Can Lung Ultrasound Act as a Diagnosis and Monitoring Tool in Children with Community Acquired Pneumonia? Correlation with Risk Factors, Clinical Indicators and Biologic Results
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
- -
- Age between 1 month and 18 years;
- -
- Diagnosis of pneumonia by a specialist doctor;
- -
- Lung ultrasound examination;
- -
- Informed consent signed by a legal guardian.
- -
- Age below 1 month or over 18 years;
- -
- Children admitted with pneumonia but without LUS examination;
- -
- Hospital-acquired pneumonia;
- -
- Lack of informed consent from the patients’ guardians;
- -
- Outpatients with the same diagnosis.
2.2. Data Collection
2.3. Paraclinical Examination
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Study Group
3.2. Anthropometric Measurements (Weight, Ideal Weight, IP, Height, BMI)
3.3. Patient-Related Risk Factors for CAP
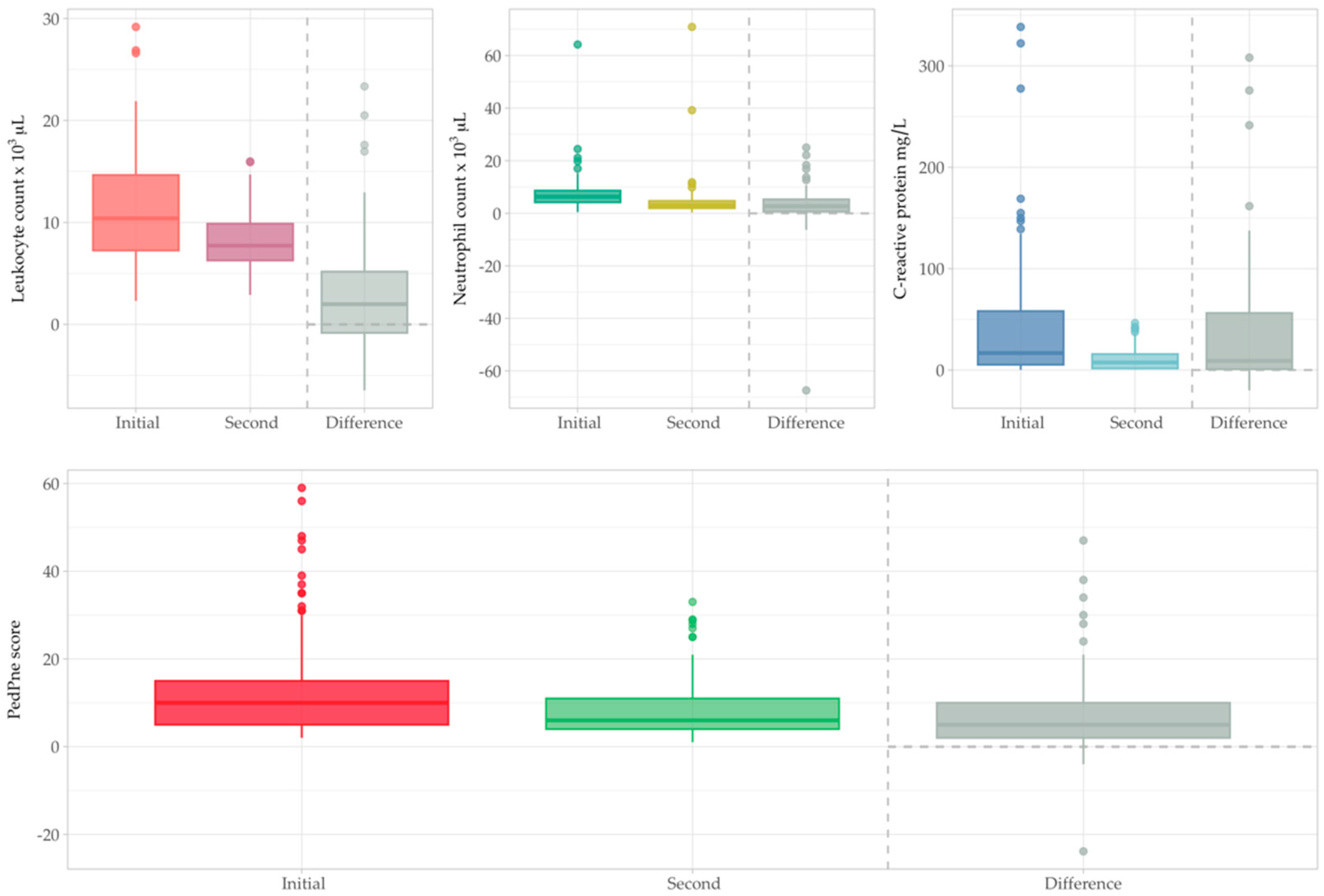
3.4. Initial Examination
3.5. Second Examination
3.6. A Predictive Model for Hospital Stay
+ β_3 × Smoker parents + β_4 × Collective + β_5 × Ponderal Index
+ β_6 × Environment + β_7 × Antibiotic use at home
+ β_8 × Neutrophil %
4. Discussion
4.1. Baseline Characteristics of Study Group (Age, Sex, Environment Origin)
4.2. Anthropometric Measurements (Weight, Ideal Weight, IP, Height, BMI)
4.3. Patient-Related Risk Factors for Pneumonia
4.4. Initial Examination (Clinical, Inflammatory Markers and Imaging)
4.5. Second Examination
4.6. A Predictive Model for Hospital Stay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Le Roux, D.M.; Zar, H.J. Community-acquired pneumonia in children—A changing spectrum of disease. Pediatr. Radiol. 2017, 47, 1392–1398. [Google Scholar] [CrossRef]
- GBD 2013 Collaboration. Global and national burden of diseases and injuries among children and adolescents between 1990 and 2013: Findings from the Global Burden of Disease 2013 Study. JAMA Pediatr. 2016, 170, 267. [Google Scholar] [CrossRef]
- Plesca, D.A. Community acquired pneumonia. In Tratat de Pediatrie; Medichub: Bucharest, Romania, 2021; pp. 339–347. ISBN 978-606-95260-0-2. [Google Scholar]
- Leung, A.K.; Wong, A.H.; Hon, K.L. Community-acquired pneumonia in children. Recent Pat. Inflamm. Allergy Drug Discov. 2018, 12, 136–144. [Google Scholar] [CrossRef]
