Patient-Reported Outcomes in Intraoral Bone Block Augmentation Compared to GBR Procedures Prior to Implant Placement: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Objectives
| Patient | Systemically healthy adult patient with alveolar ridge deficiencies; |
| Intervention | Bone block augmentation using bone from the retromolar area (ABB); |
| Comparison | Guided bone regeneration (GBR); |
| Outcome | PROMs such as pain, edema, hematoma, neurosensory disturbance, willingness to repeat, OHIP-14. |
2.2. Eligibility Criteria
- Human clinical studies published in English or articles when a direct translation was possible;
- Intraoral bone grafts;
- At least 18-years old patients;
- Systemically healthy patients;
- Patients with alveolar ridge deficiency;
- Studies reporting on bone augmentation;
- Data on at least one outcome variable of interest.
- Extraoral bone grafts;
- Sinus floor elevation procedures;
- Bone ring techniques;
- Distraction osteogenesis;
- Alveolar ridge splitting;
- Full edentulous patients;
- Patients taking anti-resorptive drugs;
- Patients who underwent radiation therapy;
- Patients with pathologies affecting bone metabolism (i.e., osteoporosis, osteopenia, rheumatoid arthritis);
- Containing insufficient information on the surgical protocol and bone augmentation procedures.
2.3. Information Sources and Search Strategy
2.4. Data Extraction
2.5. Risk of Bias Quality Assessment
2.6. Data Analysis
3. Results
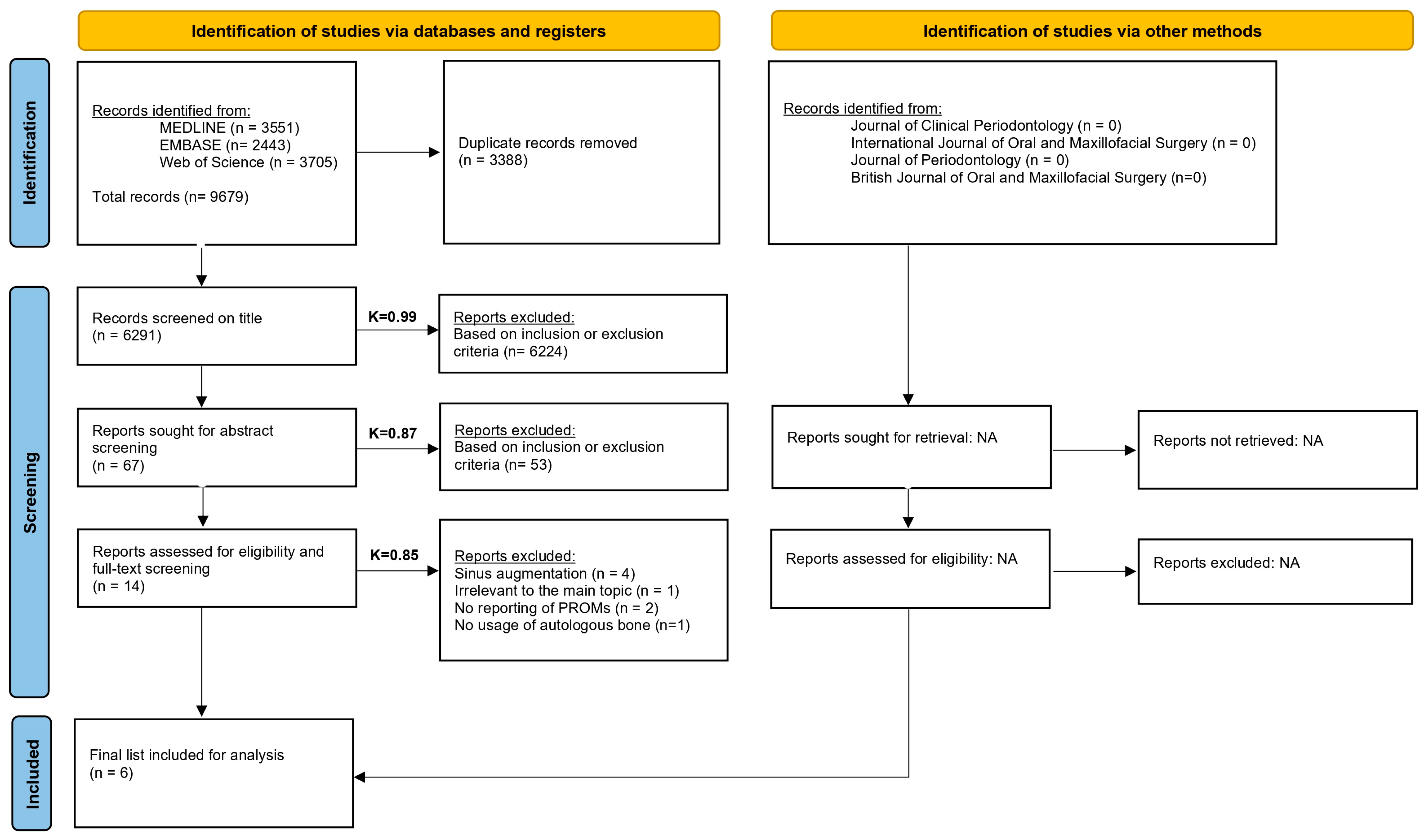
3.1. Search
3.2. Description of the Selected Studies
3.3. Risk of Bias Assessment
| Author | Study Design | Follow-Up Points | No. of Patients | No. of Dropouts | No. of Bone Grafts | No. of Implants | Mean Age (y) | Type of Graft | Augmentation Site | Test Group | Control Group | Outcome Variables |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bayram et al., 2024 [32] | rCT | Week 1 1 year | 70 | 0 | 70 | 70 | 44.96 | Autograft | Mandible or Maxilla | ABB | NR | Neurosensory disturbances |
| Shiezadeh et al., 2023 [36] | RCT | 26 Weeks | 25 | 2 | 13 | 37 | 36.27–36.5 | Autograft Vs. Xenograft | Mandible or Maxilla | ABB (Collagen Membrane) | GBR (Collagen membrane) | Neurosensory disturbances in donor sites (ABB only) and recipient sites (ABB and GBR) swelling |
| Thoma et al., 2019 [37] | rCC | Mean follow-up of 10 years | 38 | 0 | 19 | 67 | 51.9–71.1 | Autograft | Mandible or Maxilla | ABB with Bio-Oss© and Bio-Guide© | NB | Sensory problems Willingness to repeat the procedure Likelihood to recommend the procedure |
| Lorenz et al., 2025 [34] | PCT | Pretreatment Weeks 1, 3, 12, and 24 Time of implant placement Definitive prosthesis 6 months 8 months 1 year | 46 | 8 | 119 | 91 | 50 | Autograft and Xenograft | Mandible | GBR | NR | Patient-reported pain perceptions using VAS and OHIP-14 |
