Visual Field Examinations for Retinal Diseases: A Narrative Review
Abstract
1. Introduction
2. Principles and Methodologies of VF Testing
2.1. Basic Concepts of Perimetry
2.2. Traditional Perimetry Techniques
2.2.1. Confrontation VF Test
2.2.2. Kinetic Perimetry (e.g., Goldmann Perimetry)
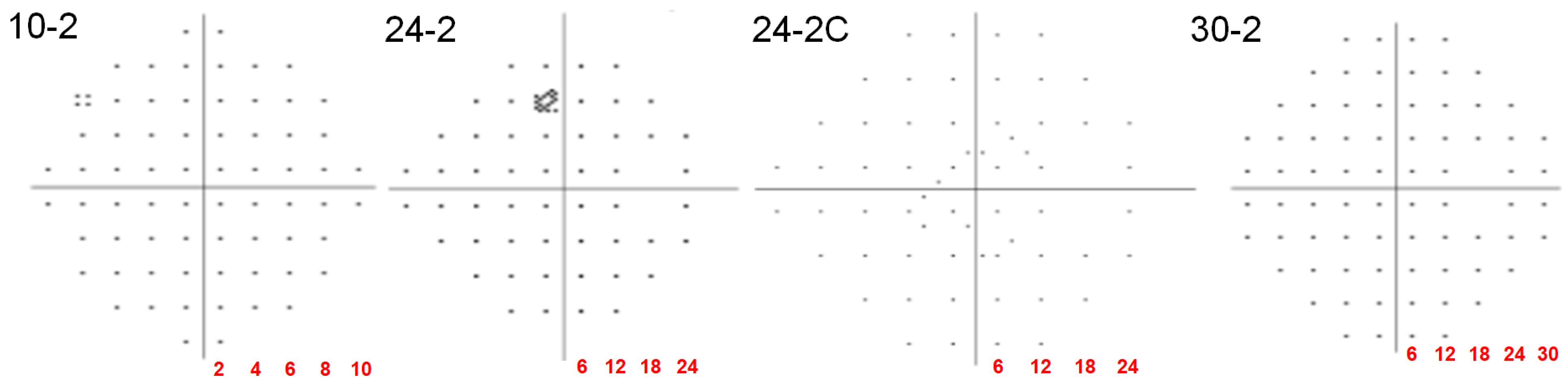
2.2.3. Automated Static Perimetry
2.2.4. Frequency Doubling Technology (FDT)
2.3. Advanced and Specialized Perimetry Techniques
2.3.1. Microperimetry
2.3.2. Amsler Grid
3. VF Findings in Retinal Diseases
3.1. AMD
3.2. DR and DME
3.3. Drug-Induced Retinopathies
3.4. Retinitis Pigmentosa (RP)
3.5. Inherited Macular Dystrophy
3.6. Central Serous Chorioretinopathy (CSCR)
3.7. Retinal Vein Occlusion (RVO)
3.8. Macular Hole
3.9. Miscellaneous Retinal Diseases
4. Clinical Utility of VF Examinations in Retinal Diseases
4.1. Diagnosis
4.2. Monitoring Disease Progression or Recovery
4.3. Prognosis
5. Challenges and Limitations
5.1. Patient Factors
5.2. Media Opacities and Comorbidities
5.3. Normative Database and Floor/Ceiling Effects
5.4. Technical and Environmental Factors
5.5. Disease-Specific Limitations
6. Emerging Technologies and Future Directions
6.1. AI and Machine Learning in VF Analysis
6.2. Virtual Reality (VR) Perimetry
6.3. Home-Based Perimetry
6.4. Implementation Barriers for Emerging Technologies
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rai, B.B.; Sabeti, F.; Carle, C.F.; Maddess, T. Visual Field Tests: A Narrative Review of Different Perimetric Methods. J. Clin. Med. 2024, 13, 2458. [Google Scholar] [CrossRef]
- Johnson, C.A.; Wall, M.; Thompson, H.S. A history of perimetry and visual field testing. Optom. Vis. Sci. 2011, 88, E8–E15. [Google Scholar] [CrossRef]
- McKendrick, A.M. Recent developments in perimetry: Test stimuli and procedures. Clin. Exp. Optom. 2005, 88, 73–80. [Google Scholar] [CrossRef]
- Fogel-Levin, M.; Sadda, S.R.; Rosenfeld, P.J.; Waheed, N.; Querques, G.; Freund, B.K.; Sarraf, D. Advanced retinal imaging and applications for clinical practice: A consensus review. Surv. Ophthalmol. 2022, 67, 1373–1390. [Google Scholar] [CrossRef]
- Hood, D.C.; Kardon, R.H. A framework for comparing structural and functional measures of glaucomatous damage. Prog. Retin. Eye Res. 2007, 26, 688–710. [Google Scholar] [CrossRef]
- Phu, J.; Khuu, S.K.; Nivison-Smith, L.; Kalloniatis, M. Standard automated perimetry for glaucoma and diseases of the retina and visual pathways: Current and future perspectives. Prog. Retin. Eye Res. 2025, 104, 101307. [Google Scholar] [CrossRef]
- Johnson, L.N.; Baloh, F.G. The accuracy of confrontation visual field test in comparison with automated perimetry. J. Natl. Med. Assoc. 1991, 83, 895–898. [Google Scholar]
- Ruia, S.; Tripathy, K. Humphrey Visual Field. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Ahn, S.J.; Seo, E.J.; Kim, K.E.; Kim, Y.J.; Lee, B.R.; Kim, J.G.; Yoon, Y.H.; Lee, J.Y. Long-Term Progression of Pericentral Hydroxychloroquine Retinopathy. Ophthalmology 2021, 128, 889–898. [Google Scholar] [CrossRef]
- Kim, K.E.; Ryu, S.J.; Kim, Y.H.; Seo, Y.; Ahn, S.J. Visual field examinations using different strategies in Asian patients taking hydroxychloroquine. Sci. Rep. 2022, 12, 14778. [Google Scholar] [CrossRef]