- Ciuca, I.M.; Dediu, M.; Marc, M.S.; Lukic, M.; Horhat, D.I.; Pop, L.L. Lung ultrasound is more sensitive for hospitalized consolidated pneumonia diagnosis compared to CXR in children. Children 2021, 8, 659. [Google Scholar] [CrossRef]
- Andronikou, S.; Lambert, E.; Halton, J.; Hilder, L.; Crumley, I.; Lyttle, M.D.; Kosack, C. Guidelines for the use of chest radiographs in community-acquired pneumonia in children and adolescents. Pediatr. Radiol. 2017, 47, 1405–1411. [Google Scholar] [CrossRef]
- Urbankowska, E.; Krenke, K.; Drobczyński, Ł.; Korczyński, P.; Urbankowski, T.; Krawiec, M.; Kraj, G.; Brzewski, M.; Kulus, M. Lung ultrasound in the diagnosis and monitoring of community acquired pneumonia in children. Respir. Med. 2015, 109, 1207–1212. [Google Scholar] [CrossRef]
- Ciuca, I.M. COVID-19 in children: An ample review. Risk Manag. Healthc. Policy 2020, 13, 661–669. [Google Scholar] [CrossRef]
- Balk, D.S.; Lee, C.; Schafer, J.; Welwarth, J.; Hardin, J.; Novack, V.; Yarza, S.; Hoffmann, B. Lung ultrasound compared to chest X-ray for diagnosis of pediatric pneumonia: A meta-analysis. Pediatr. Pulmonol. 2018, 53, 1130–1139. [Google Scholar] [CrossRef]
- Shah, V.P.; Tunik, M.G.; Tsung, J.W. Prospective evaluation of point-of-care ultrasonography for the diagnosis of pneumonia in children and young adults. JAMA Pediatr. 2013, 167, 119–125. [Google Scholar] [CrossRef]
- Stadler, J.A.; Andronikou, S.; Zar, H.J. Lung ultrasound for the diagnosis of community-acquired pneumonia in children. Pediatr. Radiol. 2017, 47, 1412–1419. [Google Scholar] [CrossRef]
- Riccabona, M. Ultrasound of the chest in children (mediastinum excluded). Eur. Radiol. 2008, 18, 390–399. [Google Scholar] [CrossRef]
- Costa, F.; Titolo, A.; Ferrocino, M.; Biagi, E.; Dell’Orto, V.; Perrone, S.; Esposito, S. Lung Ultrasound in Neonatal Respiratory Distress Syndrome: A Narrative Review of the Last 10 Years. Diagnostics 2024, 14, 2793. [Google Scholar] [CrossRef]
- Trias-Sabria, P.; Molina-Molina, M.; Aso, S.; Argudo, M.H.; Diez-Ferrer, M.; Sabater, J.; Dorca, J.; Santos, S.; Suarez-Cuartin, G. Lung ultrasound score to predict outcomes in COVID-19. Respir. Care 2021, 66, 1263–1270. [Google Scholar] [CrossRef]
