Management of Aberrant Internal Carotid Artery Injury Caused During Otologic Procedures: Systematic Review and Multicenter Case Series
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Case Series
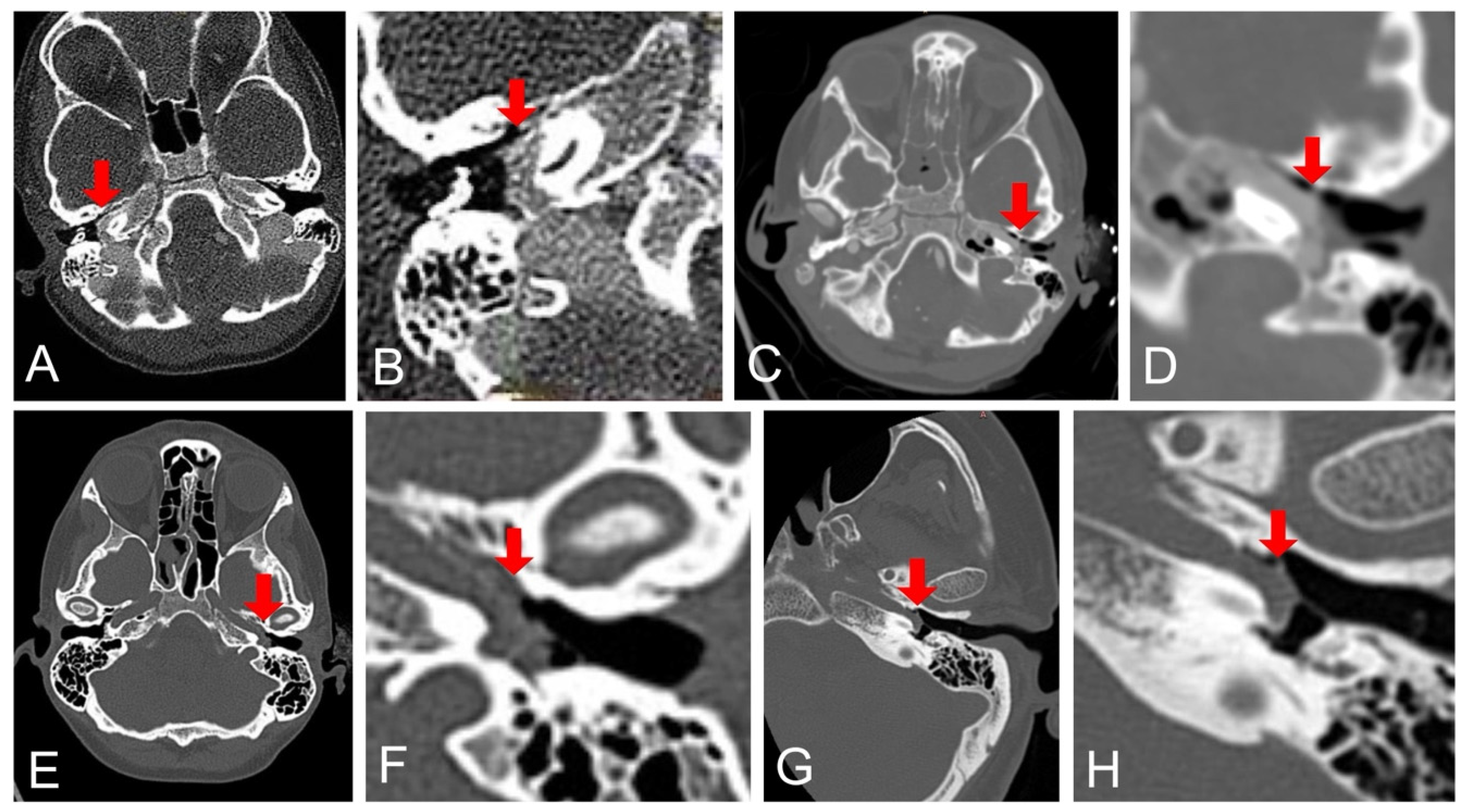
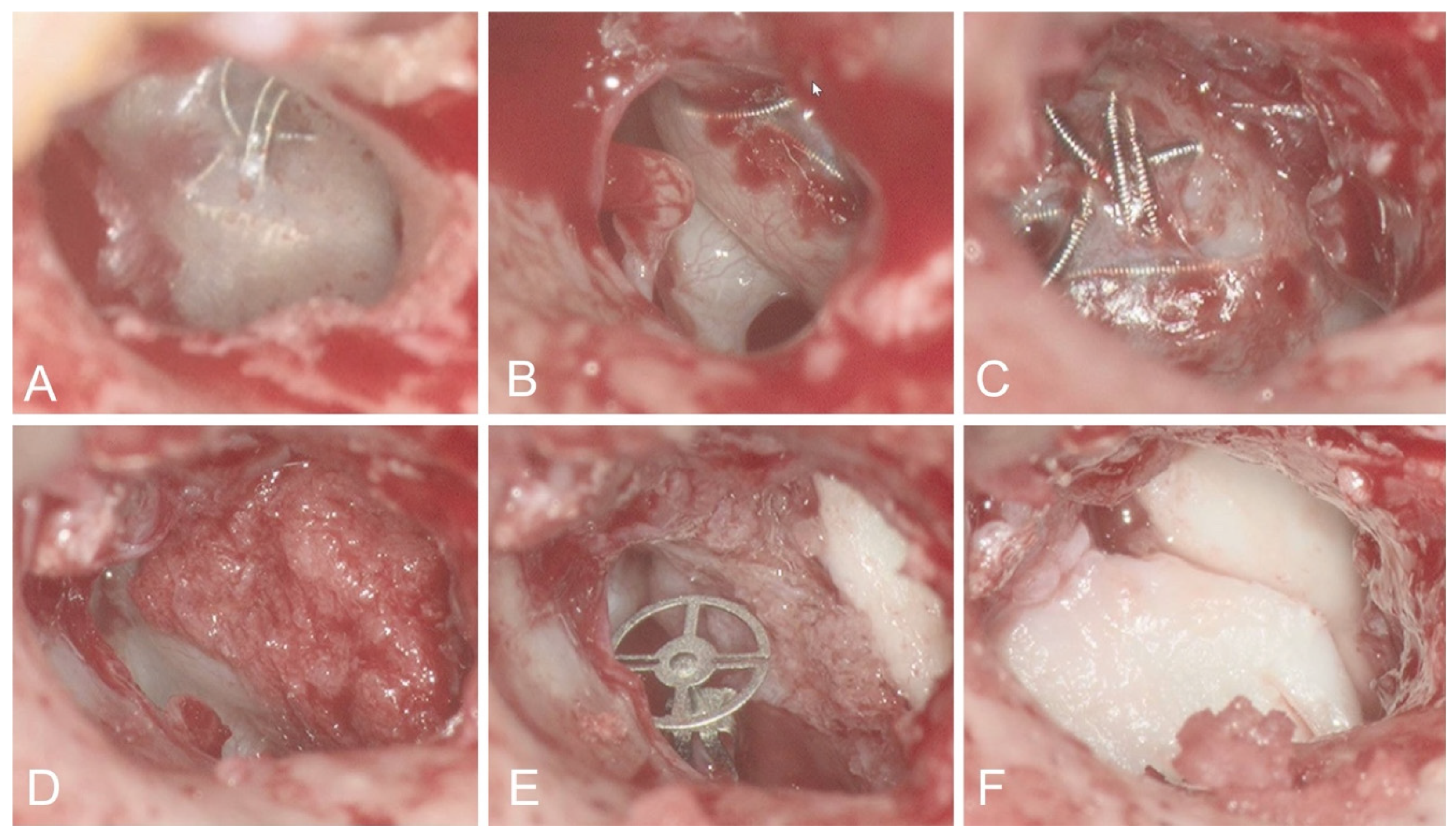
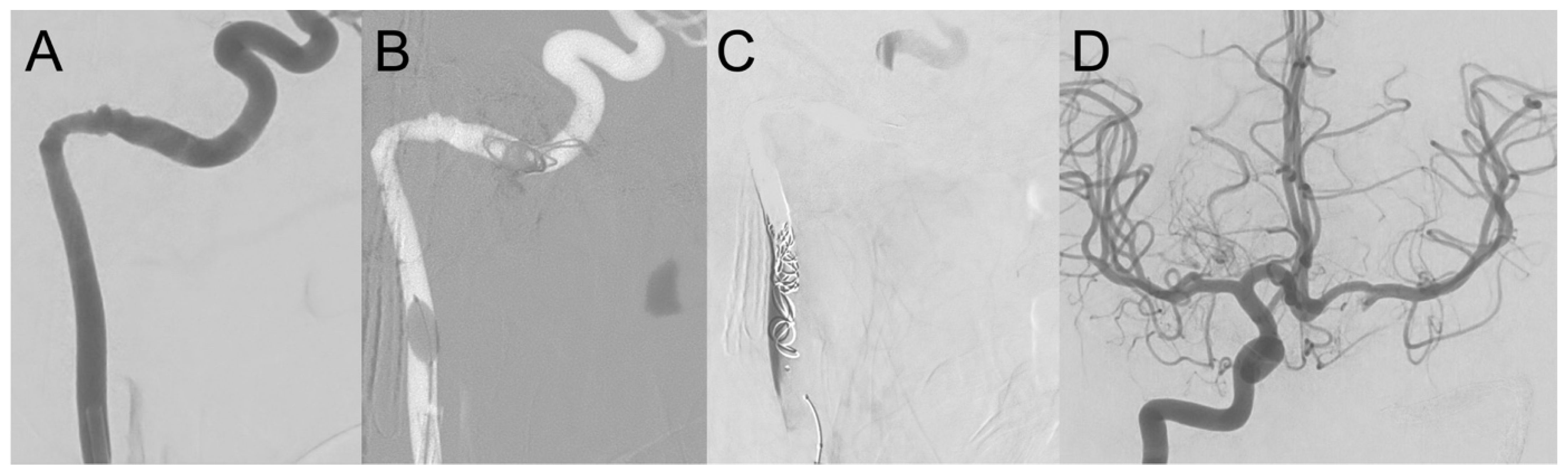
3.1.1. Case 1
3.1.2. Case 2
3.1.3. Case 3
3.1.4. Case 4
3.2. Systematic Review
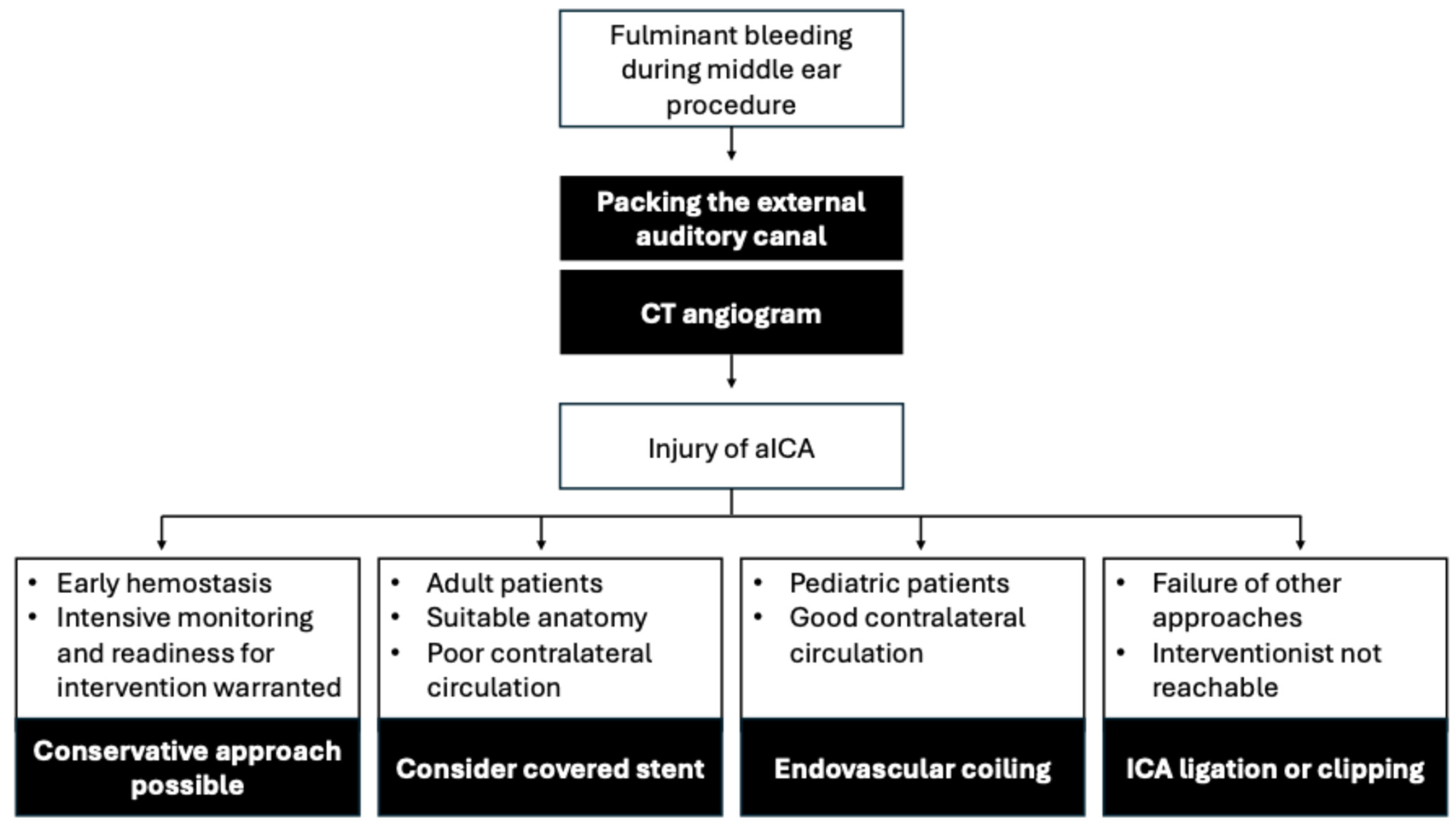
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sauvaget, E.; Paris, J.; Kici, S.; Kania, R.; Guichard, J.P.; Chapot, R.; Thomassin, J.M.; Herman, P.; Tran Ba Huy, P. Aberrant Internal Carotid Artery in the Temporal Bone: Imaging Findings and Management. Arch. Otolaryngol. Head Neck Surg. 2006, 132, 86–91. [Google Scholar] [CrossRef]
- Saito, H.; Chikamori, Y.; Yanagihara, N. Aberrant Carotid Artery in the Middle Ear. Arch. Otorhinolaryngol. 1975, 209, 83–87. [Google Scholar] [CrossRef]
- Bonnard, D.; De Monès, E.; Sagardoy, T.; Franco-Vidal, V.; Darrouzet, V.; Fierens, S. Transtympanic Pseudoaneurysm of the Internal Carotid Artery Complicating a Myringotomy in a Four-Year Old Child: Case Report and Literature Review. Am. J. Otolaryngol. 2017, 38, 713–717. [Google Scholar] [CrossRef]
- Hunt, J.T.; Andrews, T.M. Management of Aberrant Internal Carotid Artery Injuries in Children. Am. J. Otolaryngol. 2000, 21, 50–54. [Google Scholar] [CrossRef]
- Lasjaunias, P.; Moret, J. Normal and Non-Pathological Variations in the Angiographic Aspects of the Arteries of the Middle Ear. Neuroradiology 1978, 15, 213–219. [Google Scholar] [CrossRef]
- Lasjaunias, P.; Santoyo-Vazquez, A. Segmental Agenesis of the Internal Carotid Artery: Angiographic Aspects with Embryological Discussion. Anat. Clin. 1984, 6, 133–141. [Google Scholar] [CrossRef]
- Song, Y.-S.; Yuan, Y.-Y.; Wang, G.-J.; Dai, P.; Han, D.-Y. Aberrant Internal Carotid Artery Causing Objective Pulsatile Tinnitus and Conductive Hearing Loss. Acta Oto-Laryngol. 2012, 132, 1126–1130. [Google Scholar] [CrossRef]