- Katz, J.; Sommer, A. Asymmetry and variation in the normal hill of vision. Arch. Ophthalmol. 1986, 104, 65–68. [Google Scholar] [CrossRef]
- Wood, J.M.; Swann, P.G.; Stavrou, E.P. Visual fields in glaucoma: A clinical overview. Clin. Exp. Optom. 2000, 83, 128–135. [Google Scholar] [CrossRef]
- Wall, M.; Woodward, K.R.; Doyle, C.K.; Zamba, G. The effective dynamic ranges of standard automated perimetry sizes III and V and motion and matrix perimetry. Arch. Ophthalmol. 2010, 128, 570–576. [Google Scholar] [CrossRef]
- Hepworth, L.R.; Rowe, F.J. Programme choice for perimetry in neurological conditions (PoPiN): A systematic review of perimetry options and patterns of visual field loss. BMC Ophthalmol. 2018, 18, 241. [Google Scholar] [CrossRef]
- Xu, M.; Zhai, Y.; MacDonald, I.M. Visual Field Progression in Retinitis Pigmentosa. Investig. Ophthalmol. Vis. Sci. 2020, 61, 56. [Google Scholar] [CrossRef]
- Grover, S.; Fishman, G.A.; Brown, J., Jr. Patterns of visual field progression in patients with retinitis pigmentosa. Ophthalmology 1998, 105, 1069–1075. [Google Scholar] [CrossRef]
- Delgado, M.F.; Nguyen, N.T.; Cox, T.A.; Singh, K.; Lee, D.A.; Dueker, D.K.; Fechtner, R.D.; Juzych, M.S.; Lin, S.C.; Netland, P.A.; et al. Automated perimetry: A report by the American Academy of Ophthalmology. Ophthalmology 2002, 109, 2362–2374. [Google Scholar] [CrossRef]
- Marmor, M.F.; Kellner, U.; Lai, T.Y.; Melles, R.B.; Mieler, W.F.; American Academy of Ophthalmology. Recommendations on Screening for Chloroquine and Hydroxychloroquine Retinopathy (2016 Revision). Ophthalmology 2016, 123, 1386–1394. [Google Scholar] [CrossRef]
- Nishijima, E.; Fukai, K.; Sano, K.; Noro, T.; Ogawa, S.; Okude, S.; Tatemichi, M.; Lee, G.C.; Iwase, A.; Nakano, T. Comparative Analysis of 24-2C, 24-2, and 10-2 Visual Field Tests for Detecting Mild-Stage Glaucoma With Central Visual field Defects. Am. J. Ophthalmol. 2024, 268, 275–284. [Google Scholar] [CrossRef]
- Phu, J.; Kalloniatis, M. Comparison of 10-2 and 24-2C Test Grids for Identifying Central Visual Field Defects in Glaucoma and Suspect Patients. Ophthalmology 2021, 128, 1405–1416. [Google Scholar] [CrossRef]
- Chakravarti, T.; Moghadam, M.; Proudfoot, J.A.; Weinreb, R.N.; Bowd, C.; Zangwill, L.M. Agreement Between 10-2 and 24-2C Visual Field Test Protocols for Detecting Glaucomatous Central Visual Field Defects. J. Glaucoma 2021, 30, e285–e291. [Google Scholar] [CrossRef]
- Bengtsson, B.; Heijl, A. Evaluation of a new perimetric threshold strategy, SITA, in patients with manifest and suspect glaucoma. Acta Ophthalmol. Scand. 1998, 76, 268–272. [Google Scholar] [CrossRef]
- Heijl, A.; Patella, V.M.; Chong, L.X.; Iwase, A.; Leung, C.K.; Tuulonen, A.; Lee, G.C.; Callan, T.; Bengtsson, B. A New SITA Perimetric Threshold Testing Algorithm: Construction and a Multicenter Clinical Study. Am. J. Ophthalmol. 2019, 198, 154–165. [Google Scholar] [CrossRef]
- Heijl, A.; Lindgren, G.; Olsson, J. Normal variability of static perimetric threshold values across the central visual field. Arch. Ophthalmol. 1987, 105, 1544–1549. [Google Scholar] [CrossRef]