- Mongodi, S.; De Luca, D.; Colombo, A.; Stella, A.; Santangelo, E.; Corradi, F.; Gargani, L.; Rovida, S.; Volpicelli, G.; Bouhemad, B.; et al. Quantitative lung ultrasound: Technical aspects and clinical applications. Anesthesiology 2021, 134, 949–965. [Google Scholar] [CrossRef]
- Krishna, D.; Khera, D.; Toteja, N.; Sureka, B.; Choudhary, B.; Ganakumar, V.M.; Singh, K. Point-of-care thoracic ultrasound in children with bronchiolitis. India. J. Pediatr. 2022, 89, 1079–1085. [Google Scholar] [CrossRef]
- Elabbas, A.; Choudhary, R.; Gullapalli, D.; Mistry, S.; Farzana, M.H.; Mallick, A.H.; Kevu, E.P.; Asif, J.; Mostafa, J.A. Lung ultrasonography beyond the diagnosis of pediatrics pneumonia. Cureus 2022, 14, e22460. [Google Scholar] [CrossRef]
- Fu, B.; Zhang, P.; Zhang, J. Diagnosis and prognosis evaluation of severe pneumonia by lung ultrasound score combined with serum inflammatory markers. Mediterr. J. Hematol. Infect. Dis. 2023, 15, e2023057. [Google Scholar] [CrossRef]
- Ciuca, I.M.; Dediu, M.; Pop, L.L. Pediatric pneumonia (PedPne) lung ultrasound score and inflammatory markers: A pilot study. Pediatr. Pulmonol. 2022, 57, 576–582. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Buda, N.; Ciuca, I.M.; Dong, Y.; Fang, C.; Feldkamp, A.; Jüngert, J.; Kosiak, W.; Mentzel, H.J.; Pienar, C.; et al. Lung ultrasound in children, WFUMB review paper (part 2). Med. Ultrason. 2021, 23, 443–452. [Google Scholar] [CrossRef]
- Jaworska, J.; Buda, N.; Ciuca, I.M.; Dong, Y.; Fang, C.; Feldkamp, A.; Jüngert, J.; Kosiak, W.; Mentzel, H.J.; Pienar, C.; et al. Ultrasound of the pleura in children, WFUMB review paper. Med. Ultrason. 2021, 23, 339–347. [Google Scholar] [CrossRef]
- Pérez, A.W.; Gonçalves, J.F.; Pérez, J.H.; Fariña, Y.R.; Rodriguez, J.D. Prognostic value of lung ultrasound and its link with inflammatory biomarkers in patients with SARS-CoV-2 infection. Respir. Med. Res. 2021, 79, 100809. [Google Scholar]
- Reissig, A.; Gramegna, A.; Aliberti, S. The role of lung ultrasound in the diagnosis and follow-up of community-acquired pneumonia. Eur. J. Intern. Med. 2012, 23, 391–397. [Google Scholar] [CrossRef]
- Child and Teen BMI Calculator, 26 June 2024. Available online: https://www.cdc.gov/healthyweight/assessing/bmi/childrens_bmi/about_childrens_bmi.html (accessed on 20 December 2024).
