Effect of an Optimized Clinical Pathway Protocol Including Fascia Iliaca Compartment Block on Delirium and Postoperative Complications in Elderly Hip Fracture Patients
Abstract
1. Introduction
2. Materials and Methods
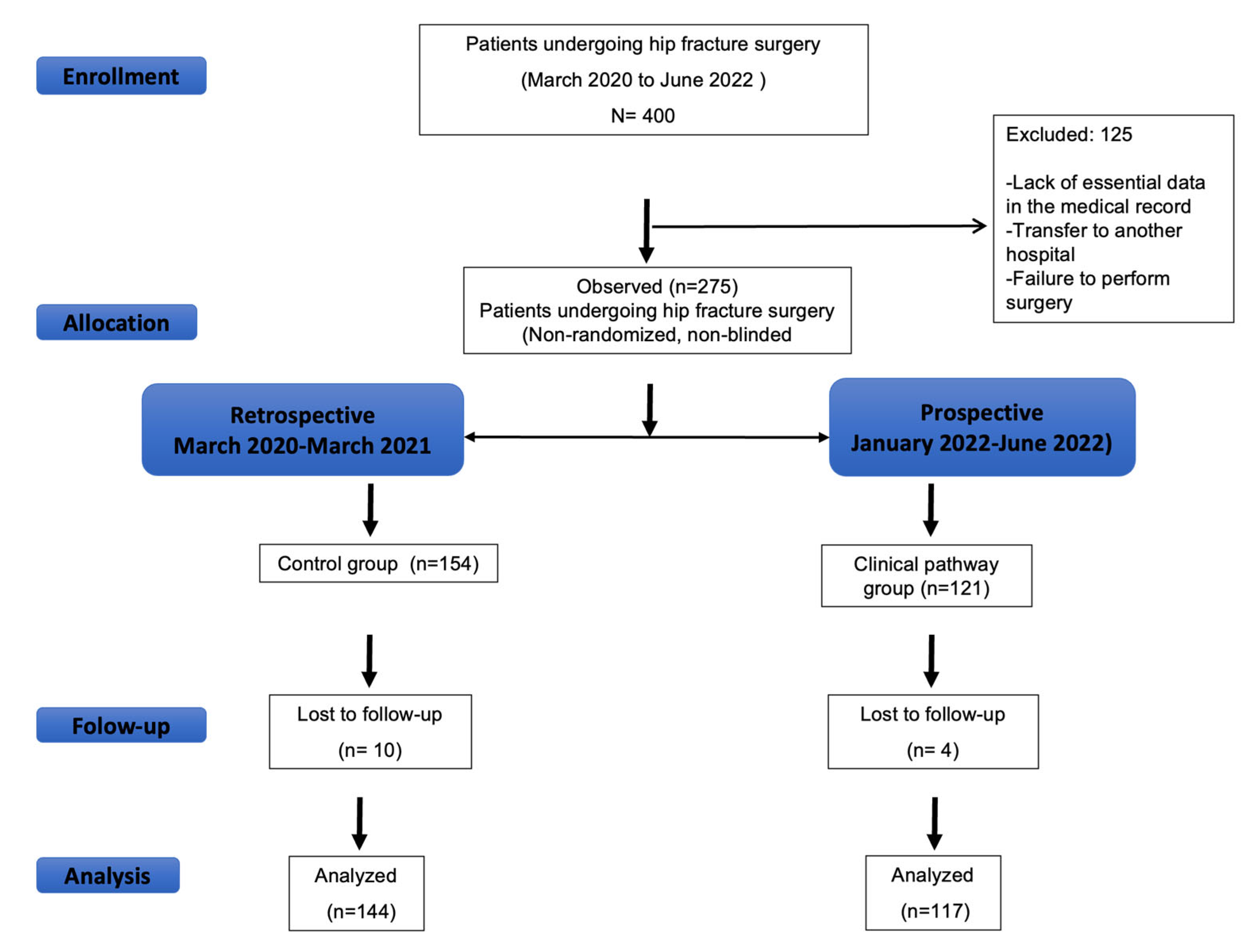
2.1. Study Population
2.2. Optimized Clinical Pathway Protocol
- o
- In patients receiving warfarin, treatment was discontinued, and vitamin K was administered. Spinal anesthesia was allowed provided that the International Normalized Ratio (INR) was <1.5.
