Sex Differences and Long-Term Outcomes in Patients with Left Bundle Branch Area Pacing Compared with Right Ventricular Pacing
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Procedural Techniques for Left Bundle Branch Area Pacing
2.3. Study Endpoint
2.4. Statistical Analyses
3. Results
3.1. Baseline Characteristics of Total Cohort with RVP and LBBAP
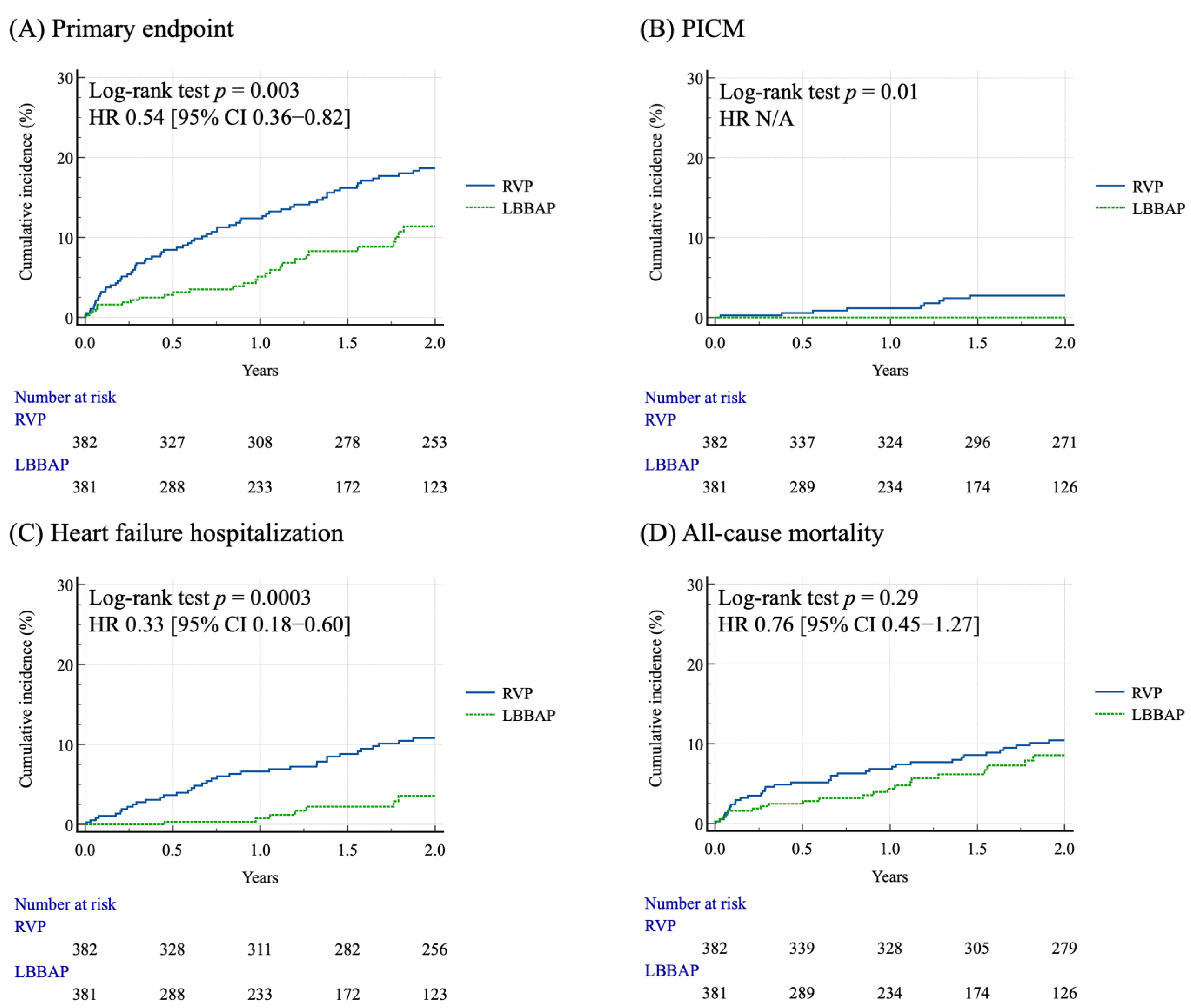
3.2. Clinical Outcomes of the Matched Cohort After PSM
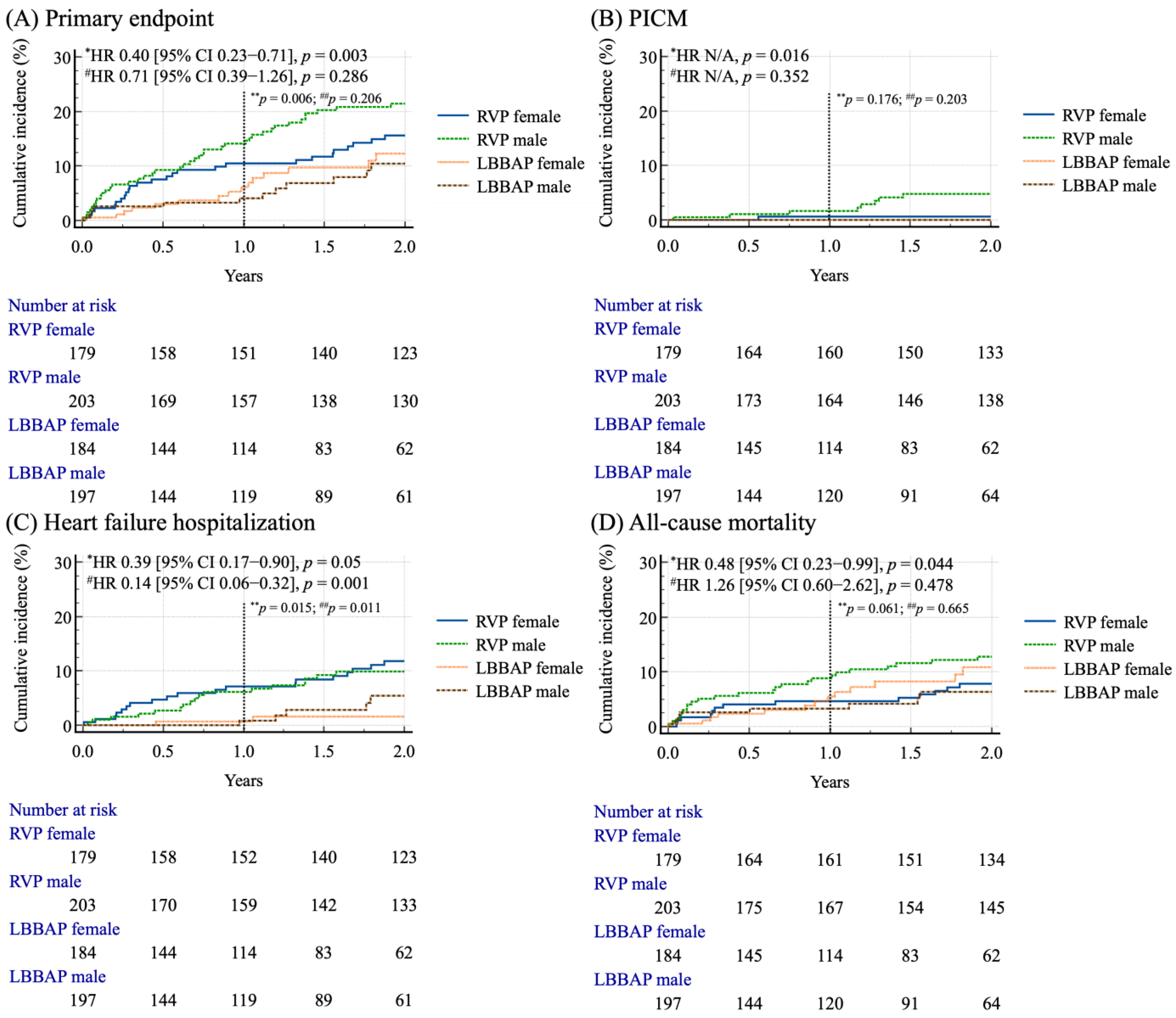
3.3. Sex-Based Survival Analysis in Matched Cohort
3.4. Identification of Predictors of Primary Composite Endpoint and Heart Failure Hospitalization
4. Discussion
4.1. Sex-Based Demographic Disparities
4.2. Sex-Based and Pacing-Modality-Related Outcomes
4.3. Sex-Based and Pacing-Modality-Related Survival Analysis
4.4. Potential Influencing Factors for HFH, PICM, and All-Cause Mortality in the Matched Cohort
4.5. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACS | Acute coronary syndrome |
| CRT | Cardiac resynchronization therapy |
| CIED | Cardiac implantable electronic devices |
| HFH | Heart failure hospitalization |
| LBBAP | Left bundle branch area pacing |
| PICM | Pacing-induced cardiomyopathy |
| RVP | Right ventricular pacing |
References
- Furman, S.; Schwedel, J.B. An intracardiac pacemaker for Stokes-Adams seizures. N. Engl. J. Med. 1959, 261, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, T.Z.; Chao, C.J. Adverse effects of right ventricular pacing on cardiac function: Prevalence, prevention and treatment with physiologic pacing. Trends Cardiovasc. Med. 2023, 33, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, S.S.; Syed, T.; Vijayaraman, P. Pacing induced cardiomyopathy: Recognition and management. Heart 2023, 109, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Park, H.W. New Insights into Pacing Induced Cardiomyopathy. Rev. Cardiovasc. Med. 2024, 25, 118. [Google Scholar] [CrossRef]
- Li, F.; Zhang, Y.; Huang, J.; Peng, S.L.; Jin, M.C.; Geng, C.; Ravi, V.; Sharma, P.S.; Vijayaraman, P.; Li, H. Lower Risk of New-Onset Atrial Fibrillation in Conduction System Pacing Compared With Right Ventricular Pacing. Pacing Clin. Electrophysiol. 2025, 48, 202–215. [Google Scholar] [CrossRef]
- Liu, B.; Dai, W.; Lou, Y.; Li, Y.; Wu, Y.; Du, J. Risk of Atrial Fibrillation Following Left Bundle Branch Area Pacing versus Right Ventricular Pacing and Biventricular Pacing: A Systematic Review and Meta-Analysis. Rev. Cardiovasc. Med. 2023, 24, 220. [Google Scholar] [CrossRef]