- Browning, D.J. Hydroxychloroquine and chloroquine retinopathy: Screening for drug toxicity. Am. J. Ophthalmol. 2002, 133, 649–656. [Google Scholar] [CrossRef]
- Heijl, A.; Leske, M.C.; Bengtsson, B.; Hyman, L.; Bengtsson, B.; Hussein, M.; Early Manifest Glaucoma Trial, G. Reduction of intraocular pressure and glaucoma progression: Results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol. 2002, 120, 1268–1279. [Google Scholar] [CrossRef] [PubMed]
- Kass, M.A.; Heuer, D.K.; Higginbotham, E.J.; Johnson, C.A.; Keltner, J.L.; Miller, J.P.; Parrish, R.K., 2nd; Wilson, M.R.; Gordon, M.O. The Ocular Hypertension Treatment Study: A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch. Ophthalmol. 2002, 120, 701–713; discussion 829–830. [Google Scholar] [CrossRef]
- Lichter, P.R.; Musch, D.C.; Gillespie, B.W.; Guire, K.E.; Janz, N.K.; Wren, P.A.; Mills, R.P.; Group, C.S. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology 2001, 108, 1943–1953. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.A.; Samuels, S.J. Screening for glaucomatous visual field loss with frequency-doubling perimetry. Investig. Ophthalmol. Vis. Sci. 1997, 38, 413–425. [Google Scholar]
- Liu, S.; Yu, M.; Weinreb, R.N.; Lai, G.; Lam, D.S.; Leung, C.K. Frequency-doubling technology perimetry for detection of the development of visual field defects in glaucoma suspect eyes: A prospective study. JAMA Ophthalmol. 2014, 132, 77–83. [Google Scholar] [CrossRef]
- Bao, Y.K.; Yan, Y.; Gordon, M.; McGill, J.B.; Kass, M.; Rajagopal, R. Visual Field Loss in Patients With Diabetes in the Absence of Clinically-Detectable Vascular Retinopathy in a Nationally Representative Survey. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4711–4716. [Google Scholar] [CrossRef]
- Rohrschneider, K.; Springer, C.; Bultmann, S.; Volcker, H.E. Microperimetry—Comparison between the micro perimeter 1 and scanning laser ophthalmoscope—Fundus perimetry. Am. J. Ophthalmol. 2005, 139, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Montesano, G.; Gervasoni, A.; Ferri, P.; Allegrini, D.; Migliavacca, L.; De Cilla, S.; Rossetti, L. Structure-function relationship in early diabetic retinopathy: A spatial correlation analysis with OCT and microperimetry. Eye 2017, 31, 931–939. [Google Scholar] [CrossRef]
- Midena, E.; Vujosevic, S. Microperimetry in diabetic retinopathy. Saudi J. Ophthalmol. 2011, 25, 131–135. [Google Scholar] [CrossRef]
- Vujosevic, S.; Bottega, E.; Casciano, M.; Pilotto, E.; Convento, E.; Midena, E. Microperimetry and fundus autofluorescence in diabetic macular edema: Subthreshold micropulse diode laser versus modified early treatment diabetic retinopathy study laser photocoagulation. Retina 2010, 30, 908–916. [Google Scholar] [CrossRef]
- Parravano, M.; De Geronimo, D.; Sacconi, R.; Giannini, D.; Costanzo, E.; Fragiotta, S.; Viggiano, P.; Varano, M.; Querques, G. Impact of Intravitreal Anti-VEGF Therapy on Microperimetry of the Retinal Nonperfusion Areas of Patients with Proliferative Diabetic Retinopathy. Ophthalmol. Ther. 2022, 11, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Schuchard, R.A. Validity and interpretation of Amsler grid reports. Arch. Ophthalmol. 1993, 111, 776–780. [Google Scholar] [CrossRef]
