Abstract
Background/Objectives: Tension-type headache (TTH) is the most prevalent form of primary headache. The etiology of TTH is not yet fully understood, although it is associated with the presence of myofascial trigger points (MTPs) in cervical and facial muscles. Dry needling (DN) therapy has emerged as an effective and safe non-pharmacological option for pain relief, but there are a lack of systematic reviews focused on its specific characteristics in TTH. The aim of this paper is to examine the characteristics and methodologies of DN in managing TTH. Methods: A scoping review was conducted with inclusion criteria considering studies that evaluated DN interventions in adults with TTH, reporting target muscles, diagnostic criteria, and technical features. The search was performed using PubMed, Embase, Scopus, and the Web of Science, resulting in the selection of seven studies after a rigorous filtering and evaluation process. Results: The included studies, primarily randomized controlled trials, involved a total of 309 participants. The most frequently treated muscles were the temporalis and trapezius. Identification of MTPs was mainly performed through manual palpation, although diagnostic criteria varied. DN interventions differed in technique. All studies included indicated favorable outcomes with improvements in headache symptoms. No serious adverse effects were reported, suggesting that the technique is safe. However, heterogeneity in protocols and diagnostic criteria limits the comparability of results. Conclusions: The evidence supports the use of DN in key muscles such as the temporalis and trapezius for managing TTH, although the diversity in methodologies and diagnostic criteria highlights the need for standardization. The safety profile of the method is favorable, but further research is necessary to define optimal protocols and improve reproducibility. Implementing objective diagnostic criteria and uniform protocols will facilitate advances in clinical practice and future research, ultimately optimizing outcomes for patients with TTH.
1. Introduction
Headache is one of the most prevalent disorders worldwide, affecting approximately 2.81 billion people in 2021, with women aged 15 to 49 years being the most impacted [1]. Due to its potential to cause disability and incur high financial costs, headaches have become a significant public health challenge [2].
Headache classification is based on the International Classification of Headache Disorders, 3rd edition (ICHD-3). It categorizes headaches into two main groups: primary and secondary. Primary headaches are disorders in themselves, with no identified cause, while secondary headaches are caused by or occur due to another condition. Primary headache disorders include migraine, tension-type headache (TTH), trigeminal autonomic cephalalgias, and others [3]. Among these, TTH is recognized as the most prevalent form of primary headache, affecting between 26% and 38% of the global population [4]. This disorder manifests as bilateral, dull, non-pulsating, and diffuse pain of mild-to-moderate intensity in the head or neck, which can significantly decrease quality of life and increase disability levels [2,4]. Despite its high incidence, the etiology and underlying mechanisms of TTH remain poorly understood [5]. Although current research suggests that muscle activity and facilitation of nociceptive pain processing play important roles in the pathogenesis of this condition, findings have been controversial [6]. While electromyography studies have reported normal muscle activity in patients with TTH, increased activity has also been observed in myofascial trigger points (MTPs). Therefore, it has been suggested that MTPs may contribute to this condition [5,6,7].
MTPs are defined as irritable points in skeletal muscle that exhibit a palpable hypersensitive nodule within a taut band. They can cause pain, referred tenderness in other areas, and motor dysfunction [8,9]. Recent clinical evidence has demonstrated the presence of MTPs in specific muscles of the neck and shoulders in individuals with TTH, suggesting that these points may contribute to peripheral nociceptive inputs. Specifically, MTPs located in muscles innervated by the C1–C3 nerves and the trigeminal nerve may be responsible for peripheral nociceptive input, generating an afferent overload in the caudal nucleus of the trigeminal nerve. This overload can lead to referred pain in the orofacial region, consistent with the neuronal convergence theory. In this context, hyperexcitability of nociceptive pathways appears to play a crucial role, as sensitization of these pathways may explain the increased muscle sensitivity observed in TTH [8,9,10].
The treatment of TTH includes both pharmacological and non-pharmacological approaches. One non-pharmacological method that has demonstrated a good cost-effectiveness ratio [11], along with low morbidity and mortality [12], is dry needling (DN). DN involves inserting a solid, filiform needle into the skin to target MTPs, aiming to disrupt dysfunctional motor endplates and alleviate neuromusculoskeletal pain [13]. The use of DN has gained traction in clinical practice [14]; however, to date, there has been no review of the literature focusing solely on the characteristics of DN interventions in the context of TTH.
Given these considerations, this review aims to comprehensively analyze the current literature on TTH and its relationship with MTPs, with a particular emphasis on DN interventions. By synthesizing recent research findings, we seek to examine the characteristics and methodologies of DN in managing this prevalent headache disorder.