- Veasey, R.A.; Arya, A.; Silberbauer, J.; Sharma, V.; Lloyd, G.W.; Patel, N.R.; Sulke, A.N. The relationship between right ventricular pacing and atrial fibrillation burden and disease progression in patients with paroxysmal atrial fibrillation: The long-MinVPACE study. Europace 2011, 13, 815–820. [Google Scholar] [CrossRef]
- Chen, H.C.; Liu, W.H.; Chen, Y.L.; Lee, W.C.; Fang, Y.N.; Chong, S.Z.; Chen, M.C. Left bundle branch pacing preserved left ventricular myocardial work in patients with bradycardia. Front. Cardiovasc. Med. 2023, 10, 1201841. [Google Scholar] [CrossRef]
- Yin, L.; Wang, L.; Meng, J.; Liu, Q.; Zhang, Y.; Zhao, Y.; Li, M.; You, L. A systematic review and meta-analysis of the impact of left bundle branch area pacing on right ventricular function. Front. Cardiovasc. Med. 2025, 12, 1545757. [Google Scholar] [CrossRef]
- Kono, H.; Kuramitsu, S.; Fukunaga, M.; Korai, K.; Nagashima, M.; Hiroshima, K.; Ando, K. Outcomes of left bundle branch area pacing compared to His bundle pacing and right ventricular apical pacing in Japanese patients with bradycardia. J. Arrhythm. 2024, 40, 333–341. [Google Scholar] [CrossRef]
- Vijayaraman, P.; Longacre, C.; Kron, J.; Subzposh, F.; Zimmerman, P.; Butler, K.; Crossley, G.H.; Ellenbogen, K.A. Conduction system pacing associated with reduced heart failure hospitalizations and all-cause mortality compared with traditional right ventricular pacing in the Medicare population. Heart Rhythm 2025, 22, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Leventopoulos, G.; Travlos, C.K.; Aronis, K.N.; Anagnostopoulou, V.; Patrinos, P.; Papageorgiou, A.; Perperis, A.; Gale, C.P.; Davlouros, P. Safety and efficacy of left bundle branch area pacing compared with right ventricular pacing in patients with bradyarrhythmia and conduction system disorders: Systematic review and meta-analysis. Int. J. Cardiol. 2023, 390, 131230. [Google Scholar] [CrossRef] [PubMed]
- Tun, H.N.; Khan, H.; Chernikova, D.; Mareev, Y.; Chakrabarti, S.; Thant, M.; Cannata, A. Conduction system pacing: Promoting the physiology to prevent heart failure. Heart Fail. Rev. 2023, 28, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.K.; Patton, K.K.; Lau, C.P.; Dal Forno, A.R.J.; Al-Khatib, S.M.; Arora, V.; Birgersdotter-Green, U.M.; Cha, Y.M.; Chung, E.H.; Cronin, E.M.; et al. 2023 HRS/APHRS/LAHRS guideline on cardiac physiologic pacing for the avoidance and mitigation of heart failure. Heart Rhythm 2023, 20, e17–e91. [Google Scholar] [CrossRef]