- o
- Direct oral anticoagulants (DOACs):
- For patients taking apixaban, edoxaban, or rivaroxaban, spinal anesthesia was permitted after a cessation period equivalent to at least two elimination half-lives of the respective drug.
- For patients on dabigatran, a minimum withholding period of 48 h post-last dose was required before spinal anesthesia could be administered.
- In individuals with renal insufficiency, these drug cessation intervals were adjusted according to the degree of renal impairment.
- o
- Antiplatelet medication was discontinued upon hospital admission except aspirin
- o
- Surgical procedures were not delayed due to antiplatelet therapy cessation and the use of general anesthesia was encouraged.
- o
- Avoid psychotropic drugs, opioids, anticholinergics, and antihistamines.
- o
- Transfusion was considered when hemoglobin (Hb) levels fell to 8–9 g/dL. For patients with cardiac disease, a higher transfusion threshold of 9–10 g/dL was generally advised.
- o
- Actively prevent hypothermia.
- o
- Treat hypotensive events promptly.
- o
- Furthermore, the protocol stipulated the administration of tranexamic acid and the avoidance of routine urinary catheterization.
2.3. Control Group
2.4. Data Collection and Outcomes
2.5. Outcomes
2.6. Statistical Analysis
2.7. Sample Size
2.8. AI-Assisted Manuscript Preparation
3. Results
Postoperative Management and Complications
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dyer, S.M.; Crotty, M.; Fairhall, N.; Magaziner, J.; Beaupre, L.A.; Cameron, I.D.; Sherrington, C.; Fragility Fracture Network (FFN) Rehabilitation Research Special Interest Group. A critical review of the long-term disability outcomes following hip fracture. BMC Geriatr. 2016, 16, 158. [Google Scholar] [CrossRef]
- Moerman, S.; Mathijssen, N.M.; Tuinebreijer, W.E.; Nelissen, R.G.; Vochteloo, A.J. Less than one-third of hip fracture patients return to their prefracture level of instrumental activities of daily living in a prospective cohort study of 480 patients. Geriatr. Gerontol. Int. 2018, 18, 1244–1248. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Delirium: Prevention, Diagnosis and Management Clinical Guideline CG103. Available online: https://www.nice.org.uk/guidance/cg103 (accessed on 18 January 2023).
- Chen, Y.; Liang, S.; Wu, H.; Deng, S.; Wang, F.; Lunzhu, C.; Li, J. Postoperative delirium in geriatric patients with hip fractures. Front. Aging Neurosci. 2022, 14, 1068278. [Google Scholar] [CrossRef]
- Inouye, S.K.; Westendorp, R.G.; Saczynski, J.S. Delirium in elderly people. Lancet 2014, 383, 911–922. [Google Scholar] [CrossRef]
- Griffiths, R.; Babu, S.; Dixon, P.; Freeman, N.; Hurford, D.; Kelleher, E.; Moppett, I.; Ray, D.; Sahota, O.; Shields, M.; et al. Guideline for the management of hip fractures 2020: Guideline by the Association of Anaesthetists. Anaesthesia 2021, 76, 225–237. [Google Scholar] [CrossRef]