2. Materials and Methods
This scoping review was conducted to explore and synthesize the literature describing the evidence on target muscles and specific intervention characteristics of dry needling in the treatment of tension-type headache. The protocol was developed following the framework proposed by Arksey and O’Malley (2005) and further refined by Levac et al. (2010) [15,16]. To ensure the quality of reporting, the PRISMA extension for Scoping Reviews (PRISMA-ScR) checklist was used [17].
Following Arksey and O’Malley’s five-stage framework [15], the review included the following: (a) identifying the research question; (b) identifying relevant studies; (c) selecting the studies; (d) charting the data; and (e) collating, summarizing, and reporting the results.
2.1. Identifying the Research Question
The main research question was as follows: What are the target muscles and specific intervention characteristics of dry needling in patients with tension-type headache, according to the available evidence?
Sub-questions included the following:
- What diagnostic criteria have been used to identify the presence or absence of trigger points in the target muscles for dry needling in patients with tension-type headache?
- What specific methodology has been applied in the administration of dry needling interventions?
- What are the potential adverse effects or unwanted reactions reported following dry needling application in muscles related to tension-type headache?
The study was guided by the Participant–Concept–Context (PCC) framework [18]. The population included adults diagnosed with any form of head and neck pain; the concept was orofacial pain or headache, and the context included any clinical or research setting.
2.2. Identifying Relevant Studies
2.2.1. Eligibility Criteria
Inclusion Criteria:
- Studies addressing tension-type headache as the primary diagnosis;
- Studies evaluating dry needling interventions, applied alone or as part of a combined treatment (provided the effect of dry needling is specified);
- Studies reporting the targeted muscles, diagnostic criteria for muscles, and/or characteristics of the dry needling protocol (frequency, duration, technique, adverse effects, or unwanted reactions);
- Articles published in English, French, or Spanish;
- Quantitative or mixed-methods studies: clinical trials, quasi-experimental studies, case reports, and case series;
- No restriction on the year of publication.
Exclusion Criteria:
- Studies that do not clearly differentiate dry needling from other techniques;
- Studies where tension-type headache is not the primary diagnosis or is unspecified;
- Systematic reviews, meta-analyses, letters to the editor, editorials, or commentaries;
- Studies conducted on animals or non-human models.
2.2.2. Information Sources
A comprehensive search was conducted in the following databases: PubMed, Embase, Scopus, and Web of Science. Additionally, references for included studies were manually screened to identify further relevant publications.
2.2.3. Search Strategy
A sensitive and comprehensive search strategy combining controlled vocabulary and free-text terms was developed for each database:
- PubMed: Used MeSH terms (“Dry Needling”[MeSH Terms] OR “Dry Needling”[Title/Abstract]) AND (“Headache, Tension-Type”[MeSH Terms] OR “tension-type headache”[Title/Abstract] OR “headache”[Title/Abstract] OR “cervicogenic headache”[Title/Abstract]) AND (“Myofascial Pain Syndromes”[MeSH Terms] OR “trigger points”[Title/Abstract])
- Embase: Used EMTREE terms and synonyms (‘dry needling’/exp OR ‘dry needling’) AND (‘tension-type headache’/exp OR ‘tension-type headache’ OR ‘headache’ OR ‘cervicogenic headache’) AND (‘myofascial trigger point’/exp OR ‘trigger point’)
- Web of Science: Used the Topic (TS) field for free-text search:
- TS = (“dry needling” OR “intramuscular stimulation”) AND
- TS = (“tension-type headache” OR “tension headache” OR “TTH” OR “headache” OR “cervicogenic headache”) AND
- TS = (“myofascial trigger points” OR “trigger points” OR “myofascial pain”)
- Scopus: TITLE-ABS-KEY(“dry needling” OR “intramuscular stimulation”) AND
- TITLE-ABS-KEY(“tension-type headache” OR “tension headache” OR “TTH” OR “headache” OR “cervicogenic headache”) AND
- TITLE-ABS-KEY(“myofascial trigger points” OR “trigger points” OR “myofascial pain”)
2.3. Study Selection
After removing duplicates, titles and abstracts were screened independently by two reviewers (EA and CB). Full-text articles were retrieved for potentially relevant records and assessed against the eligibility criteria. Discrepancies were resolved through discussion or by a third reviewer (CR)
2.4. Data Charting
2.4.1. Data Extraction
A standardized form was developed to collect key information from the included studies. Two reviewers independently extracted the data (AB and EA). Any disagreements were resolved by consensus or consultation with a third reviewer (CB).