- Hashim, N.D.; Jang, S.H.; Moon, I.S. Endoscopic Intervention of Aberrant Carotid Artery in the Middle Ear. Otol. Neurotol. 2021, 42, e82. [Google Scholar] [CrossRef]
- Windfuhr, J.P. Aberrant Internal Carotid Artery in the Middle Ear. Ann. Otol. Rhinol. Laryngol. Suppl. 2004, 192, 1–16. [Google Scholar] [CrossRef]
- Steele, D.W.; Adam, G.P.; Di, M.; Halladay, C.H.; Balk, E.M.; Trikalinos, T.A. Effectiveness of Tympanostomy Tubes for Otitis Media: A Meta-Analysis. Pediatrics 2017, 139, e20170125. [Google Scholar] [CrossRef]
- Smith, N.; Greinwald, J.J. To Tube or Not to Tube: Indications for Myringotomy with Tube Placement. Curr. Opin. Otolaryngol. Head Neck Surg. 2011, 19, 363. [Google Scholar] [CrossRef]
- Rimmer, J.; Giddings, C.E.B.; Weir, N. History of Myringotomy and Grommets. J. Laryngol. Otol. 2007, 121, 911–916. [Google Scholar] [CrossRef]
- Schwam, Z.G.; Cosetti, M.K. Endoscopic Myringoplasty and Type I Tympanoplasty. Otolaryngol. Clin. North Am. 2021, 54, 75–88. [Google Scholar] [CrossRef]
- Darouassi, Y.; Aljalil, A.; Ennouali, A.; Hanine, M.A.; Chebraoui, Y.; Bouaity, B.; Touati, M.M.; Ammar, H. Prognostic Factors of Myringoplasty: Study of a 140 Cases Series and Review of the Literature. Pan Afr. Med. J. 2019, 33, 323. [Google Scholar] [CrossRef]
- Aggarwal, R.; Saeed, S.R.; Green, K.J.M. Myringoplasty. J. Laryngol. Otol. 2006, 120, 429–432. [Google Scholar] [CrossRef]
- Aladeyelu, O.S.; Olojede, S.O.; Lawal, S.K.; Mbatha, W.-B.E.; Sibiya, A.L.; Rennie, C.O. Influence of Pneumatization on Morphology of Temporal Bone-Related Vasculatures and Their Morphometric Relationship with Ear Regions: A Computed Tomography Study. Sci. Rep. 2023, 13, 1996. [Google Scholar] [CrossRef]
- Visvanathan, V.; Morrissey, M.S.C. Anatomical Variations of the Temporal Bone on High-Resolution Computed Tomography Imaging: How Common Are They? J. Laryngol. Otol. 2015, 129, 634–637. [Google Scholar] [CrossRef]
- Koesling, S.; Kunkel, P.; Schul, T. Vascular Anomalies, Sutures and Small Canals of the Temporal Bone on Axial CT. Eur. J. Radiol. 2005, 54, 335–343. [Google Scholar] [CrossRef]