- Shenkin, S.D.; Fox, C.; Godfrey, M.; Siddiqi, N.; Goodacre, S.; Young, J.; Anand, A.; Gray, A.; Smith, J.; Ryan, T.; et al. Protocol for validation of the 4AT, a rapid screening tool for delirium: A multicentre prospective diagnostic test accuracy study. BMJ Open 2018, 8, e015572. [Google Scholar] [CrossRef]
- Bellelli, G.; Morandi, A.; Davis, D.H.; Mazzola, P.; Turco, R.; Gentile, S.; Ryan, T.; Cash, H.; Guerini, F.; Torpilliesi, T.; et al. Validation of the 4AT, a new instrument for rapid delirium screening: A study in 234 hospitalised older people. Age Ageing 2014, 43, 496–502. [Google Scholar] [CrossRef]
- Rockwood, K.; Fox, R.A.; Stolee, P.; Robertson, D.; Beattie, B.L. Frailty in elderly people: An evolving concept. CMAJ 1994, 150, 489–495. [Google Scholar]
- Chuan, A.; Zhao, L.; Tillekeratne, N.; Alani, S.; Middleton, P.M.; Harris, I.A.; McEvoy, L.; Ní Chróinín, D. The effect of a multidisciplinary care bundle on the incidence of delirium after hip fracture surgery: A quality improvement study. Anaesthesia 2020, 75, 63–71. [Google Scholar] [CrossRef]
- Björkelund, K.B.; Hommel, A.; Thorngren, K.G.; Gustafson, L.; Larsson, S.; Lundberg, D. Reducing delirium in elderly patients with hip fracture: A multi-factorial intervention study. Acta Anaesthesiol. Scand. 2010, 54, 678–688. [Google Scholar] [CrossRef]
- Marcantonio, E.R.; Flacker, J.M.; Wright, R.J.; Resnick, N.M. Reducing delirium after hip fracture: A randomized trial. J. Am. Geriatr. Soc. 2001, 49, 516–522. [Google Scholar] [CrossRef]
- Ranhoff, A.H.; Saltvedt, I.; Frihagen, F.; Raeder, J.; Maini, S.; Sletvold, O. Interdisciplinary care of hip fractures: Orthogeriatric models, alternative models, interdisciplinary teamwork. Best Pract. Res. Clin. Rheumatol. 2019, 33, 205–226. [Google Scholar] [CrossRef]
- Mouzopoulos, G.; Vasiliadis, G.; Lasanianos, N.; Nikolaras, G.; Morakis, E.; Kaminaris, M. Fascia iliaca block prophylaxis for hip fracture patients at risk for delirium: A randomized placebo-controlled study. J. Orthop. Traumatol. 2009, 10, 127–133. [Google Scholar] [CrossRef]
- Steenberg, J.; Møller, A.M. Systematic review of the effects of fascia iliaca compartment block on hip fracture patients before operation. Br. J. Anaesth. 2018, 120, 1368–1380. [Google Scholar] [CrossRef]
- Wennberg, P.; Möller, M.; Herlitz, J.; Kenne Sarenmalm, E. Fascia iliaca compartment block as a preoperative analgesic in elderly patients with hip fractures-effects on cognition. BMC Geriatr. 2019, 19, 252. [Google Scholar] [CrossRef]