| Sakkas et al., 2016 [35] | rCS | NR | 86 | 0 | 104 | 155 | 37.9 | Autograft | Mandible or Maxilla | ABB with Bio-Guide© | NR | Neurosensory disturbance |
| Korsch et al., 2014 [33] | RCT | Day 1, 3, 7, 14, and 28 | 30 | 0 | 30 | NR | 47.9–52.1 | Autograft and Xenograft | Maxilla | ABB | GBR | Pain and swelling in the donor and recipient sites, postoperative analgesic usage and patient-reported predominant symptom |
| D1 | D2 | D3 | D4 | D5 | Overall | |
|---|---|---|---|---|---|---|
| Shiezadeh et al., 2014 [36] | ☑ | ☒ | ☑ | ☑ | ☐ | ☒ |
| Korsch et al., 2014 [33] | ☐ | ☑ | ☑ | ☑ | ☑ | ☐ |
| Author | Selection | Comparability | Outcome | Total | Overall Quality | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Representatives of the exposed cohort/case (Maximum: ✸) | Selection of the non-exposed cohort/case (Maximum: ✸) | Ascertainment of exposure (Maximum: ✸) | Demonstration that outcome of interest was not present at the start of the study (Maximum: ✸) | Compare ability of cohorts on the basis of the design or analysis (Maximum: ✸✸) | Assessment of outcome (Maximum: ✸) | Was follow-up long enough for outcomes to occur (Maximum: ✸) | Adequacy of follow-up cohorts (Maximum: ✸) | |||
| Lorenz et al., 2024 [34] | ✸ | ✸ | ✸ | ✸ | ✸ | ✸ | ✸ | 7 | Good | |
| Bayram et al., 2024 [32] | ✸ | ✸ | ✸ | ✸✸ | ✸ | ✸ | ✸ | 8 | Good | |
| Sakkas et al., 2016 [35] | ✸ | ✸ | ✸ | ✸ | ✸ | 5 | Poor | |||
| Author | Selection | Comparability | Outcome | Total | Overall Quality | |||||
| Is the case definition adequate? (Maximum: ✸) | Representativeness of the cases? (Maximum: ✸) | Selection of controls (Maximum: ✸) | Definition of controls (Maximum: ✸) | Comparability of cases and controls on the basis of the design or analysis (Maximum: ✸✸) | Ascertainment of exposure (Maximum: ✸) | Same method of ascertainment for cases and controls (Maximum: ✸) | Non-response rate (Maximum: ✸) | |||
| Thoma et al., 2024 [37] | ✸ | ✸ | ✸ | ✸ | ✸ | ✸ | ✸ | ✸ | 8 | Good |
3.4. Outcomes
3.4.1. Pain
3.4.2. Swelling
3.4.3. Neurosensory Disturbance
| Author | Treatments | Pain | Swelling | Neurosensory Disturbance | Patient-Reported Predominant Symptom (Pain/Swelling) | Postoperative Analgesic Usage | OHIP-14 (Quality of Life) | Willingness to Repeat | Likelihood to Recommend | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D | R | NS | D | R | D | R | NS | Ds | Rs | |||||||
| Bayram et al., 2024 [32] | ABB | NR | NR | NR | NR | NR | NR | W1–Y1: Decrease | NR | NR | NR | NR | NR | NR | ||
| Shiezadeh et al., 2023 [36] | ABB | GBR | NR | NR | NR | NR | Swelling until 4 weeks FU | 0/11 | 0/11 | NR | NR | NR | NR | NR | NR | NR |