- Wadhavkar, N.; Goldrich, D.Y.; Roychowdhury, S.; Kwong, K. Laceration of Aberrant Internal Carotid Artery Following Myringotomy: A Case Report and Review of Literature. Ann. Otol. Rhinol. Laryngol. 2022, 131, 555–561. [Google Scholar] [CrossRef]
- Brodish, B.N.; Woolley, A.L. Major Vascular Injuries in Children Undergoing Myringotomy for Tube Placement. Am. J. Otolaryngol. 1999, 20, 46–50. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological Quality of Case Series Studies: An Introduction to the JBI Critical Appraisal Tool. JBI Evid. Synth. 2020, 18, 2127. [Google Scholar] [CrossRef]
- Glauser, G.; Walcott, B.P.; Choudhri, O.A. Parent Vessel Occlusion via the Balloon-Assisted, Dual Microcatheter Technique. Asian J. Neurosurg. 2020, 15, 726–729. [Google Scholar] [CrossRef]
- Schutt, C.; Dissanaike, S.; Marchbanks, J. Case Report: Inadvertent Carotid Artery Injury during Myringotomy as a Result of Carotid Artery Dehiscence. Ear Nose Throat J. 2013, 92, E35–E37. [Google Scholar] [CrossRef]
- Henriksen, S.D.; Kindt, M.W.; Pedersen, C.B.; Nepper-Rasmussen, H.J. Pseudoaneurysm of a Lateral Internal Carotid Artery in the Middle Ear. Int. J. Pediatr. Otorhinolaryngol. 2000, 52, 163–167. [Google Scholar] [CrossRef]
- Knox, W.J.; Milburn, J.M.; Dawson, R. Bilateral Aberrant Internal Carotid Arteries: Treatment of a Hemorrhagic Complication. Am. J. Otolaryngol. 2007, 28, 212–217. [Google Scholar] [CrossRef]
- Macht, S.; Mathys, C.; Schipper, J.; Turowski, B. Initial Experiences with the Amplatzer Vascular Plug 4 for Permanent Occlusion of the Internal Carotid Artery in the Skull Base in Patients with Head and Neck Tumors. Neuroradiology 2012, 54, 61–64. [Google Scholar] [CrossRef]
- Saylam, G.; Tulgar, M.; Saatci, I.; Korkmaz, H. Iatrogenic Carotid Artery Pseudoaneurysm Presenting with Conductive Hearing Loss. Am. J. Otolaryngol. 2009, 30, 141–144. [Google Scholar] [CrossRef]
- Jain, R.; Marotta, T.R.; Redekop, G.; Vancouver, D.W.A. Management of Aberrant Internal Carotid Artery Injury: A Real Emergency. Otolaryngol. Head Neck Surg. 2002, 127, 470–473. [Google Scholar] [CrossRef]
