Subscapularis CT-Scan Evaluation in Patients with Proximal Humerus Fracture: Reverse Total Shoulder Arthroplasty Versus Hemi-Arthroplasty
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
- Age greater than 60;
- Proximal humeral fracture with displaced three- or four-part (according to Neer’s classification [3]) confirmed by shoulder X-ray and pre-operative CT scan;
- Absence of neurological disease or cognitive dysfunction.
- Fracture-dislocation;
- History of rheumatic diseases;
- Presence of neurological disease or cognitive dysfunction;
- Pathologic or open fractures;
- Associated neurovascular injury;
- Degenerated or irreparable subscapularis tendon.
2.2. Surgical Technique
2.3. Clinical Evaluation
2.4. CT Evaluation
2.5. Statistical Analysis
3. Results
3.1. Demographics
3.2. Clinical Assessment
3.3. Range of Motion
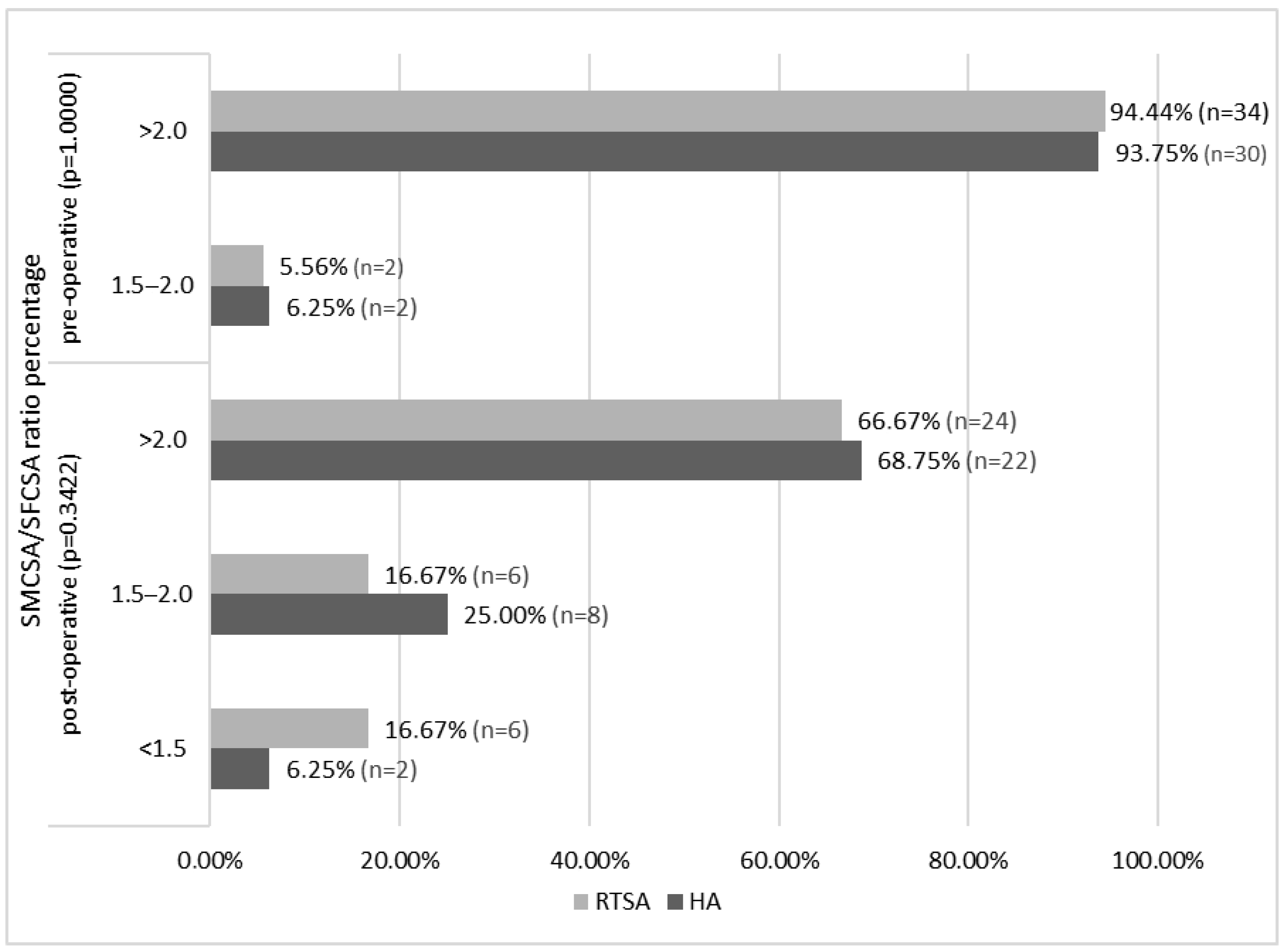
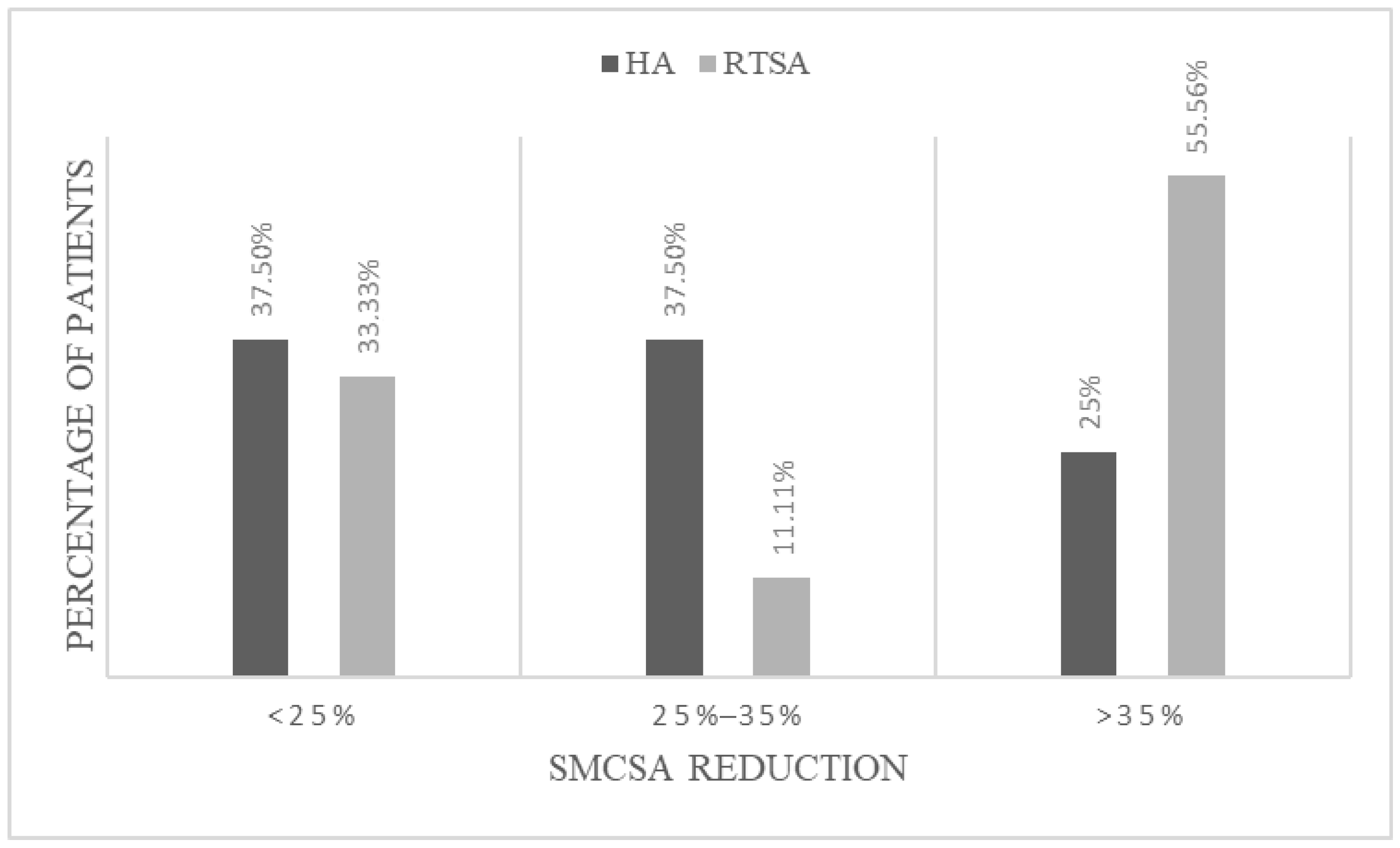
3.4. Radiographic Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HA | Hemiarthroplasty |
| RTSA | Reverse Total Shoulder Arthroplasty |
| SMCSA | Subscapularis muscle cross-sectional area |
| SFCSA | Supraspinatus fossa cross-sectional area |
| PROs | Patient reported outcomes |
| ROM | Range of motion |
| CT | Computed Tomography |
| ER | External rotation |
| IR | Internal rotation |
| VAS | Visual Analog Scale |
| ASES | American Shoulder and Elbow Surgeons score |
References
- Calvo, E.; Morcillo, D.; Foruria, A.M.; Redondo-Santamaría, E.; Osorio-Picorne, F.; Caeiro, J.R.; GEIOS-SECOT Outpatient Osteoporotic Fracture Study Group. Nondisplaced Proximal Humeral Fractures: High Incidence among Outpatient-Treated Osteoporotic Fractures and Severe Impact on Upper Extremity Function and Patient Subjective Health Perception. J. Shoulder Elb. Surg. 2011, 20, 795–801. [Google Scholar] [CrossRef]