- Okubo, Y.; Sakai, T.; Miyamoto, S.; Uotani, Y.; Oguri, N.; Furutani, M.; Miyauchi, S.; Okamura, S.; Tokuyama, T.; Nakano, Y. Mid-term clinical outcomes of left bundle branch area pacing compared to accurate right ventricular septal pacing. J. Interv. Card. Electrophysiol. 2025, 68, 55–63. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Qiu, C.; Wang, Z.; Li, H.; Pang, K.; Yao, Y.; Liu, Z.; Xie, R.; Chen, Y.; et al. Clinical Outcomes in Patients With Left Bundle Branch Area Pacing vs. Right Ventricular Pacing for Atrioventricular Block. Front. Cardiovasc. Med. 2021, 8, 685253. [Google Scholar] [CrossRef]
- Diaz, J.C.; Gabr, M.; Tedrow, U.B.; Duque, M.; Aristizabal, J.; Marin, J.; Nino, C.; Bastidas, O.; Koplan, B.A.; Hoyos, C.; et al. Improved all-cause mortality with left bundle branch area pacing compared to biventricular pacing in cardiac resynchronization therapy: A meta-analysis. J. Interv. Card. Electrophysiol. 2024, 67, 1463–1476. [Google Scholar] [CrossRef]
- Linde, C.; Bongiorni, M.G.; Birgersdotter-Green, U.; Curtis, A.B.; Deisenhofer, I.; Furokawa, T.; Gillis, A.M.; Haugaa, K.H.; Lip, G.Y.H.; Van Gelder, I.; et al. Sex differences in cardiac arrhythmia: A consensus document of the European Heart Rhythm Association, endorsed by the Heart Rhythm Society and Asia Pacific Heart Rhythm Society. Europace 2018, 20, 1565–1565ao. [Google Scholar] [CrossRef]
- Ehdaie, A.; Cingolani, E.; Shehata, M.; Wang, X.; Curtis, A.B.; Chugh, S.S. Sex Differences in Cardiac Arrhythmias: Clinical and Research Implications. Circ. Arrhythm. Electrophysiol. 2018, 11, e005680. [Google Scholar] [CrossRef]
- MacFadden, D.R.; Crystal, E.; Krahn, A.D.; Mangat, I.; Healey, J.S.; Dorian, P.; Birnie, D.; Simpson, C.S.; Khaykin, Y.; Pinter, A.; et al. Sex differences in implantable cardioverter-defibrillator outcomes: Findings from a prospective defibrillator database. Ann. Intern. Med. 2012, 156, 195–203. [Google Scholar] [CrossRef]