- Su, D.; Greenberg, A.; Simonson, J.L.; Teng, C.C.; Liebmann, J.M.; Ritch, R.; Park, S.C. Efficacy of the Amsler Grid Test in Evaluating Glaucomatous Central Visual Field Defects. Ophthalmology 2016, 123, 737–743. [Google Scholar] [CrossRef]
- Frisen, L. The Amsler grid in modern clothes. Br. J. Ophthalmol. 2009, 93, 714–716. [Google Scholar] [CrossRef]
- Robison, C.D.; Jivrajka, R.V.; Bababeygy, S.R.; Fink, W.; Sadun, A.A.; Sebag, J. Distinguishing wet from dry age-related macular degeneration using three-dimensional computer-automated threshold Amsler grid testing. Br. J. Ophthalmol. 2011, 95, 1419–1423. [Google Scholar] [CrossRef]
- Bjerager, J.; Schneider, M.; Potapenko, I.; van Dijk, E.H.C.; Faber, C.; Grauslund, J.; Pfau, K.; Huemer, J.; Muttuvelu, D.V.; Rasmussen, M.L.R.; et al. Diagnostic Accuracy of the Amsler Grid Test for Detecting Neovascular Age-Related Macular Degeneration: A Systematic Review and Meta-analysis. JAMA Ophthalmol. 2023, 141, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Keenan, T.D.L.; Cukras, C.A.; Chew, E.Y. Age-Related Macular Degeneration: Epidemiology and Clinical Aspects. Adv. Exp. Med. Biol. 2021, 1256, 1–31. [Google Scholar] [CrossRef]
- Trinh, M.; Kalloniatis, M.; Khuu, S.K.; Nivison-Smith, L. Retinal sensitivity changes in early/intermediate AMD: A systematic review and meta-analysis of visual field testing under mesopic and scotopic lighting. Eye 2024, 38, 1827–1835. [Google Scholar] [CrossRef]
- Hobbs, S.D.; Tripathy, K.; Pierce, K. Wet Age-Related Macular Degeneration (AMD). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Holz, F.G.; Strauss, E.C.; Schmitz-Valckenberg, S.; van Lookeren Campagne, M. Geographic atrophy: Clinical features and potential therapeutic approaches. Ophthalmology 2014, 121, 1079–1091. [Google Scholar] [CrossRef]
- Pondorfer, S.G.; Wintergerst, M.W.M.; Gorgi Zadeh, S.; Schultz, T.; Heinemann, M.; Holz, F.G.; Finger, R.P. Association of Visual Function Measures with Drusen Volume in Early Stages of Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2020, 61, 55. [Google Scholar] [CrossRef]
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef]
- Yau, J.W.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, H.W.; Verbraak, F.D.; Kok, P.H.; Stehouwer, M.; Garvin, M.K.; Sonka, M.; DeVries, J.H.; Schlingemann, R.O.; Abramoff, M.D. Early neurodegeneration in the retina of type 2 diabetic patients. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2715–2719. [Google Scholar] [CrossRef] [PubMed]
- Pinilla, I.; Ferreras, A.; Idoipe, M.; Sanchez-Cano, A.I.; Perez-Garcia, D.; Herrera, L.X.; Pinilla, M.J.; Abecia, E. Changes in frequency-doubling perimetry in patients with type I diabetes prior to retinopathy. Biomed. Res. Int. 2013, 2013, 341269. [Google Scholar] [CrossRef] [PubMed]
- Lobefalo, L.; Verrotti, A.; Mastropasqua, L.; Della Loggia, G.; Cherubini, V.; Morgese, G.; Gallenga, P.E.; Chiarelli, F. Blue-on-yellow and achromatic perimetry in diabetic children without retinopathy. Diabetes Care 1998, 21, 2003–2006. [Google Scholar] [CrossRef]
- Melles, R.B.; Marmor, M.F. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014, 132, 1453–1460. [Google Scholar] [CrossRef]