- Hashiguchi, H.; Iwashita, S.; Ohkubo, A.; Takai, S. The Outcome of Hemiarthroplasty for Proximal Humeral Fractures Is Dependent on the Status of the Rotator Cuff. Int. Orthop. 2015, 39, 1115–1119. [Google Scholar] [CrossRef]
- Neer, C.S. Displaced Proximal Humeral Fractures. I. Classification and Evaluation. J. Bone Jt. Surg. Am. 1970, 52, 1077–1089. [Google Scholar] [CrossRef]
- Maier, D.; Jaeger, M.; Izadpanah, K.; Strohm, P.C.; Suedkamp, N.P. Proximal Humeral Fracture Treatment in Adults. J. Bone Jt. Surg. 2014, 96, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, C.; Berizzi, A.; Biz, C.; Camporese, A. Treatment of Proximal Humeral Fractures with Reverse Shoulder Arthroplasty in Elderly Patients. Musculoskelet. Surg. 2015, 99, 39–44. [Google Scholar] [CrossRef]
- Armstrong, A.; Lashgari, C.; Teefey, S.; Menendez, J.; Yamaguchi, K.; Galatz, L.M. Ultrasound Evaluation and Clinical Correlation of Subscapularis Repair after Total Shoulder Arthroplasty. J. Shoulder Elb. Surg. 2006, 15, 541–548. [Google Scholar] [CrossRef]
- Jawa, A.; Colliton, E.M. Role of Subscapularis Tendon Repair in Reverse Total Shoulder Arthroplasty. J. Am. Acad. Orthop. Surg. 2021, 29, 604–608. [Google Scholar] [CrossRef]
- Miller, S.L.; Hazrati, Y.; Klepps, S.; Chiang, A.; Flatow, E.L. Loss of Subscapularis Function after Total Shoulder Replacement: A Seldom Recognized Problem. J. Shoulder Elb. Surg. 2003, 12, 29–34. [Google Scholar] [CrossRef]
- Chalmers, P.N.; Rahman, Z.; Romeo, A.A.; Nicholson, G.P. Early Dislocation after Reverse Total Shoulder Arthroplasty. J. Shoulder Elb. Surg. 2014, 23, 737–744. [Google Scholar] [CrossRef]
- Matthewson, G.; Kooner, S.; Kwapisz, A.; Leiter, J.; Old, J.; MacDonald, P. The Effect of Subscapularis Repair on Dislocation Rates in Reverse Shoulder Arthroplasty: A Meta-Analysis and Systematic Review. J. Shoulder Elb. Surg. 2019, 28, 989–997. [Google Scholar] [CrossRef]
