Multiple Organ Failure as a Strong Predictor of Mortality in Patients with Hypoxic Hepatitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Definitions of Predisposing Conditions
2.4. Definitions of Organ Failure
2.5. Statistical Analysis
2.6. Ethics Statement
3. Results
3.1. Patient Characteristics
3.2. Organ Failure and Mortality
3.3. Multiple Organ Failure in Hypoxic Hepatitis
3.4. Types of Organ Failure According to Predisposing Conditions
3.5. Predictors Associated with 30-Day Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Henrion, J. Hypoxic hepatitis. Liver Int. 2012, 32, 1039–1052. [Google Scholar] [CrossRef]
- Tapper, E.B.; Sengupta, N.; Bonder, A. The Incidence and Outcomes of Ischemic Hepatitis: A Systematic Review with Meta-analysis. Am. J. Med. 2015, 128, 1314–1321. [Google Scholar] [CrossRef]
- Aboelsoud, M.M.; Javaid, A.I.; Al-Qadi, M.O.; Lewis, J.H. Hypoxic hepatitis—Its biochemical profile, causes and risk factors of mortality in critically-ill patients: A cohort study of 565 patients. J. Crit. Care 2017, 41, 9–15. [Google Scholar] [CrossRef]
- Van den Broecke, A.; Van Coile, L.; Decruyenaere, A.; Colpaert, K.; Benoit, D.; Van Vlierberghe, H.; Decruyenaere, J. Epidemiology, causes, evolution and outcome in a single-center cohort of 1116 critically ill patients with hypoxic hepatitis. Ann. Intensive Care 2018, 8, 15. [Google Scholar] [CrossRef]
- Drolz, A.; Horvatits, T.; Roedl, K.; Rutter, K.; Staufer, K.; Haider, D.G.; Zauner, C.; Heinz, G.; Schellongowski, P.; Kluge, S.; et al. Outcome and features of acute kidney injury complicating hypoxic hepatitis at the medical intensive care unit. Ann. Intensive Care 2016, 6, 61. [Google Scholar] [CrossRef]
- Jager, B.; Drolz, A.; Michl, B.; Schellongowski, P.; Bojic, A.; Nikfardjam, M.; Zauner, C.; Heinz, G.; Trauner, M.; Fuhrmann, V. Jaundice increases the rate of complications and one-year mortality in patients with hypoxic hepatitis. Hepatology 2012, 56, 2297–2304. [Google Scholar] [CrossRef]
- Fuhrmann, V.; Kneidinger, N.; Herkner, H.; Heinz, G.; Nikfardjam, M.; Bojic, A.; Schellongowski, P.; Angermayr, B.; Schoniger-Hekele, M.; Madl, C.; et al. Impact of hypoxic hepatitis on mortality in the intensive care unit. Intensive Care Med. 2011, 37, 1302–1310. [Google Scholar] [CrossRef]
- Fuhrmann, V.; Kneidinger, N.; Herkner, H.; Heinz, G.; Nikfardjam, M.; Bojic, A.; Schellongowski, P.; Angermayr, B.; Kitzberger, R.; Warszawska, J.; et al. Hypoxic hepatitis: Underlying conditions and risk factors for mortality in critically ill patients. Intensive Care Med. 2009, 35, 1397–1405. [Google Scholar] [CrossRef]