- Kim Kim, S.Y.; Jo, H.Y.; Na, H.S.; Han, S.H.; Do, S.H.; Shin, H.J. The effect of peripheral nerve block on postoperative delirium in older adults undergoing hip surgery: A systematic review and meta-analysis of randomized controlled trials. J. Clin. Med. 2023, 12, 2459. [Google Scholar] [CrossRef]
- Verbeek, T.; Adhikary, S.; Urman, R.; Liu, H. The application of fascia iliaca compartment block for acute pain control of hip fracture and surgery. Curr. Pain Headache Rep. 2021, 25, 22. [Google Scholar] [CrossRef]
- Wan, H.Y.; Li, S.Y.; Ji, W.; Yu, B.; Jiang, N. Fascia iliaca compartment block for perioperative pain management of geriatric patients with hip fractures: A systematic review of randomized controlled trials. Pain Res. Manag. 2020, 2020, 8503963. [Google Scholar] [CrossRef]
- Schmid, S.; Blobner, M.; Haas, B.; Lucke, M.; Neumaier, M.; Anetsberger, A.; Jungwirth, B. Perioperative multi-system optimization protocol in elderly hip fracture patients: A randomized-controlled trial. Can. J. Anaesth. 2019, 66, 1472–1482. [Google Scholar] [CrossRef]
- Vidán, M.T.; Sánchez, E.; Alonso, M.; Montero, B.; Ortiz, J.; Serra, J.A. An intervention integrated into daily clinical practice reduces the incidence of delirium during hospitalization in elderly patients. J. Am. Geriatr. Soc. 2009, 57, 2029–2036. [Google Scholar] [CrossRef]
- You, D.; Xu, Y.; Krzyzaniak, H.; Korley, R.; Carrier, M.; Schneider, P. Safety of expedited-surgery protocols in anticoagulant-treated patients with hip fracture: A systematic review and meta-analysis. Can. J. Surg. 2023, 66, E170–E180. [Google Scholar] [CrossRef]
- Cassinello, C.; Ferrandis, R.; Gómez-Luque, A.; Hidalgo, F.; Llau, J.V.; Yanes-Vidal, G.; Sierra, P. Perioperative management of the patients with hip fracture under anticoagulant or antiaggregants treatment. Consensus recommendations from the hemostasis section of SEDAR. Rev. Esp. Anestesiol. Reanim. (Engl. Ed.) 2025, 72, 501651. [Google Scholar] [CrossRef]
- Lees, D.; Harrison, W.D.; Ankers, T.; A’Court, J.; Marriott, A.; Shipsey, D.; Chaplin, A.; Reed, M.R. Fascia iliaca compartment block for hip fractures: Experience of integrating a new protocol across two hospital sites. Eur. J. Emerg. Med. 2016, 23, 12–18. [Google Scholar] [CrossRef]
- Reguant, F.; Arnau, A.; Lorente, J.V.; Maestro, L.; Bosch, J. Efficacy of a multidisciplinary approach on postoperative morbidity and mortality of elderly patients with hip fracture. J. Clin. Anesth. 2019, 53, 11–19. [Google Scholar] [CrossRef]
- Chang, Y.; Ragheb, S.M.; Oravec, N.; Kent, D.; Nugent, K.; Cornick, A.; Hiebert, B.; Rudolph, J.L.; MacLullich, A.M.J.; Arora, R.C.; et al. Diagnostic accuracy of “4AT” delirium screening tool for the postoperative cardiac surgery ward. J. Thorac. Cardiovasc. Surg. 2023, 165, 1151–1160.e8. [Google Scholar] [CrossRef]

| Clinical Pathway N = 117 | Control N = 144 | p | |