- Pearce, W.A.; Chen, R.; Jain, N. Pigmentary Maculopathy Associated with Chronic Exposure to Pentosan Polysulfate Sodium. Ophthalmology 2018, 125, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- Lindeke-Myers, A.; Hanif, A.M.; Jain, N. Pentosan polysulfate maculopathy. Surv. Ophthalmol. 2022, 67, 83–96. [Google Scholar] [CrossRef]
- Bourla, D.H.; Sarraf, D.; Schwartz, S.D. Peripheral retinopathy and maculopathy in high-dose tamoxifen therapy. Am. J. Ophthalmol. 2007, 144, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef]
- Grover, S.; Fishman, G.A.; Anderson, R.J.; Tozatti, M.S.; Heckenlively, J.R.; Weleber, R.G.; Edwards, A.O.; Brown, J., Jr. Visual acuity impairment in patients with retinitis pigmentosa at age 45 years or older. Ophthalmology 1999, 106, 1780–1785. [Google Scholar] [CrossRef]
- Allikmets, R. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat. Genet. 1997, 17, 122. [Google Scholar] [CrossRef]
- Zhang, K.; Garibaldi, D.C.; Kniazeva, M.; Albini, T.; Chiang, M.F.; Kerrigan, M.; Sunness, J.S.; Han, M.; Allikmets, R. A novel mutation in the ABCR gene in four patients with autosomal recessive Stargardt disease. Am. J. Ophthalmol. 1999, 128, 720–724. [Google Scholar] [CrossRef]
- Fujinami, K.; Zernant, J.; Chana, R.K.; Wright, G.A.; Tsunoda, K.; Ozawa, Y.; Tsubota, K.; Robson, A.G.; Holder, G.E.; Allikmets, R.; et al. Clinical and molecular characteristics of childhood-onset Stargardt disease. Ophthalmology 2015, 122, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Strauss, R.W.; Munoz, B.; Ho, A.; Jha, A.; Michaelides, M.; Cideciyan, A.V.; Audo, I.; Birch, D.G.; Hariri, A.H.; Nittala, M.G.; et al. Progression of Stargardt Disease as Determined by Fundus Autofluorescence in the Retrospective Progression of Stargardt Disease Study (ProgStar Report No. 9). JAMA Ophthalmol. 2017, 135, 1232–1241. [Google Scholar] [CrossRef]
- Dhooge, P.P.A.; Runhart, E.H.; Lambertus, S.; Bax, N.M.; Groenewoud, J.M.M.; Klevering, B.J.; Hoyng, C.B. Correlation of Morphology and Function of Flecks Using Short-Wave Fundus Autofluorescence and Microperimetry in Patients With Stargardt Disease. Transl. Vis. Sci. Technol. 2021, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.S.; Georgiou, M.; Kalitzeos, A.; Moore, A.T.; Michaelides, M. Progressive cone and cone-rod dystrophies: Clinical features, molecular genetics and prospects for therapy. Br. J. Ophthalmol. 2019, 103, 711–720. [Google Scholar] [CrossRef]
- Daruich, A.; Matet, A.; Dirani, A.; Bousquet, E.; Zhao, M.; Farman, N.; Jaisser, F.; Behar-Cohen, F. Central serous chorioretinopathy: Recent findings and new physiopathology hypothesis. Prog. Retin. Eye Res. 2015, 48, 82–118. [Google Scholar] [CrossRef]
- Zhang, X.; Lim, C.Z.F.; Chhablani, J.; Wong, Y.M. Central serous chorioretinopathy: Updates in the pathogenesis, diagnosis and therapeutic strategies. Eye Vis. 2023, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- van Rijssen, T.J.; van Dijk, E.H.C.; Yzer, S.; Ohno-Matsui, K.; Keunen, J.E.E.; Schlingemann, R.O.; Sivaprasad, S.; Querques, G.; Downes, S.M.; Fauser, S.; et al. Central serous chorioretinopathy: Towards an evidence-based treatment guideline. Prog. Retin. Eye Res. 2019, 73, 100770. [Google Scholar] [CrossRef]