- Huang, W.; Chen, X.; Su, L.; Wu, S.; Xia, X.; Vijayaraman, P. A beginner’s guide to permanent left bundle branch pacing. Heart Rhythm 2019, 16, 1791–1796. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Qian, Z.; Zou, F.; Wang, Y.; Zhang, X.; Qiu, Y.; Hou, X.; Zhou, X.; Vijayaraman, P.; Zou, J. Differentiating left bundle branch pacing and left ventricular septal pacing: An algorithm based on intracardiac electrophysiology. J. Cardiovasc. Electrophysiol. 2022, 33, 448–457. [Google Scholar] [CrossRef] [PubMed]
- De Silva, K.; Nassar, N.; Badgery-Parker, T.; Kumar, S.; Taylor, L.; Kovoor, P.; Zaman, S.; Wilson, A.; Chow, C.K. Sex-Based Differences in Selected Cardiac Implantable Electronic Device Use: A 10-Year Statewide Patient Cohort. J. Am. Heart Assoc. 2022, 11, e025428. [Google Scholar] [CrossRef]
- Vijayarajan, V.; Kritharides, L.; Brieger, D.; Cheng, Y.Y.; Chow, V.; Ng, A.C.C. Sex differences in rates of permanent pacemaker implantation and in-hospital complications: A statewide cohort study of over 7 million persons from 2009–2018. PLoS ONE 2022, 17, e0272305. [Google Scholar] [CrossRef]
- Eccleston, D.; Cehic, D.; Young, G.; Lin, T.; Pavia, S.; Chowdhury, E.K.; Reid, C.; Liew, D.; King, B.; Tan, I.; et al. Sex differences in Cardiac electronic device implantation: Outcomes from an Australian multi-centre clinical quality registry. Int. J. Cardiol. Heart Vasc. 2021, 35, 100828. [Google Scholar] [CrossRef]
- Riesenhuber, M.; Spannbauer, A.; Rauscha, F.; Schmidinger, H.; Boszotta, A.; Pezawas, T.; Schukro, C.; Gwechenberger, M.; Stix, G.; Anvari, A.; et al. Sex Differences and Long-Term Outcome in Patients With Pacemakers. Front. Cardiovasc. Med. 2020, 7, 569060. [Google Scholar] [CrossRef]
- Costa, S.; Saguner, A.M.; Gasperetti, A.; Akdis, D.; Brunckhorst, C.; Duru, F. The Link Between Sex Hormones and Susceptibility to Cardiac Arrhythmias: From Molecular Basis to Clinical Implications. Front. Cardiovasc. Med. 2021, 8, 644279. [Google Scholar] [CrossRef]
- Sivasinprasasn, S.; Shinlapawittayatorn, K.; Chattipakorn, S.C.; Chattipakorn, N. Estrogenic Impact on Cardiac Ischemic/Reperfusion Injury. J. Cardiovasc. Transl. Res. 2016, 9, 23–39. [Google Scholar] [CrossRef]
- Savergnini, S.Q.; Reis, A.M.; Santos, R.A.; Santos, P.E.; Ferreira, A.J.; Almeida, A.P. Effects of short-term administration of estradiol on reperfusion arrhythmias in rats of different ages. Braz. J. Med. Biol. Res. 2012, 45, 1248–1254. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Zhao, Y.; Gong, D.; Wang, D.; Li, C.; Zhao, H. Protective effects of estrogen against reperfusion arrhythmias following severe myocardial ischemia in rats. Circ. J. 2010, 74, 634–643. [Google Scholar] [CrossRef]