|---|---|---|---|
| Age (yr) | 83.40 ± 9.9; 86 [78.5–90.0] | 84.2 ± 9.5; 86 [80.0–90.0] | 0.43 |
| Older than 90 years; n (%) | 33 (28.2) | 42 (29.2) | 0.86 |
| Weight | 63 ± 13 | 62.6 ± 13.8 | 0.79 |
| Height | 159.1 ± 9.26 | 159.96 ± 8.65 | 0.49 |
| Body mass index (kg/m2). | 24.89 ± 4.56 | 24.63 ± 4.76 | 0.65 |
| ASA; n (%) | |||
| I | 1 (0.9) | - | 0.25 |
| II | 24 (20.5) | 30 (20.8) | |
| III | 84 (71.8) | 95 (66) | |
| IV | 8 (6.8) | 19 (13.2) | |
| Sex; n (%) | |||
| Female | 83 (70.9) | 105 (72.9) | 0.72 |
| Male | 34 (29.1) | 39 (27.1) | |
| Comorbidity; n (%) | 116 (99.1) | 138 (95.8) | 0.1 |
| Origin; n (%) | 0.63 | ||
| Domicile | 100 (85.5) | 120 (83.3) | |
| Residence | 17 (14.5) | 24 (16.7) | |
| Diabetes; n (%) | 28 (23.9) | 34 (23.6) | 0.95 |
| Diabetes treatment; n (%) | |||
| Oral antidiabetics | 16 (59.3) | 12 (61.8) | 0.12 |
| Insulin | 5 (18.5) | 11 (32.4) | |
| No treatment | 6 (22.2) | 2 (5.9) | |
| Glucose (g/dL) | 122 [101.0–142.0] | 117 [98.0–144.0] | 0.63 |
| Hemoglobin (mg/dL) | 12.5 [11.35–13.45] | 12.7 [11.40–13.67] | 0.59 |
| Hypertension; n (%) | 82 (70.1) | 102 (70.8) | 0.89 |
| Hypertension treatment; n (%) (ACE-I or ARAII) | 50 (57.5) | 63 (48.1) | 0.17 |
| Asthma/COPD; n (%) | 17 (14.5) | 11 (7.6) | 0.07 |
| OSAHS; n (%) | 5 (4.3) | 2 (1.4) | 0.15 |
| Active smoker; n (%) | 12 (10.4) | 11 (7.9) | 0.48 |
| Chronic kidney failure; n (%) | 17 (14.5) | 17(11.8) | 0.51 |
| Serum creatinine (mg/dL) | 0.87 [0.74–1.13] | 0.8 [0.68–1.07] | 0.075 |
| Estimated GFR; mL.min −1.1.73 m−2 | 47 [33.0–61.0] | 50 [36.0–62.0] | 0.26 |
| Liver disease | 6 (5.1) | 6 (4.2) | 0.71 |
| Coronary artery disease | 10 (8.5) | 13 (9) | 0.89 |
| Heart failure | 18 (15.4) | 22 (15.3) | 0.98 |
| Valvular heart disease | 16 (13.7) | 18 (12.5) | 0.77 |
| Atrial Fibrillation | 26 (22.2) | 36 (25) | 0.60 |
| Cerebrovascular disease | 14 (12) | 17 (11.8) | 0.96 |
| Urinary tract infection; n (%) | 2 (1.7) | 5 (3.5) | 0.38 |
| Antiplatelet therapy | 21 (17.9) | 34 (23.6) | 0.26 |
| Anticoagulation therapy | 30 (25.6) | 38 (26.4) | 0.89 |
| Chronic benzodiazepines treatment | 45 (38.5) | 65 (45.1) | 0.27 |
| Admission timing | |||
| From Monday to Thursday | 80 (68.4) | 79 (54.9) | |
| On Fridays | 13 (11.1) | 24 (16.7) | 0.08 |
| On weekends | 24 (20.5) | 41 (28.5) | |
| Type hip fracture; n (%) | |||
| Subcapital femoral fracture | 49 (41.9) | 72 (50) | 0.001 |
| Pertrochanteric femoral fracture | 47 (40.2) | 70 (48.6) | |
| Basicervical femoral neck fracture | 3 (2.6) | 0 | |
| Subtrochanteric femoral fracture | 12 (10.3) | 3 (1.4) | |
| Persubchanteric femoral fracture | 5 (4.1) | 0 | |
| Other fractures | 1 (0.9) | 0 | |
| Type of surgical operation; n (%) | |||
| Hemiarthroplasty | 48 (41) | 66(43.8) | |