- Nicholson, B.; Noble, J.; Forooghian, F.; Meyerle, C. Central serous chorioretinopathy: Update on pathophysiology and treatment. Surv. Ophthalmol. 2013, 58, 103–126. [Google Scholar] [CrossRef]
- Hayreh, S.S.; Zimmerman, M.B. Branch retinal vein occlusion: Natural history of visual outcome. JAMA Ophthalmol. 2014, 132, 13–22. [Google Scholar] [CrossRef]
- Hayreh, S.S.; Podhajsky, P.A.; Zimmerman, M.B. Natural history of visual outcome in central retinal vein occlusion. Ophthalmology 2011, 118, 119–133.e2. [Google Scholar] [CrossRef]
- Ghashut, R.; Muraoka, Y.; Ooto, S.; Iida, Y.; Miwa, Y.; Suzuma, K.; Murakami, T.; Kadomoto, S.; Tsujikawa, A.; Yoshimura, N. Evaluation Of Macular Ischemia In Eyes With Central Retinal Vein Occlusion: An Optical Coherence Tomography Angiography Study. Retina 2018, 38, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Gaudric, A.; Haouchine, B.; Massin, P.; Paques, M.; Blain, P.; Erginay, A. Macular hole formation: New data provided by optical coherence tomography. Arch. Ophthalmol. 1999, 117, 744–751. [Google Scholar] [CrossRef]
- Ezra, E.; Wells, J.A.; Gray, R.H.; Kinsella, F.M.; Orr, G.M.; Grego, J.; Arden, G.B.; Gregor, Z.J. Incidence of idiopathic full-thickness macular holes in fellow eyes. A 5-year prospective natural history study. Ophthalmology 1998, 105, 353–359. [Google Scholar] [CrossRef]
- Wittich, W.; Overbury, O.; Kapusta, M.A.; Watanabe, D.H.; Faubert, J. Macular hole: Perceptual filling-in across central scotomas. Vision. Res. 2006, 46, 4064–4070. [Google Scholar] [CrossRef]
- Nicolai, M.; Franceschi, A.; De Turris, S.; Rosati, A.; Pelliccioni, P.; Pirani, V.; Pasanisi, P.; Mariotti, C. Long-term improvement of retinal sensitivity after macular hole surgery over at least 9-year-old follow-up: A case series. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 1655–1662. [Google Scholar] [CrossRef]
- Heeren, T.F.; Clemons, T.; Scholl, H.P.; Bird, A.C.; Holz, F.G.; Charbel Issa, P. Progression of Vision Loss in Macular Telangiectasia Type 2. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3905–3912. [Google Scholar] [CrossRef]
- Marmor, M.F.; Melles, R.B. Disparity between visual fields and optical coherence tomography in hydroxychloroquine retinopathy. Ophthalmology 2014, 121, 1257–1262. [Google Scholar] [CrossRef]
- Ahn, S.J.; Yang, L.; Tsunoda, K.; Kondo, M.; Fujinami-Yokokawa, Y.; Nakamura, N.; Iwata, T.; Kim, M.S.; Mun, Y.; Park, J.Y.; et al. Visual Field Characteristics in East Asian Patients With Occult Macular Dystrophy (Miyake Disease): EAOMD Report No. 3. Investig. Ophthalmol. Vis. Sci. 2022, 63, 12. [Google Scholar] [CrossRef]
- Anderson, C.; Blaha, G.R.; Marx, J.L. Humphrey visual field findings in hydroxychloroquine toxicity. Eye 2011, 25, 1535–1545. [Google Scholar] [CrossRef] [PubMed]
- Pham, B.H.; Marmor, M.F. Sequential Changes In Hydroxychloroquine Retinopathy Up To 20 Years After Stopping The Drug: Implications for Mild Versus Severe Toxicity. Retina 2019, 39, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Horie, S.; Corradetti, G.; Esmaeilkhanian, H.; Sadda, S.R.; Cheung, C.M.G.; Ham, Y.; Chang, A.; Takahashi, T.; Ohno-Matsui, K. Microperimetry in Retinal Diseases. Asia Pac. J. Ophthalmol. 2023, 12, 211–227. [Google Scholar] [CrossRef] [PubMed]