- About Body Mass Index (BMI), 20 May 2024. Available online: https://www.cdc.gov/bmi/about/index.html (accessed on 10 November 2024).
- WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development; World Health Organization: Geneva, Switzerland, 2006; p. 312.
- WHO Anthro for Personal Computers. Software for Assessing Growth and Development of the World’s Children, Department of Nutrition for Health and Development; World Health Organization: Geneva, Switzerland, 2011.
- Fleming, S.; Thompson, M.; Stevens, R.; Heneghan, C.; Plüddemann, A.; Maconochie, I.; Tarassenko, L.; Mant, D. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: A systematic review of observational studies. Lancet 2011, 377, 1011–1018. [Google Scholar] [CrossRef]
- El Brihi, J.; Pathak, S. Normal and Abnormal Complete Blood Count with Differential; StatPearls Publishing: Tampa, FL, USA, 2024. [Google Scholar]
- Andropoulos, D.B.; Gregory, G.A. (Eds.) Gregory’s Pediatric Anesthesia; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- RC Team. R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, 2013. Available online: https://www.R-project.org/ (accessed on 17 May 2024).
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Bland, J.M.; Altman, D.G. Multiple significance tests: The Bonferroni method. BMJ 1995, 310, 170. [Google Scholar]
- Qian, C.; Chen, Q.; Lin, W.; Li, Z.; Zhu, J.; Zhang, J.; Luan, L.; Zheng, B.; Zhao, G.; Tian, J.; et al. Incidence of community-acquired pneumonia among children under 5 years in Suzhou, China: A hospital-based cohort study. BMJ Open 2024, 14, e078489. [Google Scholar] [CrossRef]
- Rueda, Z.V.; Aguilar, Y.; Maya, M.A.; López, L.; Restrepo, A.; Garcés, C.; Morales, O.; Roya-Pabón, C.; Trujillo, M.; Arango, C.; et al. Etiology and the challenge of diagnostic testing of community-acquired pneumonia in children and adolescents. BMC Pediatr. 2022, 22, 169. [Google Scholar] [CrossRef]
- Oyana, T.J.; Minso, J.; Jones, T.L.; McCullers, J.A.; Arnold, S.R.; Cormier, S.A. Particulate matter exposure predicts residence in high-risk areas for community acquired pneumonia among hospitalized children. Exp. Biol. Med. 2021, 246, 1907–1916. [Google Scholar] [CrossRef]
- Kasundriya, S.K.; Dhaneria, M.; Mathur, A.; Pathak, A. Incidence and Risk Factors for Severe Pneumonia in Children Hospitalized with Pneumonia in Ujjain, India. Int. J. Environ. Res. Public Health 2020, 17, 4637. [Google Scholar] [CrossRef]
- Rees, C.A.; Colbourn, T.; Hooli, S.; King, C.; Lufesi, N.; McCollum, E.D.; Mwansambo, C.; Cutland, C.; Madhi, S.A.; Nunes, M.; et al. Derivation and validation of a novel risk assessment tool to identify children aged 2–59 months at risk of hospitalized pneumonia-related mortality in 20 countries. BMJ Glob. Health 2022, 7, e008143. [Google Scholar] [CrossRef]
- Roh, E.J.; Lee, M.H.; Lee, J.Y.; Kim, H.B.; Ahn, Y.M.; Kim, J.K.; Kim, H.Y.; Jung, S.S.; Kim, M.; Kang, E.K.; et al. Analysis of national surveillance of respiratory pathogens for community-acquired pneumonia in children and adolescents. BMC Infect. Dis. 2022, 22, 330. [Google Scholar] [CrossRef]
- Bulata-Pop, I.; Stirbu, I.; Simionescu, B.; Grama, A.; Junie, L.M. Clinical, Biological, and Radiological Findings and Management of Lower Respiratory Tract Infections in a Tertiary Hospital in Romania. Cureus 2024, 16, e67685. [Google Scholar] [CrossRef]