| Thoma et al., 2019 [37] | ABB | NB | NR | NR | NR | NR | NR | EO: 7/19 IO: 3/19 | 0/19 | NR | NR | NR | NR | NR | Median 10 (AB range 5.5–10) (NB range 7–10) | Median 10 |
| Lorenz et al., 2025 [34] | GBR | NR | NR | W1–W24: Decrease W24–M8: Stable | NR | NR | NR | NR | NR | NR | NR | NR | Pre-W1: Increase W1–Y1: Decrease | NR | NR | |
| Sakkas et al., 2016 [35] | ABB | NR | NR | NR | NR | NR | NR | NR | 11/104 (10.4%) | NR | NR | NR | NR | NR | NR | |
| Korsch et al., 2014 [33] | ABB | D1–D28: Decrease | D1–D3: Increase D3–D28: Decrease | NR | D1–D28: Decrease | D1–D3: Increase D3–D28: Decrease | NR | NR | NR | D1–D7: More swelling than pain | D1–D7: More swelling than pain | D1–D11: Decrease After D11: usage = 0 | NR | NR | NR | |
| GBR | D1–D3: Stable D3–D28: Decrease | D1–D3: Stable D3–D28: Decrease | NR | D1–D3: Increase D3–D28: Decrease | D1–D3: Increase D3–D28: Decrease | NR | NR | NR | D3: More swelling than pain | D1–D7: More swelling than pain | D1–D2: Increase D2–D11: Decrease After D11: usage = 0 | NR | NR | NR | ||
3.4.4. Patient-Reported Predominant Symptom
3.4.5. Postoperative Analgesic Usage
3.4.6. OHIP-14
3.4.7. Willingness to Repeat and Likelihood to Recommend
4. Discussion
4.1. Neurosensory Disturbance
4.2. OHIP-14
4.3. Pain and Swelling
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABB | Autologous Bone Block |
| GBR | Guided Bone Regeneration |
| PROMs | Patient-Reported Outcome Measures |
| VAS | Visual Analog Scale |
| OHIP-14 | Oral Health Impact Profile-14 |
| RCT | Randomized Controlled Trial |
| CCT | Controlled Clinical Trial |
| PCS | Prospective Case Series |
| PCT | Prospective Cohort Study |
| rCS | Retrospective Case Series |
| rCT | Retrospective Cohort Study |
| rCC | Retrospective Case–Control |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-analyses |
| PROSPERO | International Prospective Register of Systematic Reviews |
| RoB2 | Revised Cochrane Risk-of-Bias Tool for Randomized Trials |
| NOS | Newcastle Ottawa Scale |
Appendix A
| Population | Systemically healthy adult patients with alveolar ridge deficiencies. |
| Intervention | Intraoral Autologous Bone Block Augmentation (ABB). |
| Comparison | Guided Bone Regeneration (GBR). |
| Outcome | Patient-Reported Outcomes Measures (PROMs) allowing for a direct comparison of PROMs during Intraoral Bone Block Augmentation procedures and Guided Bone Regeneration. |
| Study Design | Randomized Controlled Trials, Controlled Clinical Trials (CCT), Prospective Case Series (PCS), Prospective Cohort Studies (PCT), Retrospective Case Series (rCS), Retrospective Cohort Studies (rCT), Retrospective Case–Control (rCC). |
| Languages | English, Dutch, French, German |
| MEDLINE | Embase | Web of Science | ||||
|---|---|---|---|---|---|---|