- Hamilton, M.A.; Diep, P.; Roche, C.; Flurin, P.H.; Wright, T.W.; Zuckerman, J.D.; Routman, H. Effect of Reverse Shoulder Design Philosophy on Muscle Moment Arms: Moment Arms and Rtsa Designs. J. Orthop. Res. 2015, 33, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.L.; Nayak, A.; Narayanan, M.S.; Worhacz, K.; Stowell, R.; Jacofsky, M.C.; Roche, C.P. Role of Subscapularis Repair on Muscle Force Requirements with Reverse Shoulder Arthroplasty. Bull. Hosp. Jt. Dis. 2015, 73 (Suppl. 1), S21–S27. [Google Scholar]
- De Carli, A.; Gaj, E.; Drogo, P.; Viglietta, E.; Polidori, T.; Forlenza, E.; Patel, B.H.; Ferretti, A. Computer Tomography Assessment of the Subscapularis after Reverse Shoulder Arthroplasty and Subscapularis Repair: Reduction in Subscapularis Size Do Not Affect Clinical Outcomes. Semin. Arthroplast. JSES 2021, 31, 131–138. [Google Scholar] [CrossRef]
- Fuchs, B.; Weishaupt, D.; Zanetti, M.; Hodler, J.; Gerber, C. Fatty Degeneration of the Muscles of the Rotator Cuff: Assessment by Computed Tomography versus Magnetic Resonance Imaging. J. Shoulder Elb. Surg. 1999, 8, 599–605. [Google Scholar] [CrossRef]
- Zanetti, M.; Gerber, C.; Hodler, J. Quantitative Assessment of the Muscles of the Rotator Cuff with Magnetic Resonance Imaging. Investig. Radiol. 1998, 33, 163–170. [Google Scholar] [CrossRef]
- Constant, C.R.; Murley, A.H. A Clinical Method of Functional Assessment of the Shoulder. Clin. Orthop. Relat. Res. 1987, 214, 160–164. [Google Scholar] [CrossRef]
- Terrier, A.; Ston, J.; Dewarrat, A.; Becce, F.; Farron, A. A Semi-Automated Quantitative CT Method for Measuring Rotator Cuff Muscle Degeneration in Shoulders with Primary Osteoarthritis. Orthop. Traumatol. Surg. Res. 2017, 103, 151–157. [Google Scholar] [CrossRef]