| Short intramedullary nail fixation | 45 (38.5) | 57 (39.6) | 0.19 |
| Long intramedullary nail fixation | 21 (17.9) | 15 10.4) | |
| Cannulated or compression screws | 3 (2.6) | 9 (6.3) | |
| Cemented implant (yes) *; n (%) | 45 (93.75) | 51 (77.27) | 0.02 |
| Surgery duration (min) | 66.21 ± 28.77; | 60.23 ± 24 | 0.07 |
| 60 [46.5–83.5] | 56 [45.0–70.0] |
| Clinical Pathway N = 117 | Control N = 144 | p | |
|---|---|---|---|
| Delirium status; n (%) | 18 (15.4) | 27 (18.8) | 0.47 |
| Dementia; n (%) | 39 (33.3) | 64 (44.4) | 0.11 |
| GDS scale; n (%) | |||
| 1: No cognitive decline | 77 (65.8) | 81 (56.3) | 0.40 |
| 2: Very mild cognitive decline | 5 (4.3) | 2 (1.4) | |
| 3: Mild cognitive decline | 9 (7.7) | 15 (10.4) | |
| 4: Moderate cognitive decline | 5 (4.3) | 7 (4.9) | |
| 5: Moderately severe cognitive decline | 10 (8.5) | 16 (11.1) | |
| 6: Severe cognitive decline | 10 (8.5) | 20 (13.9) | |
| 7: Very severe cognitive decline | 1 (0.9) | 3 (2.1) | |
| Barthel index; n (%) | |||
| <20: total dependency | 6 (5.1) | 13 (9) | 0.61 |
| 20–60: severe dependency | 26 (22.2) | 33 (22.9) | |
| 61–90: moderate dependency | 41 (35) | 51 (35.4) | |
| >90: mild dependency | 44 (37.6) | 47 (32.6) | |
| Clinical Frailty Scale *; n (%) | |||
| 1: very fit | 7 (6) | 10 (6.9) | |
| 2: Well | 20 (17.1) | 24 (16.7) | 0.008 |
| 3: Well, with treated comorbid disease | 23 (19.7) | 19 (13.2) | |
| 4: Apparently vulnerable | 25 (21.4) | 21 (14.6) | |
| 5: Mildly frail | 23 (19.7) | 16 (11.1) | |
| 6: Moderately frail | 17 (14.5) | 45 (32.6) | |
| 7: Severely frail | 2 (1.7) | 9 (6.5) |
| Clinical Pathway N = 117 | Control N = 144 | Effect Size (95%CI) | p | |
|---|---|---|---|---|
| Time to preoperative assessment (hours) | 32.86 ± 28.97; 21 [14.0–44.0] | 49.14 ± 56.15; 36 [19.0–62.0] | –8.56 [–15 to –3] | 0.002 |
| Time to surgery (days) | 2.68 ± 1.87; 2 [2.0, 3.0] | 3.44± 2.44; 3 [2.0–4.0] | –1 [–1 to 0] | 0.001 |
| Time to surgery (hours) | 66.50 ± 45.49; 52.46 [41.75–76.50] | 79.23 ± 55.41; 69 [44.0–99.75] | -–9.5 [–19.5 to –1.52] | 0.02 |
| Surgery within 48 h | 49 (41.9) | 43 (29.9) | 1.69 [1.01 to 2.82] | 0.043 |
| Time to surgery in patients on antiplatelet or anticoagulants therapy (days) | 2.76 ± 1.42; 2.5 [2.0–4.0] | 4.03 ± 2,8; 3 [2.0–5.0] | –1 [–1 to 0] | 0.006 |
| Time to surgery in patients on antiplatelet or anticoagulants therapy (hours) | 66.22 ± 33.8; 59.5 [41.87–87.51] | 90.86 ± 62.12; 76 [49.0–114.75] | –16.41 [–29 to –2.45] | 0.016 |
| Time to surgery in patients on anticoagulants (days) | 2.77 ± 1.17; 2 [2.0–4.0] | 4.73 ± 3.23; 4 [3.0–5.5] | –1 [–2 to –1] | 0.002 |
| Time to surgery in patients on anticoagulants (hours) | 67.42 ± 30.35; 58 [41.5–98.0] | 106.13 ± 68.71; 92 [66.0–122.0] | –26 [–47 to –9.49] | 0.005 |