| 5 | #1 AND #2 AND 3 Filters: from 2007–2024 | 3551 | #1 AND #2 AND 3 AND [2007–2024]/py | 2423 | #1 AND #2 AND #3 and 2024 or 2007 or 2008 or 2009 or 2010 or 2011 or 2023 or 2022 or 2021 or 2020 or 2019 or 2018 or 2016 or 2017 or 2015 or 2014 or 2013 or 2012 (Publication Years) | 3705 |
| 4 | #1 AND #2 AND #3 | 4555 | #1 AND #2 AND 3 | 2875 | #1 AND #2 AND #3 | 4121 |
| 3 | ((((((((((((postoperative complications[MeSH Major Topic]) OR (intraoperative complications [MeSH Major Topic])) OR (complication)) OR (patient reported outcome)) OR (success)) OR (patient related outcome)) OR (morbidity)) OR (pain)) OR (swelling)) OR (fistula)) OR (hematoma)) OR (sensory)) OR (edema) | 12,934,256 | ‘postoperative complication’/exp OR ‘postoperative complication’ OR ‘peroperative complication’/exp OR ‘peroperative complication’ OR ‘complication’/exp OR ‘complication’ OR ‘patient-reported outcome’/exp OR ‘patient-reported outcome’ OR ‘patient reported outcome measure’/exp OR ‘patient reported outcome measure’ OR ‘success’/exp OR ‘success’ OR ‘patient related outcome’ OR ((‘patient’/exp OR patient) AND related AND (‘outcome’/exp OR outcome)) OR ‘morbidity’/exp OR ‘morbidity’ OR ‘pain’/exp OR ‘pain’ OR ‘pain assessment’/exp OR ‘pain assessment’ OR ‘swelling’/exp OR ‘swelling’ OR ‘fistula’/exp OR ‘fistula’ OR ‘hematoma’/exp OR ‘hematoma’ OR ‘sensory dysfunction’/exp OR ‘sensory dysfunction’ OR ‘sensory’/exp OR ‘sensory’ OR ‘edema’/exp OR ‘edema’ | 8,422,227 | ((((((((((((ALL = (postoperative complications)) OR ALL = (intraoperative complications)) OR ALL = (complication)) OR ALL = (patient reported outcome)) OR ALL = (success)) OR ALL = (patient related outcome)) OR ALL = (morbidity)) OR ALL= (pain)) OR ALL = (swelling)) OR ALL = (fistula)) OR ALL = (hematoma)) OR ALL = (sensory)) OR ALL = (edema) | 4,264,864 |
| 2 | (((((((((((Sausage) OR (bone graft)) OR (bone block)) OR (onlay)) OR (alveolar ridge augmentation[MeSH Major Topic])) OR (shell technique)) OR (Khoury)) OR (Split bone block)) OR (bone plate)) OR (guided bone regeneration)) OR (GBR)) OR (Lateral bone augmentation) | 15,091 | ‘sausage’/exp OR sausage OR ‘bone graft’/exp OR ‘bone graft’ OR ‘bone transplantation’/exp OR ‘bone transplantation’ OR ‘bone block’/exp OR ‘bone block’ OR ‘onlay graft’/exp OR ‘onlay graft’ OR ‘onlay technique’/exp OR ‘onlay technique’ OR ‘alveolar ridge augmentation’/exp OR ‘alveolar ridge augmentation’ OR ‘shell technique’ OR ((‘shell’/exp OR shell) AND (‘technique’/exp OR technique)) OR khoury OR ‘split bone block’ OR (split AND (‘bone’/exp OR bone) AND block) OR ‘bone plate’/exp OR ‘bone plate’ OR ‘guided bone regeneration’/exp OR ‘guided bone regeneration’ OR gbr OR ‘lateral bone augmentation’ OR (lateral AND (‘bone’/exp OR bone) AND (‘augmentation’/exp OR augmentation)) | 278,771 | (((((((((((ALL = (sausage)) OR ALL = (bone graft)) OR ALL = (bone block)) OR ALL= (onlay)) OR ALL = (alveolar ridge Augmentation)) OR ALL = (shell technique)) OR ALL= (khoury)) OR ALL = (split bone block)) OR ALL = (bone plate)) OR ALL = (guided bone regeneration)) OR ALL = (GBR)) OR ALL = (Lateral bone augmentation) | 248,670 |