- Kirolos, A.; Blacow, R.M.; Parajuli, A.; Welton, N.J.; Khanna, A.; Allen, S.J.; McAllister, D.A.; Campbell, H.; Nair, H. The impact of childhood malnutrition on mortality from pneumonia: A systematic review and network meta-analysis. BMJ Glob. Health 2021, 6, e007411. [Google Scholar] [CrossRef]
- Ngocho, J.S.; de Jonge, M.I.; Minja, L.; Olomi, G.A.; Mahande, M.J.; Msuya, S.E.; Mmbaga, B.T. Modifiable risk factors for community-acquired pneumonia in children under 5 years of age in resource-poor settings: A case–control study. Trop. Med. Int. Health 2019, 24, 484–492. [Google Scholar] [CrossRef]
- Shahunja, K.M.; Ahmed, T.; Hossain, M.I.; Das, S.K.; Faruque, A.S.G.; Islam, M.M.; Chisti, M.J. Factors Associated with Pneumonia Among Overweight and Obese Under-Five Children in an Urban Hospital of a Developing Country. Glob. Pediatr. Health 2016, 3, 2333794X16672528. [Google Scholar] [CrossRef]
- Sidabutar, E.; Bustan, N.; Birawida, A.B. Analysis of risk factor for pneumonia in children less than five years in Makassar. J. Educ. Health Promot. 2024, 13, 16. [Google Scholar] [CrossRef]
- Wonodi, C.B.; Deloria-Knoll, M.; Feikin, D.R.; DeLuca, A.N.; Driscoll, A.J.; Moïsi, J.C.; Johnson, H.L.; Murdoch, D.R.; O’Brien, L.; Levine, O.S.; et al. Evaluation of risk factors for severe pneumonia in children: The Pneumonia Etiology Research for Child Health study. Clin. Infect. Dis. 2012, 54 (Suppl. S2), S124–S131. [Google Scholar] [CrossRef]
- Sutriana, V.N.; Sitaresmi, M.N.; Wahab, A. Risk factors for childhood pneumonia: A case-control study in a high prevalence area in Indonesia. Clin. Exp. Pediatr. 2021, 64, 588. [Google Scholar] [CrossRef]
- Ending Preventable Child Deaths from Pneumonia and Diarrhea by 2025: The integrated Global Action Plan for Pneumonia and Diarrhoea (GAPPD). Available online: https://www.who.int/publications/i/item/9789241505239 (accessed on 2 January 2025).
- Youn, Y.S.; Lee, K.Y.; Hwang, J.Y.; Rhim, J.W.; Kang, J.H.; Lee, J.S.; Kim, J.C. Difference of clinical features in childhood Mycoplasma pneumoniae pneumonia. BMC Pediatr. 2010, 10, 48. [Google Scholar] [CrossRef]
- Defilippi, A.; Silvestri, M.; Tacchella, A.; Giacchino, R.; Melioli, G.; Di Marco, E.; Cirillo, C.; Di Pietro, P.; Rossi, G.A. Epidemiology and clinical features of Mycoplasma pneumoniae infection in children. Respir. Med. 2008, 102, 1762–1768. [Google Scholar] [CrossRef]
- Juvén, T.; Ruuskanen, O.; Mertsola, J. Symptoms and signs of community-acquired pneumonia in children. Scand. J. Prim. Health Care 2003, 21, 52–56. [Google Scholar] [CrossRef]
- Macpherson, L.; Ogero, M.; Akech, S.; Aluvaala, J.; Gathara, D.; Irimu, G.; English, M.; Agweyu, A. Risk factors for death among children aged 5-14 years hospitalized with pneumonia: A retrospective cohort study in Kenya. BMJ Glob. Health 2019, 4, e001715. [Google Scholar] [CrossRef]
- Kevat, P.M.; Morpeth, M.; Graham, H.; Gray, A.Z. A systematic review of the clinical features of pneumonia in children aged 5-9 years: Implications for guidelines and research. J. Glob. Health 2022, 12, 10002. [Google Scholar] [CrossRef]
- Yun, K.W. Community-acquired pneumonia in children: Updated perspectives on its etiology, diagnosis, and treatment. Clin. Exp. Pediatr. 2023, 67, 80. [Google Scholar] [CrossRef]