2.4.2. Extracted Variables
The following data were extracted:
- Study characteristics: author, year, country, study design;
- Participant characteristics: headache intensity and location, sample size, age, sex;
- Target muscles;
- Diagnostic criteria;
- Characteristics of dry needling interventions: technique, frequency, duration;
- Adverse effects or unwanted reactions.
2.5. Collating, Summarizing, and Reporting the Results
A descriptive and narrative synthesis of the included studies was conducted, organized by target muscles, diagnostic criteria, and characteristics of the dry needling interventions. No formal risk of bias assessment was performed, consistent with the scoping review methodology.
3. Results
The search in all databases yielded a total of 252 articles, of which 110 duplicate articles were eliminated and 129 were ineligible. A total of 13 articles were analyzed by title and abstract. Finally, 13 articles were analyzed in full text and 6 articles were excluded because they met the exclusion criteria. See Figure 1.
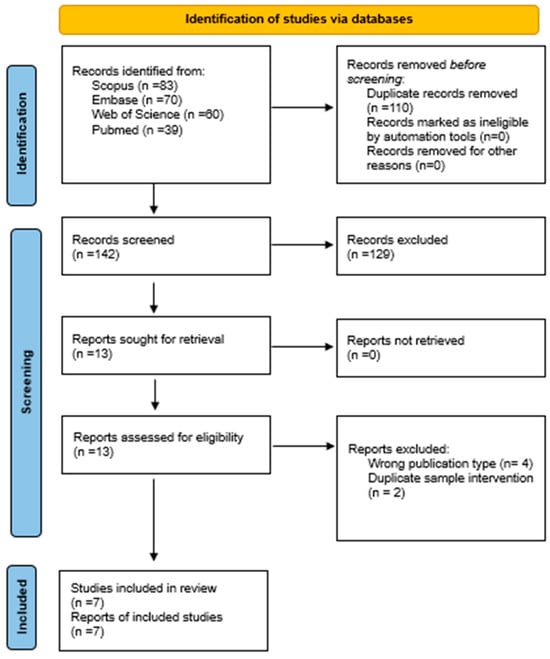
Figure 1.
PRISMA flowchart [17].
A total of seven studies were included in this review [19,20,21,22,23,24,25].
3.1. Study and Participant Characteristics
Of the 7 included studies, 5 were randomized controlled trials [19,21,23,24,25] and 2 were case reports [20,22]. Overall, the studies reviewed demonstrate a solid methodological level, with generally appropriate randomization procedures and, in some cases, well-implemented blinding strategies. The double-blind trials, such as those conducted by Gildir et al. [21] and Karakurum et al. [24], exhibit the highest methodological quality, while the single-blind studies by Kamali et al. [23] and Monti-Ballano et al. [25] provide an acceptable level of control, albeit with potential risks of bias in outcome assessment. In total, 309 subjects participated.
The characteristics of the participants can be found in Table 1.

Table 1.
Study and participant characteristics.
3.1.1. Diagnostic Criteria for TTH
One study employed the first edition of the ICHD, three studies utilized the second edition, and two studies applied the third edition. All studies adopted the diagnostic criteria of the International Headache Society that were appropriate to the time of their publication. Only one study did not specify the diagnostic criteria used, reporting instead that the diagnosis was made by a neurologist.
3.1.2. Intensity, Frequency, and Duration of Headache
In the studies analyzed, significant variations were observed in the effects of different interventions on headache intensity, frequency, and duration.
Regarding intensity, most studies reported a significant decrease following treatment. For instance, De Abreu Venancio et al. [19] observed a reduction in the Symptom Severity Index (SSI) across all three treatment groups (G1: 0.5 to 0.3; G2: 0.6 to 0.4; G3: 0.4 to 0.4). Gagnon et al. [20], in a case report, documented a progressive reduction in VAS scores from 6 to 0 over the course of five sessions. Gildir et al. [21] reported a more pronounced decrease in the intervention group (from 4.5 to 0.7) compared to the control group, which showed no improvement (from 4.6 to 4.6). Other studies, such as those by Kamali et al. [23] and Monti-Ballano et al. [25], also reported significant reductions in headache intensity, particularly in groups receiving dry needling therapy.
In terms of headache frequency, most studies recorded substantial decreases. Gildir et al. [21] reported a drop from 18.5 to 3.8 days/month in the treatment group, whereas the control group only declined from 18 to 7.9 days/month. Similarly, Kamali et al. [23] observed a reduction in frequency from 5 to 3.1 days/week in the intervention group. Karakurum et al. [24] reported a high baseline monthly frequency, with a notable reduction following intervention (headache index from 30.4 to 10.8).