| 1 | Dental Implants | 52,994 | ‘tooth implantation’/exp OR ‘tooth implantation’ OR ‘tooth implant’/exp OR ‘tooth implant’ | 48,286 | ALL = (dental implants) | 65,802 |
References
- Tan, W.L.; Wong, T.L.; Wong, M.C.; Lang, N.P. A systematic review of post-extractional alveolar hard and soft tissue dimensional changes in humans. Clin. Oral Implant. Res. 2012, 23 (Suppl. S5), 1–21. [Google Scholar] [CrossRef]
- Rozé, J.; Babu, S.; Saffarzadeh, A.; Gayet-Delacroix, M.; Hoornaert, A.; Layrolle, P. Correlating implant stability to bone structure. Clin. Oral Implant. Res. 2009, 20, 1140–1145. [Google Scholar] [CrossRef]
- Sanz, M.; Vignoletti, F. Key aspects on the use of bone substitutes for bone regeneration of edentulous ridges. Dent. Mater. 2015, 31, 640–647. [Google Scholar] [CrossRef]
- Jensen, S.S.; Aghaloo, T.; Jung, R.E.; Bertl, K.; Buser, D.; Chappuis, V.; de Stavola, L.; Monje, A.; Pispero, A.; Roccuzzo, A.; et al. Group 1 ITI Consensus Report: The role of bone dimensions and soft tissue augmentation procedures on the stability of clinical, radiographic, and patient-reported outcomes of implant treatment. Clin. Oral Implant. Res. 2023, 34, 43–49. [Google Scholar] [CrossRef]
- Couso-Queiruga, E.; Stuhr, S.; Tattan, M.; Chambrone, L.; Avila-Ortiz, G. Post-extraction dimensional changes: A systematic review and meta-analysis. J. Clin. Periodontol. 2021, 48, 127–145. [Google Scholar] [CrossRef] [PubMed]
- Urban, I.A.; Montero, E.; Monje, A.; Sanz-Sánchez, I. Effectiveness of vertical ridge augmentation interventions: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 319–339. [Google Scholar] [CrossRef]
- Barausse, C.; Tayeb, S.; Pellegrino, G.; Bonifazi, L.; Mancuso, E.; Ratti, S.; Galvani, A.; Pistilli, R.; Felice, P. The Inlay Technique in Alveolar Ridge Augmentation: A Systematic Review. J. Clin. Med. 2025, 14, 1684. [Google Scholar] [CrossRef] [PubMed]
- Gultekin, B.A.; Bedeloglu, E.; Kose, T.E.; Mijiritsky, E. Comparison of Bone Resorption Rates after Intraoral Block Bone and Guided Bone Regeneration Augmentation for the Reconstruction of Horizontally Deficient Maxillary Alveolar Ridges. Biomed. Res. Int. 2016, 2016, 4987437. [Google Scholar] [CrossRef]