- Seeto, R.K.; Fenn, B.; Rockey, D.C. Ischemic hepatitis: Clinical presentation and pathogenesis. Am. J. Med. 2000, 109, 109–113. [Google Scholar] [CrossRef]
- Waseem, N.; Chen, P.H. Hypoxic Hepatitis: A Review and Clinical Update. J. Clin. Transl. Hepatol. 2016, 4, 263–268. [Google Scholar] [CrossRef]
- Raurich, J.M.; Llompart-Pou, J.A.; Ferreruela, M.; Colomar, A.; Molina, M.; Royo, C.; Ayestaran, I.; Ibanez, J. Hypoxic hepatitis in critically ill patients: Incidence, etiology and risk factors for mortality. J. Anesth. 2011, 25, 50–56. [Google Scholar] [CrossRef]
- Lie, K.C.; Lau, C.Y.; Van Vinh Chau, N.; West, T.E.; Limmathurotsakul, D.; for Southeast Asia Infectious Disease Clinical Research, N. Utility of SOFA score, management and outcomes of sepsis in Southeast Asia: A multinational multicenter prospective observational study. J. Intensive Care 2018, 6, 9. [Google Scholar] [CrossRef]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonca, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Moreno, R.; Vincent, J.L.; Matos, R.; Mendonca, A.; Cantraine, F.; Thijs, L.; Takala, J.; Sprung, C.; Antonelli, M.; Bruining, H.; et al. The use of maximum SOFA score to quantify organ dysfunction/failure in intensive care. Results of a prospective, multicentre study. Working Group on Sepsis related Problems of the ESICM. Intensive Care Med. 1999, 25, 686–696. [Google Scholar] [CrossRef]
- American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit. Care Med. 1992, 20, 864–874. [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Drolz, A.; Horvatits, T.; Michl, B.; Roedl, K.; Schellongowski, P.; Holzinger, U.; Zauner, C.; Heinz, G.; Madl, C.; Trauner, M.; et al. Statin therapy is associated with reduced incidence of hypoxic hepatitis in critically ill patients. J. Hepatol. 2014, 60, 1187–1193. [Google Scholar] [CrossRef]
- Henrion, J.; Schapira, M.; Luwaert, R.; Colin, L.; Delannoy, A.; Heller, F.R. Hypoxic hepatitis: Clinical and hemodynamic study in 142 consecutive cases. Medicine 2003, 82, 392–406. [Google Scholar] [CrossRef]
- Birrer, R.; Takuda, Y.; Takara, T. Hypoxic hepatopathy: Pathophysiology and prognosis. Intern. Med. 2007, 46, 1063–1070. [Google Scholar] [CrossRef]