- Hess, P.L.; Hernandez, A.F.; Bhatt, D.L.; Hellkamp, A.S.; Yancy, C.W.; Schwamm, L.H.; Peterson, E.D.; Schulte, P.J.; Fonarow, G.C.; Al-Khatib, S.M. Sex and Race/Ethnicity Differences in Implantable Cardioverter-Defibrillator Counseling and Use Among Patients Hospitalized With Heart Failure: Findings from the Get With The Guidelines-Heart Failure Program. Circulation 2016, 134, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Horton, H.L.; Marinchak, R.A.; Rials, S.J.; Kowey, P.R. Gender differences in device therapy for malignant ventricular arrhythmias. Arch. Intern. Med. 1995, 155, 2342–2345. [Google Scholar] [CrossRef] [PubMed]
- Tops, L.F.; Schalij, M.J.; Bax, J.J. The effects of right ventricular apical pacing on ventricular function and dyssynchrony implications for therapy. J. Am. Coll. Cardiol. 2009, 54, 764–776. [Google Scholar] [CrossRef]
- Sweeney, M.O.; Hellkamp, A.S.; Ellenbogen, K.A.; Greenspon, A.J.; Freedman, R.A.; Lee, K.L.; Lamas, G.A.; MOde Selection Trial Investigators. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation 2003, 107, 2932–2937. [Google Scholar] [CrossRef]
- Tang, J.; Kong, N.W.; Beaser, A.; Aziz, Z.; Yeshwant, S.; Ozcan, C.; Tung, R.; Upadhyay, G.A. Clinical outcomes of conduction system pacing compared to biventricular pacing in patients with mid-range ejection fraction. J. Interv. Card. Electrophysiol. 2025, 68, 111–116. [Google Scholar] [CrossRef]
- Tedrow, U.B.; Miranda-Arboleda, A.F.; Sauer, W.H.; Duque, M.; Koplan, B.A.; Marin, J.E.; Aristizabal, J.M.; Nino, C.D.; Bastidas, O.; Martinez, J.M.; et al. Sex Differences in Left Bundle Branch Area Pacing Versus Biventricular Pacing for Cardiac Resynchronization Therapy. JACC Clin. Electrophysiol. 2024, 10, 1736–1749. [Google Scholar] [CrossRef]
- Pyatt, J.R.; Somauroo, J.D.; Jackson, M.; Grayson, A.D.; Osula, S.; Aggarwal, R.K.; Charles, R.G.; Connelly, D.T. Long-term survival after permanent pacemaker implantation: Analysis of predictors for increased mortality. Europace 2002, 4, 113–119. [Google Scholar] [CrossRef]
- Tayal, B.; Gorcsan, J., 3rd; Delgado-Montero, A.; Goda, A.; Ryo, K.; Saba, S.; Risum, N.; Sogaard, P. Comparative long-term outcomes after cardiac resynchronization therapy in right ventricular paced patients versus native wide left bundle branch block patients. Heart Rhythm 2016, 13, 511–518. [Google Scholar] [CrossRef]