- De Fauw, J.; Ledsam, J.R.; Romera-Paredes, B.; Nikolov, S.; Tomasev, N.; Blackwell, S.; Askham, H.; Glorot, X.; O′Donoghue, B.; Visentin, D.; et al. Clinically applicable deep learning for diagnosis and referral in retinal disease. Nat. Med. 2018, 24, 1342–1350. [Google Scholar] [CrossRef]
- Ting, D.S.W.; Cheung, C.Y.; Lim, G.; Tan, G.S.W.; Quang, N.D.; Gan, A.; Hamzah, H.; Garcia-Franco, R.; San Yeo, I.Y.; Lee, S.Y.; et al. Development and Validation of a Deep Learning System for Diabetic Retinopathy and Related Eye Diseases Using Retinal Images From Multiethnic Populations With Diabetes. JAMA 2017, 318, 2211–2223. [Google Scholar] [CrossRef]
- Li, F.; Wang, Z.; Qu, G.; Song, D.; Yuan, Y.; Xu, Y.; Gao, K.; Luo, G.; Xiao, Z.; Lam, D.S.C.; et al. Automatic differentiation of Glaucoma visual field from non-glaucoma visual filed using deep convolutional neural network. BMC Med. Imaging 2018, 18, 35. [Google Scholar] [CrossRef] [PubMed]
- Asaoka, R.; Murata, H.; Iwase, A.; Araie, M. Detecting Preperimetric Glaucoma with Standard Automated Perimetry Using a Deep Learning Classifier. Ophthalmology 2016, 123, 1974–1980. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.R.; Smith, N.D.; Bi, W.; Crabb, D.P. Portable Perimetry Using Eye-Tracking on a Tablet Computer-A Feasibility Assessment. Transl. Vis. Sci. Technol. 2019, 8, 17. [Google Scholar] [CrossRef]
- McLaughlin, D.E.; Savatovsky, E.J.; O′Brien, R.C.; Vanner, E.A.; Munshi, H.K.; Pham, A.H.; Grajewski, A.L. Reliability of Visual Field Testing in a Telehealth Setting Using a Head-Mounted Device: A Pilot Study. J. Glaucoma 2024, 33, 15–23. [Google Scholar] [CrossRef]
- Selvan, K.; Mina, M.; Abdelmeguid, H.; Gulsha, M.; Vincent, A.; Sarhan, A. Virtual reality headsets for perimetry testing: A systematic review. Eye 2024, 38, 1041–1064. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Kim, M.; Lee, J.Y.; Kim, M.Y.; Jeon, Y.J.; Kim, K. Feasibility of hemispatial neglect rehabilitation with virtual reality-based visual exploration therapy among patients with stroke: Randomised controlled trial. Front. Neurosci. 2023, 17, 1142663. [Google Scholar] [CrossRef]
- Blinder, K.J.; Calhoun, C.; Maguire, M.G.; Glassman, A.R.; Mein, C.E.; Baskin, D.E.; Vieyra, G.; Jampol, L.M.; Chica, M.A.; Sun, J.K.; et al. Home OCT Imaging for Newly Diagnosed Neovascular Age-Related Macular Degeneration: A Feasibility Study. Ophthalmol. Retina 2024, 8, 376–387. [Google Scholar] [CrossRef]
- Holekamp, N.M.; de Beus, A.M.; Clark, W.L.; Heier, J.S. Prospective Trial of Home Optical Coherence Tomography-Guided Management of Treatment Experienced Neovascular Age-Related Macular Degeneration Patients. Retina 2024, 44, 1714–1731. [Google Scholar] [CrossRef]
- Dolar-Szczasny, J.; Drab, A.; Rejdak, R. Home-monitoring/remote optical coherence tomography in teleophthalmology in patients with eye disorders-a systematic review. Front. Med. 2024, 11, 1442758. [Google Scholar] [CrossRef]