- Aloy-Prósper, A.; Carramolino-Cuéllar, E.; Peñarrocha-Oltra, D.; Soto-Peñaloza, D.; Peñarrocha-Diago, M. Intraoral onlay block bone grafts versus cortical tenting technique on alveolar ridge augmentations: A systematic review. Med. Oral Patol. Oral Cir. Bucal 2022, 27, e181–e190. [Google Scholar] [CrossRef]
- Chavda, S.; Levin, L. Human Studies of Vertical and Horizontal Alveolar Ridge Augmentation Comparing Different Types of Bone Graft Materials: A Systematic Review. J. Oral Implantol. 2018, 44, 74–84. [Google Scholar] [CrossRef]
- de Sousa, C.A.; Lemos, C.A.A.; Santiago-Júnior, J.F.; Faverani, L.P.; Pellizzer, E.P. Bone augmentation using autogenous bone versus biomaterial in the posterior region of atrophic mandibles: A systematic review and meta-analysis. J. Dent. 2018, 76, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Reininger, D.; Cobo-Vázquez, C.; Rosenberg, B.; López-Quiles, J. Alternative intraoral donor sites to the chin and mandibular body-ramus. J. Clin. Exp. Dent. 2017, 9, e1474–e1481. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Moreno, P.; Ávila, G.; Fernández-Barbero, J.E.; Aguilar, M.; Sánchez-Fernández, E.; Cutando, A.; Wang, H.-L. Evaluation of sinus floor elevation using a composite bone graft mixture. Clin. Oral Implant. Res. 2007, 18, 376–382. [Google Scholar] [CrossRef]
- Heimes, D.; Pabst, A.; Becker, P.; Hartmann, A.; Kloss, F.; Tunkel, J.; Smeets, R.; Kämmerer, P.W. Comparison of morbidity-related parameters between autologous and allogeneic bone grafts for alveolar ridge augmentation from patients’ perspective—A questionnaire-based cohort study. Clin. Implant. Dent. Relat. Res. 2024, 26, 170–182. [Google Scholar] [CrossRef]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef]
- Lim, G.; Lin, G.H.; Monje, A.; Chan, H.L.; Wang, H.L. Wound Healing Complications Following Guided Bone Regeneration for Ridge Augmentation: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implant. 2018, 33, 41–50. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, J.; Pickert, F.N.; Sánchez-Labrador, L.; Gf Tresguerres, F.; Martínez-González, J.M.; Meniz-García, C. Horizontal Ridge Augmentation: A Comparison between Khoury and Urban Technique. Biology 2021, 10, 749. [Google Scholar] [CrossRef]
- Brody, T. Chapter 25—Drug Safety. In Clinical Trials, 2nd ed.; FriesenPress: Altona, MB, Canada, 2016; pp. 483–568. [Google Scholar] [CrossRef]
- Douglas-de-Oliveira, D.W.; Chen, K.J. Patient-reported measures outcomes: Modern evaluation of oral health. BMC Oral Health 2023, 23, 498. [Google Scholar] [CrossRef] [PubMed]
- Gotfredsen, K. Patient-reported outcomes for bone regenerative procedures. Periodontol 2000 2023, 93, 270–276. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, j.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. 2000. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 24 July 2025).
