Sleep and Control of Breathing; European Respiratory Society: Lausanne, Switzerland, 2015; p. PA2350. [Google Scholar]
- Hnatiak, J.; Zikmund Galkova, L.; Winnige, P.; Batalik, L.; Dosbaba, F.; Ludka, O.; Krejci, J. Obstructive Sleep Apnea and a Comprehensive Remotely Supervised Rehabilitation Program: Protocol for a Randomized Controlled Trial. JMIR Res. Protoc. 2023, 12, e47460. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.M.; Kang, S.-G.; Cho, S.-J.; Lee, Y.J.; Lee, H.-J.; Kim, J.-E.; Shin, S.-H.; Park, K.H.; Kim, S.T. The quality of life of suspected obstructive sleep apnea patients is related to their subjective sleep quality rather than the apnea-hypopnea index. Sleep Breath. 2017, 21, 369–375. [Google Scholar] [CrossRef]
- Tasbakan, M.S.; Gunduz, C.; Pirildar, S.; Basoglu, O.K. Quality of life in obstructive sleep apnea is related to female gender and comorbid insomnia. Sleep Breath. 2018, 22, 1013–1020. [Google Scholar] [CrossRef]
- Kahal, H.; Tahrani, A.A.; Kyrou, I.; Dimitriadis, G.K.; Kimani, P.K.; Barber, T.M.; Nicholls, M.; Ali, A.; Weickert, M.O.; Randeva, H.S. The relationship between obstructive sleep apnoea and quality of life in women with polycystic ovary syndrome: A cross-sectional study. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820906689. [Google Scholar] [CrossRef] [PubMed]

| Variables | Mean ± SD | Min | Median | Max | n % |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years) | 60.5 ± 11.9 | 34 | 60 | 89 | |
| Sex—male (%) | 72.1% | - | - | - | |
| Sex—female (%) | 27.9% | - | - | - | |
| Body Mass Index (BMI, kg/m2) | 35.3 ± 8.0 | 22.0 | 33.5 | 68.0 | |
| BMI categories | |||||
| Normal weight (<25 kg/m2) | - | - | - | - | 1 (1.2) |
| Overweight (25–29.9 kg/m2) | - | - | - | - | 17 (19.8) |
| Obese Class I (30–34.9 kg/m2) | 27 (31.4) | ||||
| Obese Class II (35–39.9 kg/m2) | 19 (22.1) | ||||
| Obese Class III (≥40 kg/m2) | 22 (25.6) | ||||
| Variables | Mean ± SD | Min | Median | Max | n % |
|---|---|---|---|---|---|
| Moderate OSA (15–29.9 events/hour) | 23.1 ± 4.0 * | - | - | - | 30 (34.9) |
| Severe OSA (≥30 events/hour) | 52.1 ± 22.4 * | - | - | - | 56 (65.1) |
| Apnea–hypopnea index (AHI)—baseline | 42.0 ± 21.1 | 16.8 | 33.9 | 104.5 | - |
| AHI—after 7 days | 3.5 ± 4.0 | 0.2 | 2.3 | 22.5 | - |
| AHI—1 month | 2.7 ± 2.1 | 0.3 | 2.2 | 11.7 | - |
| AHI—2 months | 2.3 ± 1.6 | 0.3 | 2.0 | 9.3 | - |
| AHI—3 months | 2.2 ± 1.6 | 0.2 | 2.0 | 9.2 | - |
| AHI—4 months | 2.2 ± 1.5 | 0.3 | 1.8 | 7.7 | - |
| AHI—5 months | 2.0 ± 1.4 | 0.2 | 1.6 | 6.4 | - |
| AHI—6 months | 1.9 ± 1.3 | 0.1 | 1.6 | 5.7 | - |
| Desaturation index | 41.3 ± 22.0 | 9.9 | 36.3 | 104.1 | - |
| Minimum O2 saturation (%) | 70.8 ± 12.0 | 38 | - | 87 | - |
| Average O2 saturation (%) | 91.1 ± 4.0 | - | - | - | - |
| Variables | Mean ± SD | Min | Median | Max |
|---|---|---|---|---|
| Compliance after 7 days (%) | 75.5 ± 23.9 | 14.0 | 81.0 | 100.0 |
| Compliance >4 h/night—1 month (%) | 81.2 ± 17.4 | 6.0 | 84.0 | 100.0 |
| Compliance >4 h/night—2 months (%) | 82.1 ± 18.3 | 16.0 | 87.0 | 100.0 |
| Compliance >4 h/night—3 months (%) | 85.9 ± 13.8 | 37.0 | 90.0 | 100.0 |
| Compliance >4 h/night—4 months (%) | 88.7 ± 12.8 | 35.0 | 90.5 | 100.0 |
| Compliance >4 h/night—5 months (%) | 87.7 ± 13.1 | 37.0 | 91.0 | 100.0 |
| Compliance >4 h/night—6 months (%) | 90.5 ± 10.1 | 42.0 | 91.5 | 100.0 |