- Shi, C.; Xu, X.; Xu, Y. Systematic review and meta-analysis of the accuracy of lung ultrasound and chest radiography in diagnosing community acquired pneumonia in children. Pediatr. Pulmonol. 2024, 59, 3130–3147. [Google Scholar] [CrossRef]
- Aygün, D.; Önal, P.; Kılınç, A.A.; Aygün, F.; Şiraneci, R.; Çokuğraş, H. Can Complete Blood Count Parameters and Serum Electrolyte Levels Have a Predictive Role in Differential Diagnosis of Tuberculosis from Community-acquired Pneumonia in Children? Turk. Arch. Pediatr. 2024, 59, 289. [Google Scholar] [CrossRef]
- Yadav, K.K.; Awasthi, S. Childhood Pneumonia: What’s Unchanged, and What’s New? Indian J. Pediatr. 2023, 90, 693–699. [Google Scholar] [CrossRef]
- Gunaratnam, L.C.; Robinson, J.L.; Hawkes, M.T. Systematic review and meta-analysis of diagnostic biomarkers for pediatric pneumonia. J. Pediatr. Infect. Dis. Soc. 2021, 10, 891–900. [Google Scholar] [CrossRef]
- Simon, L.; Gauvin, F.; Amre, D.K.; Saint-Louis, P.; Lacroix, J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: A systematic review and meta-analysis. Clin. Infect. Dis. 2004, 39, 206–217. [Google Scholar] [CrossRef]
- World Health Organization. Standardization of Interpretation of Chest Radiographs for the Diagnosis of Pneumonia in Children/World Health Organization Pneumonia Vaccine Trial Investigators’ Group, 2001. Available online: http://www.who.int/iris/handle/10665/66956 (accessed on 5 January 2025).
- Campagnano, S.; Angelini, F.; Fonsi, G.B.; Novelli, S.; Drudi, F.M. Diagnostic imaging in COVID-19 pneumonia: A literature review. J. Ultrasound 2021, 24, 383–395. [Google Scholar] [CrossRef]
- Sadiq, N.; Ifikhar, A.; Tahir, M.; Mehmood, R.; Awan, A.A.; Uzair, M. Diagnostic Accuracy of Lung Ultrasound in Diagnosis of Pneumonia in Children using Chest X-RAY as Gold Standard. Pak. Armed Forces Med. J. 2021, 73, 1761. [Google Scholar] [CrossRef]
- Volpicelli, G.; Fraccalini, T.; Cardinale, L. Lung ultrasound: Are we diagnosing too much? Ultrasound J. 2023, 15, 17. [Google Scholar] [CrossRef]
- Harris, M.; Clark, J.; Coote, N.; Fletcher, P.; Harnden, A.; McKean, M.; Thomson, A. British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: Update 2011. Thorax 2011, 66 (Suppl. S2), ii1–ii23. [Google Scholar] [CrossRef]
- Liu, J.; Shi, H. Pathological and Imaging Findings of Infectious and Inflammatory Diseases of Chest. Radiol. Infect. Inflamm. Dis. 2023, 3, 17–29. [Google Scholar]
- Chelcea, R.; Dediu, M.; Dabica, D.; Laitin, S.M.D.; Ciuca, I.M. Lung Ultrasound Efficacy in Monitoring Post-SARS-CoV-2 Pneumonia and Inflammatory Biomarkers in Pediatric Patients. Medicina 2024, 60, 1296. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, Q.; Florez, I.D.; Mathew, J.L.; Shang, L.; Zhang, G.; Tian, X.; Fu, Z.; Liu, E.; Luo, Z.; et al. Short-course vs long-course antibiotic therapy for children with nonsevere community-acquired pneumonia: A systematic review and meta-analysis. JAMA Pediatr. 2022, 176, 1199–1207. [Google Scholar] [CrossRef]
- World Health Organization. Revised WHO Classification and Treatment of Pneumonia in Children at Health Facilities: Evidence Summaries. Available online: https://apps.who.int/iris/bitstream/handle/10665/137319/9789241507813_eng.pdf (accessed on 10 January 2025).