- Reilly, J.J., Jr.; Caparosa, R.J.; Latchaw, R.E.; Sheptak, P.E. Aberrant Carotid Artery Injured at Myringotomy: Control of Hemorrhage by a Balloon Catheter. JAMA 1983, 249, 1473–1475. [Google Scholar] [CrossRef]
- Andersen, P.E.; Kjeldsen, A.D. Long-Term Follow-up after Embolization of Pulmonary Arteriovenous Malformations with Detachable Silicone Balloons. Cardiovasc. Interv. Radiol. 2008, 31, 569–574. [Google Scholar] [CrossRef]
- Wang, A.Y.-C.; Chen, C.-C.; Lai, H.-Y.; Lee, S.-T. Balloon Test Occlusion of the Internal Carotid Artery with Stump Pressure Ratio and Venous Phase Delay Technique. J. Stroke Cerebrovasc. Dis. 2013, 22, e533–e540. [Google Scholar] [CrossRef]
- Abud, D.G.; Spelle, L.; Piotin, M.; Mounayer, C.; Vanzin, J.R.; Moret, J. Venous Phase Timing during Balloon Test Occlusion as a Criterion for Permanent Internal Carotid Artery Sacrifice. AJNR Am. J. Neuroradiol. 2005, 26, 2602–2609. [Google Scholar]
- Becker, T.A.; Lewis, K.L.; Berns, H.F.; Robertson, S.E.; Clark, W.E.; Wells, J.C.; Alnajrani, M.K.; Rapoport, C.; Barhouse, P.; Ramirez-Velandia, F.; et al. Aneurysm Dome and Vessel Pressure Measurements with Coiling, Stent Assisted Coiling and Flow Diversion. Acta Neurochir. 2025, 167, 8. [Google Scholar] [CrossRef]
- Abdalkader, M.; Piotin, M.; Chen, M.; Ortega-Gutierrez, S.; Samaniego, E.; Weill, A.; Norbash, A.M.; Nguyen, T.N. Coil Migration during or after Endovascular Coiling of Cerebral Aneurysms. J. Neurointerv. Surg. 2020, 12, 505–511. [Google Scholar] [CrossRef]
- Ding, D.; Liu, K.C. Management Strategies for Intraprocedural Coil Migration during Endovascular Treatment of Intracranial Aneurysms. J. Neurointerv. Surg. 2014, 6, 428–431. [Google Scholar] [CrossRef]
- Alexander, M.J.; Smith, T.P.; Tucci, D.L. Treatment of an Iatrogenic Petrous Carotid Artery Pseudoaneurysm with a Symbiot Covered Stent: Technical Case Report. Neurosurgery 2002, 50, 658–662. [Google Scholar] [CrossRef]