| Modality | Test Duration * | Area Tested | Patient Burden | Sensitivity/Specificity for Specific Diseases |
|---|---|---|---|---|
| Confrontation | <1 min per eye | ~120–150° (gross field) | Very low | Poor for subtle or early defects; not recommended for screening retinal disease. |
| Goldmann Kinetic | 5–15 min per eye | Central + peripheral (up to 90°) | Moderate | Excellent for mapping peripheral loss in RP and RVO; qualitative only. |
| Automated Static (HFA) | 5–8 min (10-2); 6–12 min (24-2/30-2) | 10–30° (protocol-dependent) | Moderate–high | 10-2: Good sensitivity (sensitivity 85–90%, specificity 85–95%) for HCQ toxicity screening; central scotoma detection in AMD. |
| Frequency-Doubling (FDT) | 2–3 min per eye | Central ~26° (19 points) | Low–moderate | Potential to detect early neural dysfunction in DR before vascular signs appear. Sensitivity of 90.5% and specificity of 97.6% for sight-threatening retinopathy. |
| Microperimetry | 6–15 min per eye | Central 2–30° | High | Maps paracentral scotomas in AMD (sensitivity 80%, specificity 30.4%); tracks GA progression. 73% sensitivity and 93% specificity for HCQ screening. |
| Amsler Grid | <1 min (self-test) | Central ~10° | Very low | Identifies metamorphopsia in wet AMD; moderate sensitivity for central defects. |
| Disease | Pathophysiology and Overview | Typical Visual Field Defect Pattern | Main Perimetry Method(s) |
|---|---|---|---|
| Age-Related Macular Degeneration (AMD) |
|
| Amsler Grid (self-monitoring) Humphrey 10-2 (central field detail) |
| Diabetic Retinopathy (DR)/Diabetic Macular Edema (DME) |
|
| Frequency Doubling Technology perimetry Blue-on-Yellow perimetry Humphrey 24-2/30-2 |
| Hydroxychloroquine Retinopathy |
|
| Humphrey 10-2 (parafoveal retinopathy) Humphrey 24-2/30-2 (pericentral patterns) |
| Retinitis Pigmentosa (RP) |
|
| Goldmann kinetic perimetry (especially for peripheral mapping) Humphrey 30-2/60-4 (central support) |
| Stargardt Disease (Fundus Flavimaculatus) |
|
| Microperimetry (early small-scotoma detection) Humphrey 10-2 (ongoing monitoring) |
| Central Serous Chorioretinopathy (CSCR) |
|
|
|
| Retinal Vein Occlusion (RVO) |
|
|
|
| Macular Hole |
|
|
|
| Category | Challenges/Limitations | Implications/Mitigation |
|---|---|---|
| Patient Factors |
|
|
| Media Opacities and Coexisting Conditions |
|
|
| Normative Database and Floor/Ceiling Effects |
|
|
| Technical and Environmental Factors |
|
|
| Disease-Specific Issues |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.E.; Ahn, S.J. Visual Field Examinations for Retinal Diseases: A Narrative Review. J. Clin. Med. 2025, 14, 5266. https://doi.org/10.3390/jcm14155266
Kim KE, Ahn SJ. Visual Field Examinations for Retinal Diseases: A Narrative Review. Journal of Clinical Medicine. 2025; 14(15):5266. https://doi.org/10.3390/jcm14155266
Chicago/Turabian StyleKim, Ko Eun, and Seong Joon Ahn. 2025. "Visual Field Examinations for Retinal Diseases: A Narrative Review" Journal of Clinical Medicine 14, no. 15: 5266. https://doi.org/10.3390/jcm14155266
APA StyleKim, K. E., & Ahn, S. J. (2025). Visual Field Examinations for Retinal Diseases: A Narrative Review. Journal of Clinical Medicine, 14(15), 5266. https://doi.org/10.3390/jcm14155266