| Sex | n | % | Min Age | Max Age | Mean Age | Median Age | SD Age | IQR Age |
|---|---|---|---|---|---|---|---|---|
| Female | 43 | 42.2% | 0.1 | 16.1 | 5.1 | 3.7 | 4.3 | 6.9 |
| Male | 59 | 57.8% | 0.3 | 17.3 | 6.2 | 4.4 | 5.2 | 7.1 |
| Total | 102 | 100% | 0.1 | 17.3 | 5.7 | 4.2 | 4.9 | 6.8 |
| All n = 102 | Feminine n = 43 | Masculine n = 59 | p Value * | |
|---|---|---|---|---|
| Age | 0.53 | |||
| <1 year | 15 | 8 (18.60%) | 7 (11.86%) | |
| ≥1 years, <3 years | 25 | 12 (27.90%) | 13 (22.03%) | |
| ≥3 years, <6 years | 25 | 8 (18.60%) | 17 (28.81%) | |
| ≥ 6 years | 37 | 15 (34.88%) | 22 (37.28%) | |
| Environment origin | 0.48 | |||
| Urban | 54 | 25 (58.13%) | 29 (49.15%) | |
| Rural | 48 | 18 (41.86%) | 30 (50.84%) | |
| Medical History | ||||
| Premature birth (gestational age ≤ 37 weeks) | 23 | 9 (20.93%) | 14 (23.72%) | 0.30 |
| Small weight at birth (birth weight ≤ 2500 g) | 14 | 5 (11.62%) | 9 (15.52%) | 0.28 |
| At least one episode of pneumonia | 45 | 19 (44.18%) | 26 (44.06%) | 0.29 |
| Incomplete Rickets prophylaxis | 34 | 19 (44.18%) | 15 (25.42%) | 0.49 |
| Smoke exposure at home | 48 | 22 (51.16%) | 26 (44.06%) | 0.56 |
| Allergy | 17 | 6 (13.95%) | 11 (18.64%) | 0.22 |
| Sex | n | Mean PI | SD PI | CI | Severe Underweight | Moderate Underweight | Mild Underweight | Eutrophic | Overweight |
|---|---|---|---|---|---|---|---|---|---|
| Female | 43 | 1.00 | 0.27 | 0.91–1.08 | 3 (2.94%) | 2 (1.96%) | 11 (10.78%) | 17 (16.66%) | 10 (9.8%) |
| Male | 59 | 1.03 | 0.31 | 0.95–1.12 | 1 (0.98%) | 6 (5.88%) | 15 (14.70%) | 21 (20.58%) | 16 (15.68%) |
| Z-Score (Value) | Percentile (%) | <5 Years n = 58 (%) | >5 Years n = 48 (%) | |
|---|---|---|---|---|
| Severe underweight for age | <−3 SD | <0.1% | 3 (5.17%) | 6 (13.63%) |
| Mild and moderate underweight for age | from −1 SD to −3 SD | 2.3–15.9% | 24 (41.37%) | 12 (27.27%) |
| Normal for age | from −1 SD to 1 SD | 15.9–84.1% | 26 (44.82%) | 18 (40.9%) |
| Possible risk of being overweight | >1 SD | >84.1% | 3 (5.17%) | 3 (6.81%) |
| Overweight for age | >2 SD | >97.7% | 2 (3.44%) | 5 (11.36%) |
| Age/ Patients in Age Group (n) | Normal Leucocyte Count (×103/mmc) | Leucocyte Range in Study Group (×103/mmc) | Leucopenia in Study Group n (%) | Leukocytosis in Study Group n (%) |
|---|---|---|---|---|
| 1 month—1 year (n = 15) | 6–17.5 | 6.7–20.6 | 1 (6.66%) | 4 (26.66%) |
| 1–12 years (n = 75) | 5–14.5 | 2.3–29.17 | 5 (6.66%) | 21 (28%) |
| 13–18 years (n = 12) | 4.5–14.5 | 4.5–15.5 | 1 (8.33%) | 1 (8.33%) |
| Age/ Patients in Age Group (n) | Absolute Neutrophil Normal Count (×103/mmc) | Neutrophil Normal Range (%) | Neutropenia in Study Group n (%) | Neutrophilia in Study Group n (%) |