- Maras, D.; Lioupis, C.; Magoufis, G.; Tsamopoulos, N.; Moulakakis, K.; Andrikopoulos, V. Covered Stent-Graft Treatment of Traumatic Internal Carotid Artery Pseudoaneurysms: A Review. Cardiovasc. Interv. Radiol. 2006, 29, 958–968. [Google Scholar] [CrossRef]
- Gnagi, S.H.; Chong, B.W.; Schraff, S.A. Aberrant Carotid Artery Injury during Myringotomy. Int. J. Pediatr. Otorhinolaryngol. Extra 2016, 13, 47–49. [Google Scholar] [CrossRef]
- Takano, K.; Wanibuchi, M.; Ito, F.; Himi, T. Pseudoaneurysm of an Aberrant Internal Carotid Artery in the Middle Ear Caused by Myringotomy. Auris Nasus Larynx 2016, 43, 698–701. [Google Scholar] [CrossRef]
- Welling, D.B.; Glasscock, M.E.; Tarasidis, N. Management of Carotid Artery Hemorrhage in Middle Ear Surgery. Otolaryngol. Head Neck Surg. 1993, 109, 996–999. [Google Scholar] [CrossRef]
- Sinnreich, A.I.; Parisier, S.C.; Cohen, N.L.; Berreby, M. Arterial Malformations of the Middle Ear. Otolaryngol. Head Neck Surg. 1984, 92, 194–206. [Google Scholar] [CrossRef]
- Goodman, R.S.; Cohen, N.L. Aberrant Internal Carotid Artery in the Middle Ear. Ann. Otol. Rhinol. Laryngol. 1981, 90, 67–69. [Google Scholar] [CrossRef]
- Goldman, N.C.; Singleton, G.T.; Holly, E.H. Aberrant Internal Carotid Artery Presenting as a Mass in the Middle Ear. Arch. Otolaryngol. 1971, 94, 269–273. [Google Scholar] [CrossRef]
- Baines, H.E.; Allcock, J.M.; Babb, J.W. Aberrant Carotid Artery Simulating a Tumor of the Middle Ear. Can. J. Otolaryngol. 1974, 3, 212–215. [Google Scholar]
- Friedberg, E.B.; Corn, D.; Prologo, J.D.; Fleishon, H.; Pyatt, R.; Duszak, R.; Cook, P. Access to Interventional Radiology Services in Small Hospitals and Rural Communities: An ACR Membership Intercommission Survey. J. Am. Coll. Radiol. 2019, 16, 185–193. [Google Scholar] [CrossRef]
- Guan, J.J.; Elhakim, T.; Matsumoto, M.M.; McKeon, T.; Laage-Gaupp, F.; Iqbal, S.; Patel, P.J.; Pereira, P.; Tam, A.L.; Binkert, C.; et al. Results of a Global Survey on the State of Interventional Radiology 2024. J. Vasc. Interv. Radiol. 2025, 36, 751–760.e5. [Google Scholar] [CrossRef]
- Ruggles, R.L.; Reed, R.C. Symposium on Ear Surgery. V. Treatment of Aberrant Carotid Arteries in the Middle Ear: A Report of Two Cases. Laryngoscope 1972, 82, 1199–1205. [Google Scholar] [CrossRef]