| Preoperative complications | 20 (17.1) | 47 (32.6) | 0.42 [0.23 to 0.77] | 0.004 |
| Delayed surgery due to medical complication; n (%) | 8 (6.8) | 17 (11.8) | 0.50 [0.14 to 1.27] | 0.17 |
| Clinical Pathway N = 117 | Control N = 144 | Effect Size (95%CI) | p | |
|---|---|---|---|---|
| Orthopedic Anesthesiologist; n (%) | 47 (40.2) | 38 (26.6) | 1.85 [1.09 to 3.13] | 0.02 |
| Type of anesthesia; n (%) | ||||
| Spinal | 109 (94) | 136 (95.1) | 0.8 [0.27 to 2.37] | 0.68 |
| General | 7 (6) | 7 (4.9) | ||
| Surgical drains; n (%) | 18(15.4) | 39(27.1) | 0.49 [0.26 to 0.91] | 0.023 |
| Urinary catheters | 17 (14.5) | 21 (14.6) | 0.99 [0.49 to 1.9] | 0.99 |
| Hypothermia prevention; n (%) | 62 (53.8) | 69 (47) | 1.26 [0.76 to 2.09] | 0.35 |
| Spinal opioids; n (%) | 83 (76.1) | 88 (66.7) | 1.59 [0.90 to2.82] | 0.10 |
| Intravenous opioids; n (%) | 25 (21.7) | 52 (37.1) | 0.47 [0.26 to 0.82] | 0.008 |
| Benzodiazepine sedation; n (%) | 22(19) | 42 (29) | 0.55 [0.30 to 0.99] | 0.047 |
| PONV prophylaxis; n (%) | 66 (56.4) | 51 (35.4) | 2.36 [1.41 to 3.89] | 0.001 |
| Intraoperative fluid volume crystalloid (mL) | 622.97 ± 243.99 500 [500.0–750.0] | 613.21 ± 287.67 500 [500.0–700.0] | 0 [–50 to 100] | 0.18 |
| Intraoperative fluid volume colloid (mL) | 394.44 ± 121.13; 475 [250.0–500.0] | 373.33 ± 137.40 400 [250.0–500.0] | 0 [0 to 0] | 0.81 |
| RBC transfusion; n (%) | 11(9.4) | 13 (9) | 1.04 [0.45 to 2.42] | 0.91 |
| RBC transfusion units (total) | 1.6 ± 0.69; 1 [2.0–2.0] | 1.25 ± 0.45 1 [1.0–1.0] | 0 [0 to 1] | 0.20 |
| Tranexamic administration; n (%) | 20 (17.5) | 4 (2.9) | 7.44 [2.4 to 22.48] | 0.001 |
| Intraoperative complications; n (%) | 28 (24.1) | 64 (45.7) | 0.37 [0.22 to 064] | 0.001 |
| Intraoperative hypotension; n (%) | 23 (20) | 51 (36.4) | 0.43 [0.24 to 0.77] | 0.004 |
| Administration of vasopressors; n (%) | 23 (20) | 57 (40.7) | 0.36 [0.20 to 0.64] | 0.001 |
| Arrythmia; n (%) | 2 (1.7) | 9 (6.4) | 0.25 [0.05 to 1.2] | 0.053 |
| Clinical Pathway N = 117 | Control N = 144 | Effect Size (95%CI) | p | |
|---|---|---|---|---|
| Postoperative care unit stay (h) | 2.68 ± 1.19; 2.4 [2.0, 3.0] | 2.94 ± 1.70; 2.5 [2.0–3.1] | 0 [–4 to 0] | 0.16 |
| Standardized analgesic protocol; n (%) | 109 (93.2) | 38 (26.4) | 38 [16.94 to 85.25] | 0.001 |
| Early mobilization; n (%) | 87 (74.4) | 59 (41.3) | 4.12 [2.42 to 7.28] | 0.001 |
| Time to mobilization (hours) | 31.2 ± 29.3 21 [18.0–38.0] | 37.14 ± 23 24 [22.0–47.0] | –5.7 [–8 to –3] | 0.001 |
| Time to ambulation (hours) | 78.4 ± 62.4; 65 [39.0–115.0] | 88.6 ± 51.7 72 [48.0–120.0] | –14 [–26 to –2] | 0.028 |
| Early food and drink intake; n (%) | 102 (87.2) | 91(63.6) | 3.49 [1.86 to 6.56] | 0.001 |
| Glucose control; n (%) | 96 (82.1) | 140 (97.2) | 0.13 [0.04 to 0.39] | 0.001 |