|---|---|---|---|---|
| 1 month–1 year (n = 15) | 1–9 | 20–48 | - | 5 (33.33%) |
| 1–6 years (n = 51) | 1.5–8 | 37–71 | 6 (11.74%) | 15 (29.41%) |
| 7–18 years (n = 36) | 1.8–8 | 33–76 | - | 13 (36.11%) |
| Variable | Estimate | Std. Error | z-Value | p-Value |
|---|---|---|---|---|
| Intercept | 1.86 | 0.30 | 6.17 | <0.001 * |
| Weight | 0.01 | 0.01 | 1.63 | 0.10 |
| Smoker at Home | −0.74 | 0.29 | −2.54 | 0.01 * |
| Smoker Parents | 0.57 | 0.29 | 1.94 | 0.05 |
| Nursery (vs. home) | −0.40 | 0.17 | −2.42 | 0.02 * |
| Kindergarten (vs. home) | −0.38 | 0.15 | −2.61 | 0.01 * |
| School (vs. home) | −0.46 | 0.18 | −2.56 | 0.01 * |
| High School (vs. home) | −0.68 | 0.33 | −2.03 | 0.04 * |
| Ponderal Index | −0.72 | 0.23 | −3.17 | <0.01 * |
| Urban Environment (vs. Rural) | 0.19 | 0.10 | 1.85 | 0.06 |
| Antibiotic Use at Home | 0.20 | 0.10 | 1.88 | 0.06 |
| Neutrophil % (24 h) | 0.01 | 0.00 | 1.46 | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isac, R.; Cugerian-Ratiu, A.-M.; Micsescu-Olah, A.-M.; Bodescu, A.D.; Vlad, L.-A.; Zaroniu, A.M.; Gafencu, M.; Doros, G. Can Lung Ultrasound Act as a Diagnosis and Monitoring Tool in Children with Community Acquired Pneumonia? Correlation with Risk Factors, Clinical Indicators and Biologic Results. J. Clin. Med. 2025, 14, 5304. https://doi.org/10.3390/jcm14155304
Isac R, Cugerian-Ratiu A-M, Micsescu-Olah A-M, Bodescu AD, Vlad L-A, Zaroniu AM, Gafencu M, Doros G. Can Lung Ultrasound Act as a Diagnosis and Monitoring Tool in Children with Community Acquired Pneumonia? Correlation with Risk Factors, Clinical Indicators and Biologic Results. Journal of Clinical Medicine. 2025; 14(15):5304. https://doi.org/10.3390/jcm14155304
Chicago/Turabian StyleIsac, Raluca, Alexandra-Monica Cugerian-Ratiu, Andrada-Mara Micsescu-Olah, Alexandra Daniela Bodescu, Laura-Adelina Vlad, Anca Mirela Zaroniu, Mihai Gafencu, and Gabriela Doros. 2025. "Can Lung Ultrasound Act as a Diagnosis and Monitoring Tool in Children with Community Acquired Pneumonia? Correlation with Risk Factors, Clinical Indicators and Biologic Results" Journal of Clinical Medicine 14, no. 15: 5304. https://doi.org/10.3390/jcm14155304
APA StyleIsac, R., Cugerian-Ratiu, A.-M., Micsescu-Olah, A.-M., Bodescu, A. D., Vlad, L.-A., Zaroniu, A. M., Gafencu, M., & Doros, G. (2025). Can Lung Ultrasound Act as a Diagnosis and Monitoring Tool in Children with Community Acquired Pneumonia? Correlation with Risk Factors, Clinical Indicators and Biologic Results. Journal of Clinical Medicine, 14(15), 5304. https://doi.org/10.3390/jcm14155304