With respect to headache duration, only a few studies provided specific data. Gildir et al. [21] documented a reduction in the experimental group from 3.9 to 0.7 h/day after the intervention. Issa and Huijbregts [22], in a case study, reported a variable headache duration ranging from 4 to 72 h. Other studies, including those by Kamali et al. [23], Karakurum et al. [24], and Monti-Ballano et al. [25], did not specify the headache duration.
The characteristics of the studies can be found in Table 1.
3.2. Intervention Characteristics
3.2.1. Target Muscles
Most of the studies analyzed included, among others, the temporalis and trapezius muscles as target areas [19,21,22,23,25]. Karakurum et al. also included the splenius capitis and splenius cervicis, in addition to the trapezius muscle [24], while Gagnon et al. focused solely on the levator scapulae muscle [20]. Only the study conducted by Monti-Ballano et al. incorporated all of the muscles known to refer pain to cranial or facial regions [25], which are typically associated with headaches according to Travell and Simons [26]. These muscles include the upper trapezius, splenius capitis and cervicis, semispinalis, rectus capitis posterior major, superior and inferior obliquus capitis, anterior and posterior occipitofrontalis, temporalis, masseter, as well as the clavicular and sternal heads of the sternocleidomastoid, zygomaticus major, and levator scapulae [25].
3.2.2. Diagnostic Criteria
The diagnostic criterion employed in most studies was the presence of active myofascial trigger points (MTPs) that reproduced headache symptoms upon manual palpation. Some studies reported using an algometer for precise digital palpation (1.5 KG pressure) [19,25]. Gildir et al. utilized flat palpation for all muscles except the trapezius, which underwent pincer palpation [21]. Only one study relied solely on the patient’s history to establish the diagnosis [24].
3.2.3. DN Intervention Characteristics
In terms of DN intervention characteristics, some authors noted the use of alcohol to disinfect the skin prior to puncturing [19,21]. Most authors provided details on the dimensions of the needles used, with calibers ranging from 0.2 to 0.3 mm and lengths from 0.13 to 0.5 mm. Some studies discussed patient positioning during the intervention, ranging from supine [22] to prone [20] or sitting [21]. Most authors elaborated on the technique applied, with some explaining the optimal angle for needle insertion [19,20], while others described various DN techniques based on the specific muscle being treated [22,25]. The average number of treatment sessions reported was three, ranging from one to five, with one session noted by Karakurum et al. and five by Gagnon et al. [20,24].
3.2.4. Outcomes
All studies included in this review indicated favorable outcomes from DN interventions, with improvements in headache symptoms being observed across the board. Abreu Venancio et al. further reported that in their study, they achieved more beneficial results with other treatments compared to DN alone, as the injection of substances into the puncture made the process less painful [19].
Gildir et al. [21] explicitly reported that DN was both effective, not only in reducing the frequency, intensity, and duration of headaches, but also in improving health-related quality of life (HRQoL).
3.2.5. Adverse Effects or Unwanted Reactions
None of the studies included in this review reported any notable adverse effects [19,20,21,22,23,24,25].
Table 2 provides a summary of the characteristics of the interventions.

Table 2.
Intervention characteristics.
4. Discussion
The main aims of this review were to assess the methodologies and clinical characteristics of DN in the management of TTH. To the best of our knowledge, the conducted scoping review has been the first to provide an expanded view of the characteristics of DN interventions in the treatment of TTH, highlighting both the target muscles and the diagnostic criteria, the specifics of the intervention, and the associated adverse effects. The comprehensive approach offered by this review and the relevant aspects it emphasizes contribute to the understanding of this technique and its clinical application.
The analysis of the included studies shows a clear predominance in the selection of the temporalis and trapezius muscles, with up to five studies implementing these interventions [19,21,22,23,25], corroborating previous findings regarding their relevance in the occurrence of MTPs related to this disorder [27,28]. This parallelism with the existing literature validates the focus on target muscles adopted in most of the analyzed studies and establishes a solid foundation for future research. However, a wide diversity is observed in the approach to other involved muscles. In other words, numerous muscles can independently or collectively trigger pain, and the different existing theories and methodologies lead to focusing on one muscle group or another. This can be understood, for example, through the study by Karakum et al. [24], which, in addition to the trapezius, concentrates on the splenius capitis and cervicis, following the radiculopathy origin described by Gunn, who attributes the pain to the paravertebral musculature [29]. Conversely, Gagnon et al. focus exclusively on the levator scapulae as the trigger point for headache in a unique case of TTH with levator scapulae syndrome, emphasizing a more isolated origin related to MTPs [20]. It is also important to highlight the study by Monti-Ballano et al., which was the only one to encompass all muscles known to refer pain to the facial or cranial regions, offering a crucial opportunity to unify criteria [25]. These results suggest, in line with the findings of Fernández-de-las-Peñas et al., the need for standardization in the selection of target muscles for DN in future research. Such standardization would not only facilitate the reproducibility of results across different contexts but also potentially enhance our understanding of the etiology and mechanisms underlying TTH [27,28]. This underlines the necessity of rigorous clinical reasoning when selecting muscles, techniques, treatment dosage, and patient profiling (e.g., chronic vs. episodic TTH, central sensitization status) [30].