- Kawamura, Y.; Sayama, T.; Maehara, N.; Nishimura, A.; Iihara, K. Ruptured Aneurysm of an Aberrant Internal Carotid Artery Successfully Treated with Simultaneous Intervention and Surgery in a Hybrid Operating Room. World Neurosurg. 2017, 102, e1–e695. [Google Scholar] [CrossRef]
- Prasad, K.C.; Basava, C.H.; Gopinathan, P.N.; Induvarsha, G.; Harshita, R.T.; Ashok, B.K. A Revisit to High Jugular Bulb: A Newer Clinical Grading. Indian J. Otolaryngol. Head Neck Surg. 2018, 70, 527–530. [Google Scholar] [CrossRef]
- Sayit, A.T.; Gunbey, H.P.; Fethallah, B.; Gunbey, E.; Karabulut, E. Radiological and Audiometric Evaluation of High Jugular Bulb and Dehiscent High Jugular Bulb. J. Laryngol. Otol. 2016, 130, 1059–1063. [Google Scholar] [CrossRef]
- Carlson, M.L.; Sweeney, A.D.; Pelosi, S.; Wanna, G.B.; Glasscock, M.E., III; Haynes, D.S. Glomus Tympanicum: A Review of 115 Cases over 4 Decades. Otolaryngol. Head Neck Surg. 2015, 152, 136–142. [Google Scholar] [CrossRef]
- Offergeld, C.; Brase, C.; Yaremchuk, S.; Mader, I.; Rischke, H.C.; Gläsker, S.; Schmid, K.W.; Wiech, T.; Preuss, S.F.; Suárez, C.; et al. Head and Neck Paragangliomas: Clinical and Molecular Genetic Classification. Clinics 2012, 67, 19–28. [Google Scholar] [CrossRef]
- Ivan, M.E.; Sughrue, M.E.; Clark, A.J.; Kane, A.J.; Aranda, D.; Barani, I.J.; Parsa, A.T. A Meta-Analysis of Tumor Control Rates and Treatment-Related Morbidity for Patients with Glomus Jugulare Tumors: Clinical Article. J. Neurosurg. 2011, 114, 1299–1305. [Google Scholar] [CrossRef]

| Year | First Author | Age | Sex | Etiology | Treatment | Neurologic Outcome | Otologic Outcome |
|---|---|---|---|---|---|---|---|
| 2025 | Spörlein (this study) | 4 | M | Myringotomy | Packing, secondary coiling after recurrent bleeding | No deficit | No hearing loss |
| 7 | M | Repeat Myringotomy | Packing and primary coiling | No deficit | Coil extrusion, air–bone gap in lower frequencies < 20 dB | ||