- Allam, J.; Ibrahim, A.; Rockey, D.C. The primary cause of markedly elevated aminotransferases in hospitalized patients with cirrhosis in ischemic hepatitis. Eur. J. Gastroenterol. Hepatol. 2024, 36, 1346–1351. [Google Scholar] [CrossRef]
- Raurich, J.M.; Perez, O.; Llompart-Pou, J.A.; Ibanez, J.; Ayestaran, I.; Perez-Barcena, J. Incidence and outcome of ischemic hepatitis complicating septic shock. Hepatol. Res. 2009, 39, 700–705. [Google Scholar] [CrossRef]
- Schupp, T.; Rusnak, J.; Weidner, K.; Ruka, M.; Egner-Walter, S.; Dudda, J.; Forner, J.; Bertsch, T.; Mashayekhi, K.; Ayoub, M.; et al. Prognostic Value of the AST/ALT Ratio versus Bilirubin in Patients with Cardiogenic Shock. J. Clin. Med. 2023, 12, 5275. [Google Scholar] [CrossRef]
- Amitrano, L.; Guardascione, M.A.; Martino, R.; Manguso, F.; Menchise, A.; Balzano, A. Hypoxic hepatitis occurring in cirrhosis after variceal bleeding: Still a lethal disease. J. Clin. Gastroenterol. 2012, 46, 608–612. [Google Scholar] [CrossRef]
- Edwards, J.D. Oxygen transport in cardiogenic and septic shock. Crit. Care Med. 1991, 19, 658–663. [Google Scholar] [CrossRef]
- Zhang, H.; Vincent, J.L. Oxygen extraction is altered by endotoxin during tamponade-induced stagnant hypoxia in the dog. Circ. Shock 1993, 40, 168–176. [Google Scholar]
- Horvatits, T.; Trauner, M.; Fuhrmann, V. Hypoxic liver injury and cholestasis in critically ill patients. Curr. Opin. Crit. Care 2013, 19, 128–132. [Google Scholar] [CrossRef]
- Rank, N.; Michel, C.; Haertel, C.; Lenhart, A.; Welte, M.; Meier-Hellmann, A.; Spies, C. N-acetylcysteine increases liver blood flow and improves liver function in septic shock patients: Results of a prospective, randomized, double-blind study. Crit. Care Med. 2000, 28, 3799–3807. [Google Scholar] [CrossRef]
- Maiwall, R.; Kumar, A.; Bhadoria, A.S.; Jindal, A.; Kumar, G.; Bhardwaj, A.; Maras, J.S.; Sharma, M.K.; Sharma, B.C.; Sarin, S.K. Utility of N-acetylcysteine in ischemic hepatitis in cirrhotics with acute variceal bleed: A randomized controlled trial. Hepatol. Int. 2020, 14, 577–586. [Google Scholar] [CrossRef]

| Characteristics | N = 1011 |
|---|---|
| Age, years | 69.0 (56.0–78.0) |
| Male gender | 611 (60.4%) |
| Diabetes | 286 (28.3%) |
| Liver cirrhosis | 130 (12.9%) |
| Hepatic decompensation | 91 (9.0%) |
| Infection | 295 (29.2%) |
| Vasopressor support | 550 (54.4%) |
| Mechanical ventilation | 380 (37.6%) |
| Renal replacement therapy | 243 (24.0%) |
| Admission at the intensive care unit | 555 (54.9%) |
| Predisposing conditions | |
| Circulatory shock | 148 (14.6%) |