- Bartols, A.; Kasprzyk, S.; Walther, W.; Korsch, M. Lateral alveolar ridge augmentation with autogenous block grafts fixed at a distance versus resorbable Poly-D-L-Lactide foil fixed at a distance: A single-blind, randomized, controlled trial. Clin. Oral Implant. Res. 2018, 29, 843–854. [Google Scholar] [CrossRef]
- Kofina, V.; Monfaredzadeh, M.; Rawal, S.Y.; Dentino, A.R.; Singh, M.; Tatakis, D.N. Patient-reported outcomes following guided bone regeneration: Correlation with clinical parameters. J. Dent. 2023, 136, 104605. [Google Scholar] [CrossRef] [PubMed]
- Meloni, S.M.; Jovanovic, S.A.; Urban, I.; Baldoni, E.; Pisano, M.; Tallarico, M. Horizontal ridge augmentation using GBR with a native collagen membrane and 1:1 ratio of particulate xenograft and autologous bone: A 3-year after final loading prospective clinical study. Clin. Implant. Dent. Relat. Res. 2019, 21, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Pieri, F.; Corinaldesi, G.; Fini, M.; Aldini, N.N.; Giardino, R.; Marchetti, C. Alveolar ridge augmentation with titanium mesh and a combination of autogenous bone and anorganic bovine bone: A 2-year prospective study. J. Periodontol. 2008, 79, 2093–2103. [Google Scholar] [CrossRef]
- Schwartz-Arad, D.; Ofec, R.; Eliyahu, G.; Ruban, A.; Sterer, N. Long Term Follow-Up of Dental Implants Placed in Autologous Onlay Bone Graft. Clin. Implant. Dent. Relat. Res. 2016, 18, 449–461. [Google Scholar] [CrossRef]
- Urban, I.; Nagursky, H.; Lozada, J. Horizontal Ridge Augmentation with a Resorbable Membrane and Particulated Autogenous Bone with or Without Anorganic Bovine Bone-Derived Mineral: A Prospective Case Series in 22 Patients. Int. J. Oral Maxillofac. Implant. 2011, 26, 404–414. [Google Scholar]
- Urban, I.A.; Lozada, J.L.; Jovanovic, S.A.; Nagursky, H.; Nagy, K. Vertical ridge augmentation with titanium-reinforced, dense-PTFE membranes and a combination of particulated autogenous bone and anorganic bovine bone-derived mineral: A prospective case series in 19 patients. Int. J. Oral Maxillofac. Implant. 2014, 29, 185–193. [Google Scholar] [CrossRef]
- Urban, I.A.; Nagursky, H.; Lozada, J.L.; Nagy, K. Horizontal ridge augmentation with a collagen membrane and a combination of particulated autogenous bone and anorganic bovine bone-derived mineral: A prospective case series in 25 patients. Int. J. Periodontics Restor. Dent. 2013, 33, 299–307. [Google Scholar] [CrossRef]
- Bayram, F.; Göçmen, G.; Özkan, Y. Evaluating risk factors and complications in mandibular ramus block grafting: A retrospective cohort study. Clin. Oral Investig. 2024, 28, 226. [Google Scholar] [CrossRef]
- Korsch, M.; Robra, B.-P.; Kasprzyk, S.; Walther, W. Patients’ perception of postoperative discomfort after bone graft: A parallel randomized clinical study of two techniques for lateral bone graft. Implantologie 2014, 22, 379–388. [Google Scholar]
- Lorenz, J.; Ghanaati, S.; Aleksic, Z.; Milinkovic, I.; Lazic, Z.; Magić, M.; Wessing, B.; Grotenclos, R.S.; Merli, M.; Mariotti, G.; et al. Horizontal Guided Bone Regeneration of the Posterior Mandible to Allow Implant Placement: 1-Year Prospective Study Results. Clin. Oral Implant. Res. 2025, 36, 100–116. [Google Scholar] [CrossRef]
- Sakkas, A.; Ioannis, K.; Winter, K.; Schramm, A.; Wilde, F. Clinical results of autologous bone augmentation harvested from the mandibular ramus prior to implant placement. An analysis of 104 cases. GMS Interdiscip. Plast. Reconstr. Surg. DGPW 2016, 5, Doc21. [Google Scholar] [CrossRef] [PubMed]