| 39 | F | Myringotomy | Packing | No deficit | Normal hearing until 4 kHz, high-frequency hypoacusis | ||
| 69 | F | Tympanoplasty | Packing | Aphasia, hemiparesis | Deterioration of air conduction by an average of 10 dB and bone conduction by an average of 30 dB, air–bone gap < 15 dB | ||
| 2022 | Wadhavkar | 4 | F | Myringotomy | Packing and primary coiling | Hemiparesis, gaze deviation | NR |
| 2018 | Hudon | 7 * | F | Myringotomy | Packing for two weeks | No deficit | Air–bone gap < 40 dB |
| 2017 | Kawamura | 31 | F | Myringotomy | Packing, high-flow bypass with radial artery graft and coiling | No deficit | "Improvement of hearing and resolution of tinnitus" |
| 2017 | Bonnard | 3 | M | Myringotomy | Packing (Planned elective stenting was abandoned after thrombosis and involution of pseudoaneurysm) | No deficit | Air–bone gap 30 dB |
| 2016 | Takano | 3 * | F | Myringotomy | Packing 1 day, followed by ICA ligation after failed low-flow and high-flow bypass | Hemiparesis | NR |
| 2016 | Gnagi | 11 | M | Repeat Myringotomy | Packing, followed by high-flow bypass | Minor facial nerve weakness | NR |
| 2013 | Schutt | 3 | F | Repeat Myringotomy | Packing 7 days | No deficit | NR |
| 2013 | Hirono | 54 | F | Spontaneous during otitis media | Packing and primary coiling | Slight left hemiparesis | NR |
| 2009 | Saylam | 28 | F | Tympanoplasty | No initial treatment necessary, secondary balloon occlusion | No deficit | NR |
| 2009 | Leuin | 7 | F | Myringotomy | Packing and primary coiling | No deficit | Coil extrusion, mild conductive hearing loss after surgical treatment |
| 2007 | Knox | 23 * | F | Tympanoplasty | Packing, secondary coiling after recurrent bleeding | No deficit | NR |
| 2006 | Sauvaget | 37 | F | Exploratory tympanotomy | Packing | NR | NR |
| 34 | F | Exploratory tympanotomy | Packing, balloon occlusion | Hemiparesis | NR | ||
| 33 | M | Exploratory tympanotomy | Packing, coiling | NR | NR | ||
| 56 | F | Exploratory tympanotomy | Packing | NR | NR | ||
| 2002 | Jain | 20 | F | Spontaneous, cholesteatoma surgery 3 years before | Packing, primary balloon occlusion | No deficit | NR |