| Cardiac dysfunction | 375 (37.1%) |
| Respiratory dysfunction | 168 (16.6%) |
| Sepsis | 293 (29.0%) |
| Others | 27 (2.7%) |
| Type of organ failures | |
| Liver failure | 73 (7.2%) |
| Renal failure | 236 (23.3%) |
| Cerebral failure | 307 (30.4%) |
| Coagulation failure | 182 (18.0%) |
| Circulatory failure | 521 (51.5%) |
| Respiratory failure | 380 (37.6%) |
| No organ failure | 290 (28.7%) |
| Initial laboratory values | |
| AST, U/L | 838.0 (557.0–1720.0) |
| ALT, U/L | 489.0 (251.0–882.0) |
| Albumin, g/dL | 2.8 (2.3–3.3) |
| Bilirubin, mg/dL | 1.4 (0.8–2.6) |
| ALP, U/L | 102.0 (71.0–161.0) |
| LDH, U/L * | 1042.0 (557.0–2046.0) |
| Creatinine, mg/dL | 1.48 (0.97–2.30) |
| PT-INR | 1.57 (1.24–2.14) |
| Platelet, ×109/L | 130.0 (66.0–204.0) |
| Median AST/ALT ratio | 2.0 (1.3–3.5) |
| Median LDH/AST ratio | 1.1 (0.7–1.9) |
| Median LDH/ALT ratio | 2.3 (1.0–4.7) |
| Peak laboratory values | |
| AST, U/L | 1316.0 (664.0–2699.0) |
| ALT, U/L | 664.0 (344.0–1439.0) |
| Median AST/ALT ratio | 2.0 (1.3–3.6) |
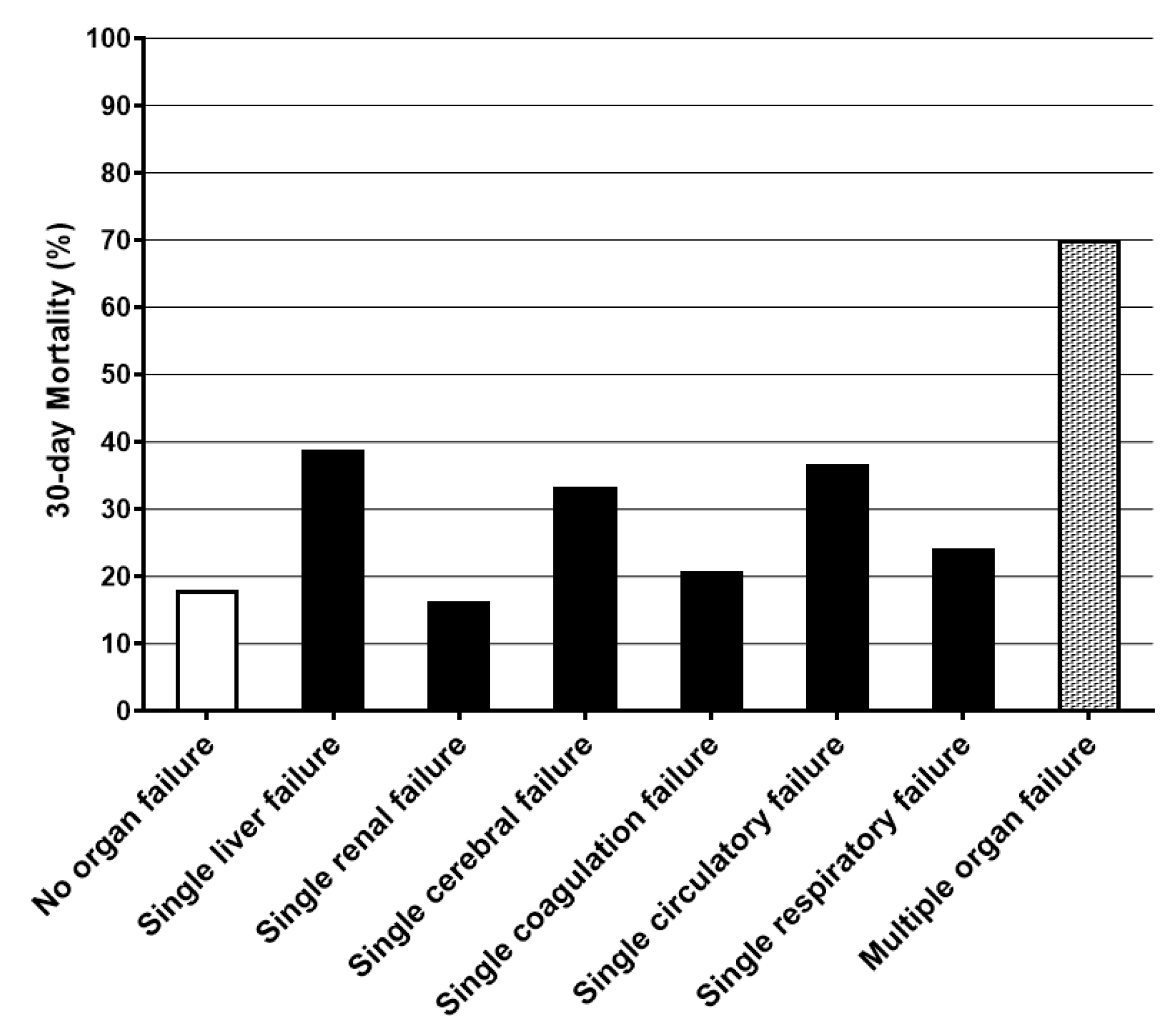
| Number of Organ Failures | Prevalence (%) | 30-Day Mortality (%) | p |
|---|---|---|---|
| No organ failure | 290 (28.7%) | 52 (17.9%) | Reference |
| Single organ failure | 225 (22.3%) | 66 (29.3%) | 0.003 |
| Liver failure | 18 | 7 (38.9%) | 0.056 |
| Renal failure | 37 | 6 (16.2%) | 1.000 |
| Cerebral failure | 21 | 7 (33.3%) | 0.089 |
| Coagulation failure | 29 | 6 (20.7%) | 0.800 |
| Circulatory failure | 87 | 32 (36.8%) | <0.001 |
| Respiratory failure | 33 | 8 (24.2%) | 0.353 |
| Multiple organ failure | 496 (49.1%) | 347 (70.0%) | <0.001 |
| Two organ failures | 203 | 122 (60.1%) | <0.001 |
| Three organ failures | 161 | 112 (69.6%) | <0.001 |
| Four organ failures or more | 132 | 113 (85.6%) | <0.001 |
| Predisposing Condition | No Organ Failure | Single Organ Failure | Multiple Organ Failure | 30-Day Mortality |
|---|---|---|---|---|
| Circulatory shock (n = 148) | 44 (29.7%) | 28 (18.9%) | 76 (51.3%) | 58 (39.2%) |
| Cardiac dysfunction (n = 375) | 140 (37.3%) | 97 (25.9%) | 138 (36.8%) | 145 (38.7%) |
| Respiratory dysfunction (n = 168) | 38 (22.6%) | 41 (24.4%) | 89 (53.0%) | 90 (53.6%) |
| Sepsis (n = 293) | 51 (17.4%) | 55 (18.8%) | 187 (63.8%) | 167 (57.0%) |
| Others (n = 27) | 17 (63.0%) | 4 (14.8%) | 6 (22.2%) | 5 (18.5%) |
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| p | HR (95% CI) | p | HR (95% CI) | |
| Age, per year | <0.001 | 1.012 (1.005–1.018) | <0.001 | 1.018 (1.011–1.025) |