- Thomazeau, H.; Rolland, Y.; Lucas, C.; Duval, J.-M.; Langlais, F. Atrophy of the Supraspinatus Belly Assessment by MRI in 55 Patients with Rotator Cuff Pathology. Acta Orthop. Scand. 1996, 67, 264–268. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, Y.; Zhang, F.; Chen, W.; Tian, Y.; Zhang, Y. Meta-Analysis Suggests That Reverse Shoulder Arthroplasty in Proximal Humerus Fractures Is a Better Option than Hemiarthroplasty in the Elderly. Int. Orthop. 2016, 40, 531–539. [Google Scholar] [CrossRef]
- Cuff, D.J.; Pupello, D.R. Comparison of Hemiarthroplasty and Reverse Shoulder Arthroplasty for the Treatment of Proximal Humeral Fractures in Elderly Patients. J. Bone Jt. Surg. 2013, 95, 2050–2055. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, Y.; Lu, Y.; Li, F.; Jiang, C. Long-Term Outcomes of Shoulder Hemiarthroplasty for Acute Proximal Humeral Fractures. Int. Orthop. 2023, 47, 1517–1526. [Google Scholar] [CrossRef]
- de Boer, F.A.; van Kampen, P.M.; Huijsmans, P.E. The Influence of Subscapularis Tendon Reattachment on Range of Motion in Reversed Shoulder Arthroplasty: A Clinical Study. Musculoskelet. Surg. 2016, 100, 121–126. [Google Scholar] [CrossRef]
- Routman, H.D. The Role of Subscapularis Repair in Reverse Total Shoulder Arthroplasty. Bull. Hosp. Jt. Dis. 2013, 71 (Suppl. 2), 108–112. [Google Scholar]
- Franceschetti, E.; de Sanctis, E.G.; Ranieri, R.; Palumbo, A.; Paciotti, M.; Franceschi, F. The Role of the Subscapularis Tendon in a Lateralized Reverse Total Shoulder Arthroplasty: Repair versus Nonrepair. Int. Orthop. 2019, 43, 2579–2586. [Google Scholar] [CrossRef] [PubMed]
- Roche, C.; Hamilton, M.; Diep, P.; Wright, T.W.; Flurin, P.-H.; Zuckerman, J.; Routman, H. Optimizing Deltoid Efficiency with Reverse Shoulder Arthroplasty Using a Novel Inset Center of Rotation Glenosphere Design. Bull. NYU Hosp. Jt. Dis. 2015, 73, S37–S41. [Google Scholar]
- Herrmann, S.; König, C.; Heller, M.; Perka, C.; Greiner, S. Reverse Shoulder Arthroplasty Leads to Significant Biomechanical Changes in the Remaining Rotator Cuff. J. Orthop. Surg. 2011, 6, 42. [Google Scholar] [CrossRef]