| Blood transfusion; n (%) | 50 (42.7) | 64 (44.4) | 0.95 [0.58 to 1.56] | 0.78 |
| Composite of 30-day mortality or major complications; n (%) | 17 (14.5) | 37 (25.7) | 0.49 [0.26 to 0.92] | 0.02 |
| Postoperative delirium; n (%) | 34 (29.3) | 63 (43.8) | 0.52 [0.31 to 0.88] | 0.015 |
| Wound infection, n (%) | 3 (2.5) | 1 (0.69) | 3.79 [0.39 to 36.9] | 0.21 |
| Urinary tract infection; n (%) | 15 (12.8) | 16 (11.1) | 1.18 [0.56 to 2.51] | 0.65 |
| Ileus; n (%) | 35 (29.9) | 42 (29.2) | 1.04 [0.61 to 1.79] | 0.86 |
| Need for reoperation; n (%) | 7 (6) | 5 (3.5) | 2.56 [0.75 to 8.75] | 0.33 |
| Readmission; n (%) | 10 (8.8) | 8 (5.7) | 2.04 [0.76 to 5.45] | 0.33 |
| Length of hospital stay; (days) | 15.50 ± 11.26; 13 [10.0–17.0] | 14.34 ± 9.6 12 [9.0–16.0] | 1 [–1 to 2] | 0.32 |
| 30-day mortality; n (%) | 5 (4.3) | 12(8.3) | 0.49 [0.16 to 1.43] | 0.18 |
| 1-year mortality; n (%) | 24 (20.5) | 28 (19.4) | 1.17 [0.64 to 2.16] | 0.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corbella-Giménez, C.; Monge-Cid, E.; Gallo-Carrasco, A.; Barros García-Imhof, J.; Sánchez-Rodríguez, F.; Díaz-García, J.; Vasserot, I.; Anadon-Baselga, M.J.; Zaballos, M. Effect of an Optimized Clinical Pathway Protocol Including Fascia Iliaca Compartment Block on Delirium and Postoperative Complications in Elderly Hip Fracture Patients. J. Clin. Med. 2025, 14, 5284. https://doi.org/10.3390/jcm14155284
Corbella-Giménez C, Monge-Cid E, Gallo-Carrasco A, Barros García-Imhof J, Sánchez-Rodríguez F, Díaz-García J, Vasserot I, Anadon-Baselga MJ, Zaballos M. Effect of an Optimized Clinical Pathway Protocol Including Fascia Iliaca Compartment Block on Delirium and Postoperative Complications in Elderly Hip Fracture Patients. Journal of Clinical Medicine. 2025; 14(15):5284. https://doi.org/10.3390/jcm14155284
Chicago/Turabian StyleCorbella-Giménez, Carmen, Elena Monge-Cid, Alba Gallo-Carrasco, Jorge Barros García-Imhof, Francisco Sánchez-Rodríguez, Jesús Díaz-García, Ignacio Vasserot, Maria José Anadon-Baselga, and Matilde Zaballos. 2025. "Effect of an Optimized Clinical Pathway Protocol Including Fascia Iliaca Compartment Block on Delirium and Postoperative Complications in Elderly Hip Fracture Patients" Journal of Clinical Medicine 14, no. 15: 5284. https://doi.org/10.3390/jcm14155284
APA StyleCorbella-Giménez, C., Monge-Cid, E., Gallo-Carrasco, A., Barros García-Imhof, J., Sánchez-Rodríguez, F., Díaz-García, J., Vasserot, I., Anadon-Baselga, M. J., & Zaballos, M. (2025). Effect of an Optimized Clinical Pathway Protocol Including Fascia Iliaca Compartment Block on Delirium and Postoperative Complications in Elderly Hip Fracture Patients. Journal of Clinical Medicine, 14(15), 5284. https://doi.org/10.3390/jcm14155284