Regarding the diagnostic criteria employed, there was a trend in six of the seven included studies towards the identification of active MTPs that reproduce TTH symptoms through manual palpation [19,20,21,22,23,25]. The variability in the palpation techniques used, ranging from digital palpation with the use of algometers [19,25] to flat palpation [21], reflects the heterogeneity in diagnostic approaches. This ambiguity may significantly influence the interpretation of the observed results, as they largely depend on the palpation process itself and the clinician’s experience. This may lead to discrepancies between the approach of different physiotherapists or even difficulties in making a differential diagnosis of other muscular injuries. Furthermore, although MTPs are defined as hyperirritable areas within a muscle that can cause local and referred pain, there is no universal consensus on the specific criteria for defining an MTP, which further complicates their diagnosis. Pain and tenderness upon palpation, as well as the interpretation of clinical signs such as the presence of palpable nodules, are highly subjective criteria that can vary greatly, potentially affecting diagnostic accuracy and, consequently, treatment effectiveness. Therefore, it is essential to address the need for establishing more standardized protocols with more objective criteria to improve the reliability of trigger point identification [31,32].
In terms of the characteristics of DN intervention, there was a consensus on the use of disinfectants and the specification of needle dimensions, reinforcing an essential aspect: the importance of safety and technical quality during the procedure. The studies employed different types of DN techniques, but all were based on the methodology described by Travell and Simons [26]. On one hand, Issa and Huijbregt described a meticulous process following the guidelines of the American Physical Therapy Association [33]. They emphasized securing the tube in the suspected area, directing the needle according to the muscle, and patient positioning. Additionally, they highlighted precautions to avoid injury to nearby structures such as the lung, carotid artery, facial nerve, and temporal artery [22]. Monti-Ballano et al., for their part, also adopted a technique based on muscle size and the presence of dangerous structures, using inward puncture and bidirectional rotation in muscles with flat bellies or proximity to vessels and nerves [25]. This underscores the need for precise knowledge of human anatomy and careful attention to safety during the intervention to prevent complications, as reported by Lee et al. in their study [34]. Abreu Venancio et al., in their study, used a multi-directional technique (up, down, lateral, and medial), repeating the puncture until the desired response was achieved, as indicated by Hong’s technique [35]. They emphasized the importance of mechanical disruption of the MTPs for treatment success, although they did not specify patient positioning or the exact technique [19]. Gagnon et al. described a more detailed technique, with the patient in the prone position, using the clamp grip and fan technique to target different points within the muscle, as well as performing periosteal tapping at the enthesis [20], a technique previously used in various musculoskeletal pathologies but with the same therapeutic approach, as seen in the studies reviewed by Tought et al. [36]. The technique of rolling or twisting the needle was also mentioned by Gagnon et al. to enhance physiological effects [20], based on the method described by Gunn [29]. On the other hand, some studies, such as those by Gildir et al. and Karakum et al., relied on specific techniques with needle retention times of 20 to 30 min [21,24]. Kamali et al. indicated that their employed technique was based on the methodology described by Demerholl and Fernandez de las Peñas in 2013 [37], although they provided few details about patient positioning or the exact technique [23]. This diversity of techniques across the different studies raises the question of whether it could affect treatment efficacy or be relevant to the outcomes. However, there are studies comparing these techniques, such as that by Taşoğlu et al., that compared a more static approach, where the needle remains inserted in the treatment area for a few minutes, versus a more dynamic approach, where the needle is repeatedly inserted in multiple directions. They concluded that both techniques were equally effective [38]. In summary, the literature demonstrates the use of a flexible and context-dependent strategy tailored to different muscles, suggesting potential variability in terms of application.
The studies reviewed show that the intervention with DN in MTPs has a positive impact, not only on the intensity and frequency of headache, but also on HRQoL and, by extension, on people’s ability to perform tasks of daily living. Gildir et al. [21] explicitly reported that DN was effective in improving HRQoL and this improvement suggests a favorable functional impact, enabling patients to resume their daily activities with greater ease. Although the remaining studies did not report direct measures of quality of life, the reduction in pain indicates a considerable positive effect on overall well-being and daily functioning, suggesting a likely recovery of the patients’ functional capacity in everyday tasks.