- Roche, C.P. Reverse Shoulder Arthroplasty Biomechanics. J. Funct. Morphol. Kinesiol. 2022, 7, 13. [Google Scholar] [CrossRef]
- Roche, C.P.; Diep, P.; Hamilton, M.; Crosby, L.A.; Flurin, P.-H.; Wright, T.W.; Zuckerman, J.D.; Routman, H.D. Impact of Inferior Glenoid Tilt, Humeral Retroversion, Bone Grafting, and Design Parameters on Muscle Length and Deltoid Wrapping in Reverse Shoulder Arthroplasty. Bull. Hosp. Jt. Dis. 2013, 71, 284–293. [Google Scholar]
- Goutallier, D.; Postel, J.M.; Bernageau, J.; Lavau, L.; Voisin, M.C. Fatty Muscle Degeneration in Cuff Ruptures. Pre- and Postoperative Evaluation by CT Scan. Clin. Orthop. 1994, 304, 78–83. [Google Scholar] [CrossRef]
- Edwards, T.B.; Williams, M.D.; Labriola, J.E.; Elkousy, H.A.; Gartsman, G.M.; O’Connor, D.P. Subscapularis Insufficiency and the Risk of Shoulder Dislocation after Reverse Shoulder Arthroplasty. J. Shoulder Elb. Surg. 2009, 18, 892–896. [Google Scholar] [CrossRef]
- Rol, M.; Favard, L.; Berhouet, J. Factors Associated with Internal Rotation Outcomes after Reverse Shoulder Arthroplasty. Orthop. Traumatol. Surg. Res. 2019, 105, 1515–1519. [Google Scholar] [CrossRef]
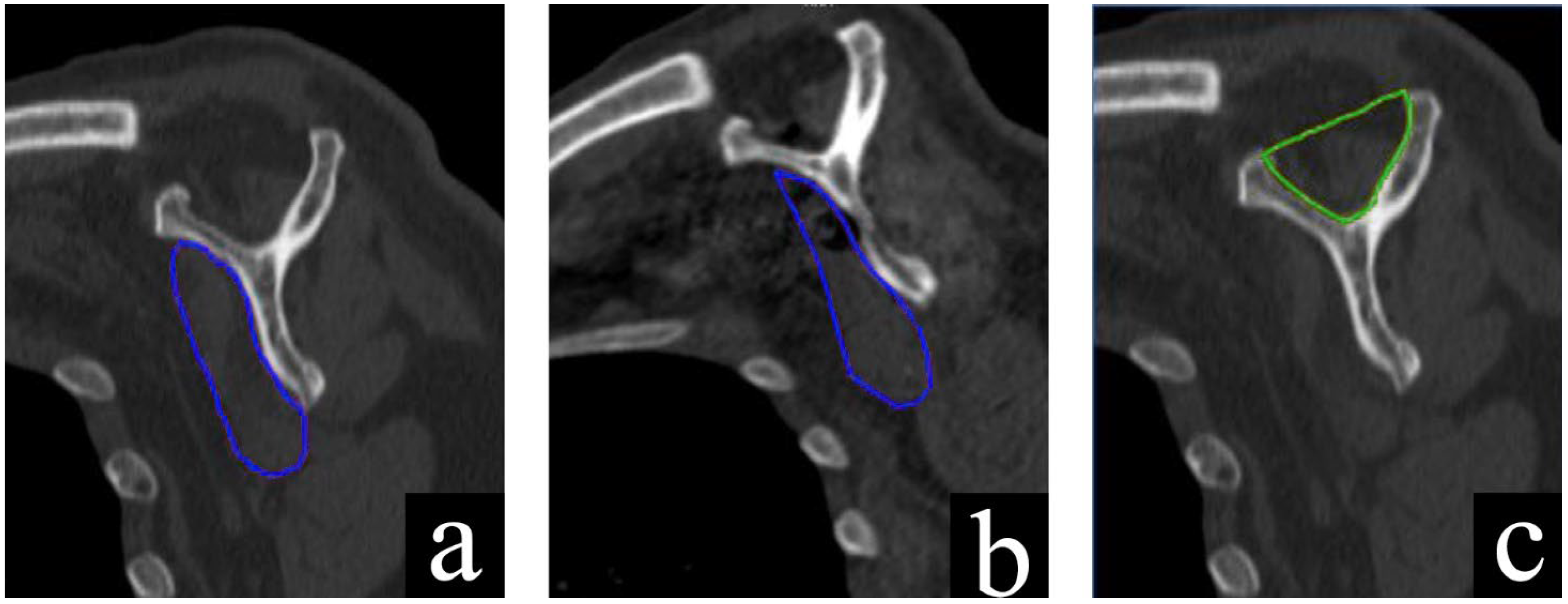
| Neer 3 | Neer 4 | AO 11-B1 | AO 11-B2 | AO 11-C1 | AO 11-C2 | |
|---|---|---|---|---|---|---|
| HA group (n = 32) | 18 (56.25%) | 14 (43.75%) | 10 (31.25%) | 8 (25.00%) | 5 (15.62%) | 9 (28.12%) |
| RTSA group (n = 36) | 21 (58.3%) | 15 (41.7%) | 11 (30.55%) | 10 (27.78%) | 7 (19.44%) | 8 (22.22%) |
| Tot. | 39 | 29 | 21 | 18 | 12 | 17 |
| Variable | HA | RTSA | p Value |
|---|---|---|---|
| Constant Score | 38.50 ± 11.73 | 58.00 ± 16.23 | 0.0001 |
| Quick Dash | 27.25 ± 23.10 | 14.75 ± 9.34 | 0.0006 |
| Simple Shoulder Test | 4.50 ± 14.75 | 8.00 ± 15.80 | 0.0129 |
| VAS (0–10) | 0.00 ± 2.12 | 2.00 ± 2.04 | 0.0311 |
| HA | RTSA | p Value | ||
|---|---|---|---|---|
| Forward flexion | 90.00 ± 37.64 | 147.50 ± 39.74 | <0.0001 | |