| Predisposing conditions | ||||
| Circulatory shock/cardiac dysfunction/others | reference | reference | ||
| Respiratory dysfunction/sepsis | <0.001 | 1.604 (1.335–1.926) | 0.586 | 1.056 (0.869–1.283) |
| AST per 100 U/L | <0.001 | 1.009 (1.006–1.013) | 0.040 | 1.004 (1.000–1.007) |
| Albumin per g/dL | <0.001 | 0.447 (0.389–0.514) | <0.001 | 0.656 (0.562–0.766) |
| PT-INR | <0.001 | 1.098 (1.056–1.142) | 0.020 | 1.061 (1.009–1.114) |
| Hepatic decompensation | <0.001 | 1.736 (1.331–2.265) | 0.214 | 1.194 (0.903–1.579) |
| MOF grades * | ||||
| Grade 0 | reference | reference | ||
| Grade 1 | <0.001 | 3.397 (2.636–4.378) | <0.001 | 2.866 (2.206–3.726) |
| Grade 2 | <0.001 | 4.619 (3.562–5.990) | <0.001 | 3.912 (2.975–5.145) |
| Grade 3 | <0.001 | 6.936 (5.335–9.017) | <0.001 | 5.008 (3.706–6.768) |
| Author | Year | N | Population | AST or ALT Cutoff (U/L) | Mortality |
|---|---|---|---|---|---|
| Henrion [18] | 2003 | 142 | ICU | >800 | 53% |
| Birrer [19] | 2007 | 322 | ICU | >400 | 45% |
| Tapper [2] | 2015 | 1782 | Meta-analysis | >300 | 50% |
| Aboelsoud [3] | 2017 | 565 | ICU | >800 | 44% |
| Broecke [4] | 2018 | 1116 | ICU | >155 (females) >185 (males) | 45% |
| Allam [20] | 2024 | 108 | Cirrhosis | >1000 | 73% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwak, J.Y.; Jeon, H.; Kwon, H.U.; Kim, J.E.; Kim, S.J.; Han, J.H.; Cha, R.R.; Lee, J.M.; Lee, S.S. Multiple Organ Failure as a Strong Predictor of Mortality in Patients with Hypoxic Hepatitis. J. Clin. Med. 2025, 14, 5286. https://doi.org/10.3390/jcm14155286
Kwak JY, Jeon H, Kwon HU, Kim JE, Kim SJ, Han JH, Cha RR, Lee JM, Lee SS. Multiple Organ Failure as a Strong Predictor of Mortality in Patients with Hypoxic Hepatitis. Journal of Clinical Medicine. 2025; 14(15):5286. https://doi.org/10.3390/jcm14155286
Chicago/Turabian StyleKwak, Ji Yoon, Hankyu Jeon, Hyeon Uk Kwon, Jae Eun Kim, Seong Je Kim, Ji Hee Han, Ra Ri Cha, Jae Min Lee, and Sang Soo Lee. 2025. "Multiple Organ Failure as a Strong Predictor of Mortality in Patients with Hypoxic Hepatitis" Journal of Clinical Medicine 14, no. 15: 5286. https://doi.org/10.3390/jcm14155286
APA StyleKwak, J. Y., Jeon, H., Kwon, H. U., Kim, J. E., Kim, S. J., Han, J. H., Cha, R. R., Lee, J. M., & Lee, S. S. (2025). Multiple Organ Failure as a Strong Predictor of Mortality in Patients with Hypoxic Hepatitis. Journal of Clinical Medicine, 14(15), 5286. https://doi.org/10.3390/jcm14155286