An encouraging aspect highlighted in this review is the scarcity of significant adverse effects associated with DN, which is a crucial point that should be communicated to gain acceptance for this technique as a safe treatment option for patients with TTH. However, the absence of reported adverse effects does not necessarily indicate that there is no risk; therefore, it is important for future research to collect data on adverse effects to provide a clearer picture of the safety of this treatment.
Another important finding of this review is the potential of DN as a therapeutic intervention that can be tailored to the specific clinical characteristics of patients with TTH, both in its episodic and chronic forms. Most of the included studies focus on heterogeneous populations, without a clear distinction between these subtypes, which limits the ability to generate specific, evidence-based recommendations for clinical practice. However, given the differing pathophysiology between episodic and chronic TTH, it would be reasonable to consider personalizing DN interventions based on headache subtypes. For instance, patients with chronic TTH may benefit from more intensive or extended protocols, involving a greater number of sessions or combining DN with other therapeutic modalities (such as therapeutic exercise or pain management education). In contrast, in episodic cases, brief and targeted interventions may be sufficient to reduce recurrence or prevent chronification.
The scoping review presented here has several limitations. Firstly, the small number of included studies restricts the breadth of the analysis and may limit the generalizability of the findings. Additionally, there was notable heterogeneity among the studies in terms of methodologies and intervention protocols, which complicates direct comparisons and synthesis of results. Furthermore, many studies lacked comprehensive reporting of key details such as specific technical procedures, which hampers the ability to draw definitive conclusions and identify best practices. With respect to diagnostic classification, the use of different editions of the International Classification of Headache Disorders (ICHD) across the included studies may significantly contribute to the clinical heterogeneity observed among the samples. The evolution of diagnostic criteria—from the broader descriptions in ICHD-1 to the more refined and flexible definitions in ICHD-3—implies that participants diagnosed with tension-type headache under different editions do not necessarily represent homogeneous populations. This variability can influence the inclusion of clinically distinct subtypes (episodic vs. chronic), the presence or absence of symptoms such as photophobia or phonophobia, and the diagnostic threshold itself. Moreover, the lack of specification regarding the diagnostic criteria used in some studies introduces an additional risk of selection bias and limits the reproducibility of the findings. Therefore, these factors must be carefully considered when interpreting the consistency and applicability of the results. Lastly, the diagnostic process for identifying MTPs is largely subjective and lacks standardization across studies, which may compromise the reliability of findings and limit their clinical applicability.
Given these limitations, it is recommended that future studies be conducted to incorporate new and more robust evidence as it becomes available, thereby strengthening the current findings. Moreover, future research should focus on the subclassification of patients based on the specific muscles affected, as this could allow for more tailored and effective intervention strategies. Standardizing intervention protocols across studies would also facilitate more meaningful comparisons and meta-analyses. Additionally, longitudinal studies with complete and detailed reporting are needed to better understand the techniques. These lines of investigation could significantly advance the field by providing clearer guidelines and improving clinical outcomes.
5. Conclusions
This scoping review has provided a comprehensive overview of the methodologies and clinical characteristics of dry needling (DN) in the management of tension-type headache (TTH), highlighting key aspects such as the target muscles, diagnostic criteria, techniques used, and potential adverse effects. A predominant trend was observed in the selection of the temporalis and trapezius muscles, supported by the previous literature, which reinforces the importance of these muscles in the pathophysiology of TTH. However, significant heterogeneity was also noted in the choice of other muscles, as well as in the techniques and protocols employed, reflecting the diversity of approaches and theories currently in practice. Variability in diagnostic criteria, especially in identifying active trigger points through palpation, along with the lack of standardized protocols, complicates result comparison and the reproducibility of interventions. Additionally, although most studies reported few adverse effects, the limited documentation regarding potential risks underscores the need for increased attention to be paid to safety and systematic data collection in future research.
Author Contributions
Conceptualization, E.A.-L. and C.B.-U.; methodology, E.A.-L., C.B.-U. and C.R.-B.; writing—original draft preparation, A.B.-V. and E.A.-L.; writing—review and editing, A.B.-V., E.A.-L. and C.B.-U.; supervision, C.R.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors declare no new data.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- GBD 2021 Diseases and Injuries Collaborators. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2133–2161.