| Variables | Mean ± SD | Min | Median | Max |
|---|---|---|---|---|
| Average usage—1 month (min/day) | 348.4 ± 85.8 | 60 | 348.0 | 538.0 |
| Average usage—2 months (min/day) | 349.9 ± 87.2 | 72 | 367.0 | 560.0 |
| Average usage—3 months (min/day) | 362.7 ± 73.7 | 193 | 365.5 | 581.0 |
| Average usage—4 months (min/day) | 371.5 ± 72.8 | 184 | 379.0 | 560.0 |
| Average usage—5 months (min/day) | 369.6 ± 73.2 | 192 | 376.0 | 562.0 |
| Average usage—6 months (min/day) | 384.2 ± 65.2 | 210 | 374.5 | 568.0 |
| Variables | Mean ± SD | Min | Median | Max | n % |
|---|---|---|---|---|---|
| Psychological Assessments | |||||
| Rosenberg Self-Esteem Scale—baseline | 20.1 ± 5.9 | 10.0 | 19.0 | 37.0 | - |
| Rosenberg Self-Esteem Scale—6 months | 30.2 ± 5.4 | 18.0 | 31.0 | 39.0 | - |
| Low self-esteem (<15) | - | - | - | - | 15 (17.4) |
| Low-normal self-esteem (15–25) | - | - | - | - | 53 (61.6) |
| Normal self-esteem (>25) | - | - | - | - | 18 (20.9) |
| STOP-BANG score | 5.0 ± 1.5 | 3.0 | 5.0 | 8.0 | - |
| Epworth Sleepiness Scale | 17.4 ± 3.9 | 8.0 | 18.0 | 24.0 | - |
| WHOQOL-BREF Domains | |||||
| Physical health—baseline | 36.7 ± 25.4 | 0 | 32.0 | 82.0 | - |
| Psychological health—baseline | 32.5 ± 27.0 | 0 | 25.0 | 79.0 | - |
| Social relationships—baseline | 20.6 ± 19.1 | 0 | 17.0 | 58.0 | - |
| Environmental health—baseline | 36.9 ± 25.4 | 0 | 36.0 | 84.0 | - |
| Physical health—6 months | 75.2 ± 21.2 | 29 | 82.0 | 100 | - |
| Psychological health—6 months | 71.9 ± 24.1 | 12 | 75.0 | 100 | - |
| Social relationships—6 months | 79.8 ± 16.5 | 33 | 83.0 | 100 | - |
| Environmental health—6 months | 75.9 ± 21.9 | 16 | 82.5 | 100 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wellmann, N.; Ancusa, V.M.; Marc, M.S.; Trusculescu, A.A.; Pescaru, C.C.; Martis, F.G.; Ciortea, I.; Crisan, A.F.; Maritescu, A.; Balica, M.A.; et al. Telemedicine-Supported CPAP Therapy in Patients with Obstructive Sleep Apnea: Association with Treatment Adherence and Clinical Outcomes. J. Clin. Med. 2025, 14, 5339. https://doi.org/10.3390/jcm14155339
Wellmann N, Ancusa VM, Marc MS, Trusculescu AA, Pescaru CC, Martis FG, Ciortea I, Crisan AF, Maritescu A, Balica MA, et al. Telemedicine-Supported CPAP Therapy in Patients with Obstructive Sleep Apnea: Association with Treatment Adherence and Clinical Outcomes. Journal of Clinical Medicine. 2025; 14(15):5339. https://doi.org/10.3390/jcm14155339
Chicago/Turabian StyleWellmann, Norbert, Versavia Maria Ancusa, Monica Steluta Marc, Ana Adriana Trusculescu, Camelia Corina Pescaru, Flavia Gabriela Martis, Ioana Ciortea, Alexandru Florian Crisan, Adelina Maritescu, Madalina Alexandra Balica, and et al. 2025. "Telemedicine-Supported CPAP Therapy in Patients with Obstructive Sleep Apnea: Association with Treatment Adherence and Clinical Outcomes" Journal of Clinical Medicine 14, no. 15: 5339. https://doi.org/10.3390/jcm14155339
APA StyleWellmann, N., Ancusa, V. M., Marc, M. S., Trusculescu, A. A., Pescaru, C. C., Martis, F. G., Ciortea, I., Crisan, A. F., Maritescu, A., Balica, M. A., & Fira-Mladinescu, O. (2025). Telemedicine-Supported CPAP Therapy in Patients with Obstructive Sleep Apnea: Association with Treatment Adherence and Clinical Outcomes. Journal of Clinical Medicine, 14(15), 5339. https://doi.org/10.3390/jcm14155339