- Prinzen, F.W.; Hunter, W.C.; Wyman, B.T.; McVeigh, E.R. Mapping of regional myocardial strain and work during ventricular pacing: Experimental study using magnetic resonance imaging tagging. J. Am. Coll. Cardiol. 1999, 33, 1735–1742. [Google Scholar] [CrossRef]
- Alizadeh, A.; Sanati, H.R.; Haji-Karimi, M.; Yazdi, A.H.; Rad, M.A.; Haghjoo, M.; Emkanjoo, Z. Induction and aggravation of atrioventricular valve regurgitation in the course of chronic right ventricular apical pacing. Europace 2011, 13, 1587–1590. [Google Scholar] [CrossRef] [PubMed]

| Before PSM (n = 1211) | After PSM (n = 764) | |||||
|---|---|---|---|---|---|---|
| Variables | RVP (n = 789) | LBBAP (n = 422) | SMD | RVP (n = 382) | LBBAP (n = 382) | SMD |
| Male | 392 (49.7) | 217 (51.4) | 0.035 | 203 (53.1) | 197 (51.6) | 0.031 |
| Age, years | 74.9 (10.8) | 74.6 (9.7) | 0.024 | 74.6 (10.5) | 74.5 (9.8) | 0.002 |
| BMI, kg/m2 | 24.8 (3.9) | 25.12 (4.0) | 0.069 | 25.0 (3.9) | 25.1 (3.9) | 0.024 |
| Hypertension | 595 (75.4) | 315 (74.6) | 0.018 | 283 (74.1) | 283 (74.1) | <0.001 |
| Diabetes | 332 (42.1) | 166 (39.3) | 0.056 | 138 (36.1) | 142 (37.2) | 0.022 |
| Dyslipidemia | 312 (39.5) | 175 (41.5) | 0.039 | 155 (40.6) | 153 (40.1) | 0.011 |
| CAD | 176 (22.3) | 84 (19.9) | 0.059 | 74 (19.4) | 77 (20.2) | 0.020 |
| HF history | 126 (16.0) | 55 (13.0) | 0.083 | 45 (11.8) | 45 (11.8) | <0.001 |
| VHD a | 133 (16.9) | 79 (18.7) | 0.049 | 66 (17.3) | 71 (18.6) | 0.034 |
| AF | 296 (37.5) | 150 (35.5) | 0.041 | 143 (37.4) | 136 (35.6) | 0.038 |
| CVA | 142 (18.0) | 81 (19.2) | 0.031 | 74 (19.4) | 74 (19.4) | <0.001 |
| CKD b | 244 (30.9) | 79 (18.7) | 0.285 | 73 (19.1) | 75 (19.6) | 0.013 |
| ESRD c | 78 (9.9) | 27 (6.4) | 0.128 | 23 (6.0) | 27 (7.1) | 0.042 |
| PAD | 22 (2.8) | 5 (1.2) | 0.115 | 9 (2.4) | 5 (1.3) | 0.078 |
| Malignancy | 161 (20.4) | 72 (17.1) | 0.086 | 68 (17.8) | 68 (17.8) | <0.001 |
| CV op history | 59 (7.5) | 33 (7.8) | 0.013 | 26 (6.8) | 29 (7.6) | 0.030 |
| COPD/asthma | 98 (12.4) | 34 (8.1) | 0.144 | 30 (7.9) | 33 (8.6) | 0.029 |
| Beta-blocker | 337 (42.7) | 207 (49.1) | 0.127 | 174 (45.5) | 183 (47.9) | 0.047 |
| RAS blockade | 469 (59.4) | 261 (61.8) | 0.049 | 231 (60.5) | 232 (60.7) | 0.005 |
| Diuretic | 268 (34.0) | 78 (18.5) | 0.358 | 76 (19.9) | 77 (20.2) | 0.007 |