- Meng, W.; Sui, L. Headache disorders: A persistent public health challenge for the under 50s. Front. Neurol. 2024, 15, 1501749. [Google Scholar] [CrossRef] [PubMed]
- Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [CrossRef] [PubMed]
- Satapathy, P.; Chauhan, S.; Gaidhane, S.; Bishoyi, A.K.; Priya, G.P.; Jayabalan, K.; Mishra, S.; Sharma, S.; Bushi, G.; Shabil, M.; et al. Trends in migraine and tension-type headaches in South Asia: Findings from the Global Burden of Disease Study 2021 (1990–2021). Front. Neurol. 2025, 16, 1514712. [Google Scholar] [CrossRef]
- Arendt-Nielsen, L.; Castaldo, M.; Mechelli, F.; Fernández-de-Las-Peñas, C. Muscle Triggers as a Possible Source of Pain in a Subgroup of Tension-type Headache Patients? Clin. J. Pain. 2016, 32, 711–718. [Google Scholar] [CrossRef]
- Bendtsen, L.; Fernández-de-la-Peñas, C. The Role of Muscles in Tension-Type Headache. Curr. Pain. Headache Rep. 2011, 15, 451–458. [Google Scholar] [CrossRef]
- de Tommaso, M.; Fernández-de-Las-Penas, C. Tension Type Headache. Curr. Rheumatol. Rev. 2016, 12, 127–139. [Google Scholar] [CrossRef]
- Belvís, R.; Irimia, P.; Seijo-Fernández, F.; Paz, J.; García-March, G.; Santos-Lasaosa, S.; Latorre, G.; González-Oria, C.; Rodríguez, R.; Pozo-Rosich, P.; et al. Neuromodulation in headache and craniofacial neuralgia: Guidelines from the Spanish Society of Neurology and the Spanish Society of Neurosurgery. Neurologia 2020, 36, 61–79. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C.; Cuadrado, M.; Arendt-Nielsen, L.; Simons, D.; Pareja, J. Myofascial Trigger Points and Sensitization: An Updated Pain Model for Tension-Type Headache. Cephalalgia 2007, 27, 383–393. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C.; Madeleine, P.; Caminero, A.; Cuadrado, M.; Arendt-Nielsen, L.; Pareja, J. Generalized Neck-Shoulder Hyperalgesia in Chronic Tension-Type Headache and Unilateral Migraine Assessed by Pressure Pain Sensitivity Topographical Maps of the Trapezius Muscle. Cephalalgia 2010, 30, 77–86. [Google Scholar] [CrossRef]
- Arias-Buría, J.L.; Martín-Saborido, C.; Cleland, J.; Koppenhaver, S.L.; Plaza-Manzano, G.; Fernández-de-las-Peñas, C. Cost-effectiveness Evaluation of the Inclusion of Dry Needling into an Exercise Program for Subacromial Pain Syndrome: Evidence from a Randomized Clinical Trial. Pain Med. 2018, 19, 2336–2347. [Google Scholar] [CrossRef]
- Brady, S.; McEvoy Johnson Dommerholt Jan Doody, C. Adverse events following trigger point dry needling: A prospective survey of chartered physiotherapists. J. Man. Manip. Ther. 2014, 22, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Justes, D.; Yarzábal-Rodríguez, R.; Doménech-García, V.; Herrero, P.; Bellosta-López, P. Effectiveness of dry needling for headache: A systematic review. Neurologia 2022, 37, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Gattie, E.; Cleland, J.A.; Snodgrass, S. The Effectiveness of Trigger Point Dry Needling for Musculoskeletal Conditions by Physical Therapists: A Systematic Review and Meta-analysis. J. Orthop. Sports Phys. Ther. 2017, 47, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Peters, M.D.J.; Godfrey, C.M.; McInerney, P.; Munn, Z.; Tricco, A.C.; Khalil, H. Chapter 11: Scoping Reviews (2020 version). In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; Joanna Briggs Institute: Adelaide, Australia, 2020. [Google Scholar]
- de Abreu Venancio, R.; Pereira, G.; Francisco, A.; Zamperini, C. Botulinum Toxin, Lidocaine, and Dry-Needling Injections in Patients with Myofascial Pain and Headaches. CRANIO® 2009, 27, 46–53. [Google Scholar] [CrossRef]
- Gagnon, P.; Dunning, J.; Bliton, P.; Charlebois, C.; Henry, N.; Gorby, P.; Mourad, F. Dry needling in the management of chronic tension-type headache associated with levator scapulae syndrome: A case report. Clin. Case Rep. 2024, 12, e8858. [Google Scholar] [CrossRef]
- Gildir, S.; Tüzün, E.H.; Eroğlu, G.; Eker, L. A randomized trial of trigger point dry needling versus sham needling for chronic tension-type headache. Medicine 2019, 98, e14520. [Google Scholar] [CrossRef]