- Shiezadeh, F.; Arab, H.R.; Khoshkam, V.; Moeintaghavi, A.; Forouzanfar, A.; Khodadadifard, L. Comparison of Guided Bone Regeneration with Periosteal Pocket Flap Technique Versus Autogenous Bone Block Graft for Horizontal Bone Augmentation: A Clinical Trial Study. Int. J. Periodontics Restor. Dent. 2023, 43, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Thoma, D.S.; Maggetti, I.; Waller, T.; Hämmerle, C.H.F.; Jung, R.E. Clinical and patient-reported outcomes of implants placed in autogenous bone grafts and implants placed in native bone: A case-control study with a follow-up of 5–16 years. Clin. Oral Implant. Res. 2019, 30, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Altiparmak, N.; Soydan, S.S.; Uckan, S. The effect of conventional surgery and piezoelectric surgery bone harvesting techniques on the donor site morbidity of the mandibular ramus and symphysis. Int. J. Oral Maxillofac. Surg. 2015, 44, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Stübinger, S.; Stricker, A.; Berg, B.I. Piezosurgery in implant dentistry. Clin. Cosmet. Investig. Dent. 2015, 7, 115–124. [Google Scholar] [CrossRef]
- Pereira, R.S.; Pavelski, M.D.; Griza, G.L.; Boos, F.; Hochuli-Vieira, E. Prospective evaluation of morbidity in patients who underwent autogenous bone-graft harvesting from the mandibular symphysis and retromolar regions. Clin. Implant. Dent. Relat. Res. 2019, 21, 753–757. [Google Scholar] [CrossRef]
- Reininger, D.; Cobo-Vázquez, C.; Monteserín-Matesanz, M.; López-Quiles, J. Complications in the use of the mandibular body, ramus and symphysis as donor sites in bone graft surgery. A systematic review. Med. Oral Patol. Oral Cir. Bucal 2016, 21, e241–e249. [Google Scholar] [CrossRef]
- Starch-Jensen, T.; Deluiz, D.; Deb, S.; Bruun, N.H.; Tinoco, E.M.B. Harvesting of Autogenous Bone Graft from the Ascending Mandibular Ramus Compared with the Chin Region: A Systematic Review and Meta-Analysis Focusing on Complications and Donor Site Morbidity. J. Oral Maxillofac. Res. 2020, 11, e1. [Google Scholar] [CrossRef]
- Poort, L.J.; van Neck, J.W.; van der Wal, K.G. Sensory testing of inferior alveolar nerve injuries: A review of methods used in prospective studies. J. Oral Maxillofac. Surg. 2009, 67, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Khoury, F.; Hanser, T. Mandibular bone block harvesting from the retromolar region: A 10-year prospective clinical study. Int. J. Oral Maxillofac. Implant. 2015, 30, 688–697. [Google Scholar] [CrossRef]
- Corrêa, N.F.; de Brito, M.J.; de Carvalho Resende, M.M.; Duarte, M.F.; Santos, F.S.; Salomé, G.M.; Ferreira, L.M. Impact of surgical wound dehiscence on health-related quality of life and mental health. J. Wound Care 2016, 25, 561–570. [Google Scholar] [CrossRef] [PubMed]
- B, A.A.; Bharat, A.; Sharma, A.; Sharma, V.; Bharat, P.; Bharat, A. Analyzing guided bone regeneration methods: A review of the literature. J. Dent. Panacea 2024, 6, 130–135. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salahi, S.; Shaar, M.K.; Pitman, J.; Vervaeke, S.; Cosyn, J.; Younes, F.; De Bruyckere, T. Patient-Reported Outcomes in Intraoral Bone Block Augmentation Compared to GBR Procedures Prior to Implant Placement: A Systematic Review. J. Clin. Med. 2025, 14, 5331. https://doi.org/10.3390/jcm14155331
Salahi S, Shaar MK, Pitman J, Vervaeke S, Cosyn J, Younes F, De Bruyckere T. Patient-Reported Outcomes in Intraoral Bone Block Augmentation Compared to GBR Procedures Prior to Implant Placement: A Systematic Review. Journal of Clinical Medicine. 2025; 14(15):5331. https://doi.org/10.3390/jcm14155331
Chicago/Turabian StyleSalahi, Sepehr, Mohamad Kamal Shaar, Jeremy Pitman, Stijn Vervaeke, Jan Cosyn, Faris Younes, and Thomas De Bruyckere. 2025. "Patient-Reported Outcomes in Intraoral Bone Block Augmentation Compared to GBR Procedures Prior to Implant Placement: A Systematic Review" Journal of Clinical Medicine 14, no. 15: 5331. https://doi.org/10.3390/jcm14155331
APA StyleSalahi, S., Shaar, M. K., Pitman, J., Vervaeke, S., Cosyn, J., Younes, F., & De Bruyckere, T. (2025). Patient-Reported Outcomes in Intraoral Bone Block Augmentation Compared to GBR Procedures Prior to Implant Placement: A Systematic Review. Journal of Clinical Medicine, 14(15), 5331. https://doi.org/10.3390/jcm14155331