| Abduction | 85.00 ± 37.87 | 95.00 ± 37.83 | 0.0175 | |
| ER | Add | 25.00 ± 13.26 | 45.00 ± 22.93 | 0.0011 |
| Abd | 30.00 ± 13.18 | 30.00 ± 26.12 | 0.0258 | |
| IR | Add | 35.00 ± 10.55 | 42.50 ± 19.51 | 0.0047 |
| Abd | 30.00 ± 13.56 | 42.50 ± 24.38 | 0.0257 | |
| Posterior IR | Lateral side of the thigh | 0 (0%) | 2 (5.56%) | 0.0046 |
| Buttock | 16 (50%) | 6 (16.67%) | ||
| Lumbosacral junction | 10 (31.25%) | 8 (22.22%) | ||
| Waist | 2 (6.25%) | 12 (33.33%) | ||
| Lumbothoracic junction | 4 (12.5%) | 8 (22.22%) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaj, E.; Redler, A.; Maggiori, A.; Pagnotta, S.; Criseo, N.; Mirle, V.; Daggett, M.; De Carli, A. Subscapularis CT-Scan Evaluation in Patients with Proximal Humerus Fracture: Reverse Total Shoulder Arthroplasty Versus Hemi-Arthroplasty. J. Clin. Med. 2025, 14, 5257. https://doi.org/10.3390/jcm14155257
Gaj E, Redler A, Maggiori A, Pagnotta S, Criseo N, Mirle V, Daggett M, De Carli A. Subscapularis CT-Scan Evaluation in Patients with Proximal Humerus Fracture: Reverse Total Shoulder Arthroplasty Versus Hemi-Arthroplasty. Journal of Clinical Medicine. 2025; 14(15):5257. https://doi.org/10.3390/jcm14155257
Chicago/Turabian StyleGaj, Edoardo, Andrea Redler, Alessandro Maggiori, Susanna Pagnotta, Natale Criseo, Vikranth Mirle, Matthew Daggett, and Angelo De Carli. 2025. "Subscapularis CT-Scan Evaluation in Patients with Proximal Humerus Fracture: Reverse Total Shoulder Arthroplasty Versus Hemi-Arthroplasty" Journal of Clinical Medicine 14, no. 15: 5257. https://doi.org/10.3390/jcm14155257
APA StyleGaj, E., Redler, A., Maggiori, A., Pagnotta, S., Criseo, N., Mirle, V., Daggett, M., & De Carli, A. (2025). Subscapularis CT-Scan Evaluation in Patients with Proximal Humerus Fracture: Reverse Total Shoulder Arthroplasty Versus Hemi-Arthroplasty. Journal of Clinical Medicine, 14(15), 5257. https://doi.org/10.3390/jcm14155257