- Issa, T.S.; Huijbregts, P.A. Physical Therapy Diagnosis and Management of a Patient with Chronic Daily Headache: A Case Report. J. Man. Manip. Ther. 2006, 14, 88E–123E. [Google Scholar] [CrossRef]
- Kamali, F.; Mohamadi, M.; Fakheri, L.; Mohammadnejad, F. Dry needling versus friction massage to treat tension type headache: A randomized clinical trial. J. Bodyw. Mov. Ther. 2019, 23, 89–93. [Google Scholar] [CrossRef]
- Karakurum, B.; Karaalin, O.; Coskun, O.; Dora, B.; Uçler, S.; Inan, L. The “dry-needle technique”: Intramuscular stimulation in tension-type headache. Cephalalgia 2001, 21, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Monti-Ballano, S.; Márquez-Gonzalvo, S.; Lucha-López, M.O.; Ferrández-Laliena, L.; Vicente-Pina, L.; Sánchez-Rodríguez, R.; Tricás-Vidal, H.J.; Tricás-Moreno, J.M. Effects of Dry Needling on Active Myofascial Trigger Points and Pain Intensity in Tension-Type Headache: A Randomized Controlled Study. J. Pers. Med. 2024, 14, 332. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.M.; Fernández-de-las-Peñas, C.F.; Finnegan, M.; Freeman, J.L. Dor e Disfunção Miofascial de Travell, Simons & Simons, 3rd ed.; Manual de Pontos-Gatilho; Artmed Editora: Rio Grande do Sul, Brazil, 2020; 998p. [Google Scholar]
- Fernández-de-Las-Peñas, C.; Ge, H.Y.; Arendt-Nielsen, L.; Cuadrado, M.L.; Pareja, J.A. The local and referred pain from myofascial trigger points in the temporalis muscle contributes to pain profile in chronic tension-type headache. Clin. J. Pain 2007, 23, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-Las-Peñas, C.; Simons, D.; Cuadrado, M.L.; Pareja, J. The role of myofascial trigger points in musculoskeletal pain syndromes of the head and neck. Curr. Pain Headache Rep. 2007, 11, 365–372. [Google Scholar] [CrossRef]
- Gunn, C.C. The Gunn Approach to the Treatment of Chronic Pain: Intramuscular Stimulation for Myofascial Pain of Radiculopathic Origin; Churchill Livingstone: London, UK, 1996; 192p. [Google Scholar]
- Fernández-de-Las-Peñas, C.; Florencio, L.L.; Plaza-Manzano, G.; Arias-Buría, J.L. Clinical Reasoning Behind Non-Pharmacological Interventions for the Management of Headaches: A Narrative Literature Review. Int. J. Environ. Res. Public Health 2020, 17, 4126. [Google Scholar] [CrossRef]
- Steen, J.P.; Jaiswal, K.S.; Kumbhare, D. Myofascial Pain Syndrome: An Update on Clinical Characteristics, Etiopathogenesis, Diagnosis, and Treatment. Muscle Nerve 2025, 71, 889–910. [Google Scholar] [CrossRef]
- Zhai, T.; Jiang, F.; Chen, Y.; Wang, J.; Feng, W. Advancing musculoskeletal diagnosis and therapy: A comprehensive review of trigger point theory and muscle pain patterns. Front. Med. 2024, 11, 1433070. [Google Scholar] [CrossRef]
- American Physical Therapy Association. Guide to Physical Therapist Practice. Second Edition. American Physical Therapy Association. Phys. Ther. 2001, 81, 9–746.
- Lee, J.H.; Lee, H.; Jo, D.J. An acute cervical epidural hematoma as a complication of dry needling. Spine 2011, 36, E891–E893. [Google Scholar] [CrossRef]
- Hong, C.Z. Lidocaine injection versus dry needling to myofascial trigger point: The importance of the local twitch response. Am. J. Phys. Med. Rehabil. 1994, 73, 256–263. [Google Scholar] [CrossRef]
- Tough, E.A.; White, A.R.; Cummings, T.M.; Richards, S.H.; Campbell, J.L. Acupuncture and dry needling in the management of myofascial trigger point pain: A systematic review and meta-analysis of randomised controlled trials. Eur. J. Pain. 2009, 13, 3–10. [Google Scholar] [CrossRef]
- Dommerholt, J.; Fernández-de-las-Peñas, C. (Eds.) Trigger Point Dry Needling: An Evidence and Clinical-Based Approach; Churchill Livingstone; Elsevier: Amsterdam, The Netherlands, 2013; 258p. [Google Scholar]
- Taşoğlu, Ö.; Şahin Onat, Ş.; Bölük, H.; Taşoğlu, İ.; Özgirgin, N. Comparision of two different dry-needling techniques in the treatment of myofascial pain syndrome. J. Turk. Soc. Algol. 2017, 29, 9–16. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).