| 2002 | Alexander | 42 | F | Exploratory tympanotomy | Packing, covered stent | No deficit | NR |
| 2000 | Hunt and Andrews | 1.5 | M | Myringotomy | Intraoperative temporary packing | No deficit | No hearing loss |
| 2000 | Henriksen | 7 | F | Repeat Myringotomy | Packing, secondary balloon embolization after recurrent bleeding | No deficit | Temporary 30 dB conductive hearing loss, then normal hearing |
| 1999 | Brodish and Woolley | 5 | M | Myringotomy | Packing, secondary coiling after recurrent bleeding | No deficit | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spörlein, A.; Arndt, S.; Jakob, T.F.; Aschendorff, A.; Demerath, T.; Taschner, C.; Balcerowiak, A.; Rusin, P.; Rauch, A.-K.; Gawęcki, W. Management of Aberrant Internal Carotid Artery Injury Caused During Otologic Procedures: Systematic Review and Multicenter Case Series. J. Clin. Med. 2025, 14, 5285. https://doi.org/10.3390/jcm14155285
Spörlein A, Arndt S, Jakob TF, Aschendorff A, Demerath T, Taschner C, Balcerowiak A, Rusin P, Rauch A-K, Gawęcki W. Management of Aberrant Internal Carotid Artery Injury Caused During Otologic Procedures: Systematic Review and Multicenter Case Series. Journal of Clinical Medicine. 2025; 14(15):5285. https://doi.org/10.3390/jcm14155285
Chicago/Turabian StyleSpörlein, Andreas, Susan Arndt, Till F. Jakob, Antje Aschendorff, Theo Demerath, Christian Taschner, Andrzej Balcerowiak, Patrycja Rusin, Ann-Kathrin Rauch, and Wojciech Gawęcki. 2025. "Management of Aberrant Internal Carotid Artery Injury Caused During Otologic Procedures: Systematic Review and Multicenter Case Series" Journal of Clinical Medicine 14, no. 15: 5285. https://doi.org/10.3390/jcm14155285
APA StyleSpörlein, A., Arndt, S., Jakob, T. F., Aschendorff, A., Demerath, T., Taschner, C., Balcerowiak, A., Rusin, P., Rauch, A.-K., & Gawęcki, W. (2025). Management of Aberrant Internal Carotid Artery Injury Caused During Otologic Procedures: Systematic Review and Multicenter Case Series. Journal of Clinical Medicine, 14(15), 5285. https://doi.org/10.3390/jcm14155285