| Statin | 349 (44.2) | 216 (51.2) | 0.140 | 187 (49.0) | 186 (48.7) | 0.005 |
| SGLT2i | 57 (7.2) | 71 (16.8) | 0.298 | 43 (11.3) | 43 (11.3) | <0.001 |
| OADs | 248 (31.4) | 147 (34.8) | 0.072 | 118 (30.9) | 122 (31.9) | 0.023 |
| Insulin | 53 (6.7) | 24 (5.7) | 0.043 | 23 (6.0) | 19 (5.0) | 0.046 |
| Stratified by Different Pacing Strategy | |||
|---|---|---|---|
| RVP (n = 382) | LBBAP (n = 382) | p Value | |
| Cardiovascular events | 70 (18.3) | 20 (5.2) | <0.001 |
| HF hospitalization | 52 (13.6) | 10 (2.6) | <0.001 |
| ACS hospitalization | 25 (6.5) | 11 (2.9) | 0.017 |
| CVA * | 13 (3.4) | 9 (2.4) | 0.387 |
| PICM | 14 (3.7) | 0 (0) | <0.001 |
| Primary outcomes | 94 (24.6) | 33 (8.6) | <0.001 |
| Cardiovascular mortality | 9 (2.4) | 2 (0.5) | 0.034 |
| All-cause mortality | 53 (13.9) | 25 (6.5) | <0.001 |
| Stratified by Different Pacing Modalities and Gender | ||||||
|---|---|---|---|---|---|---|
| RVP (n = 382) | LBBAP (n = 382) | |||||
| Male (n = 203) | Female (n = 179) | p Value | Male (n = 197) | Female (n = 185) | p Value | |
| Cardiovascular events | 36 (17.7) | 34 (19.0) | 0.751 | 10 (5.1) | 10 (5.4) | 0.885 |
| HF hospitalization | 24 (11.8) | 28 (15.6) | 0.277 | 6 (3.0) | 4 (2.2) | 0.587 |
| ACS hospitalization | 13 (6.4) | 12 (6.7) | 0.906 | 5 (2.5) | 6 (3.2) | 0.68 |
| CVA | 10 (4.9) | 3 (1.7) | 0.08 | 7 (3.6) | 2 (1.1) | 0.101 |
| PICM | 9 (4.4) | 5 (2.8) | 0.395 | 0 (0) | 0 (0) | N/A |
| Primary outcomes | 53 (26.1) | 41 (22.9) | 0.468 | 14 (7.1) | 19 (10.3) | 0.271 |
| Cardiovascular mortality | 3 (1.5) | 6 (3.4) | 0.228 | 2 (1.0) | 0 (0) | 0.103 |
| All-cause mortality | 32 (15.8) | 21 (11.7) | 0.255 | 10 * (5.1) | 15 ^ (8.1) | 0.231 |
| After PSM (n = 764) | ||||
|---|---|---|---|---|
| Variables | Univariate Analysis | Multivariate Analysis | ||
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Sex (male) | 1.04 (0.73–1.47) | 0.814 | ||
| Age (years) | 1.04 (1.02–1.06) | <0.001 | 1.04 (1.01–1.06) | <0.001 |
| BMI | 0.95 (0.92–1.01) | 0.137 | ||
| Hypertension | 1.84 (1.16–2.92) | 0.009 | 1.06 (0.65–1.73) | 0.8 |
| Diabetes mellitus | 1.51 (1.06–2.15) | 0.02 | 1.22 (0.84–1.78) | 0.287 |
| Dyslipidemia | 0.75 (0.52–1.09) | 0.134 | ||
| Coronary artery disease | 2.32 (1.59–3.38) | <0.001 | 2.24 (1.49–3.37) | <0.001 |
| Heart failure | 3.37 (2.22–5.12) | <0.001 | 1.64 (1.002–2.59) | 0.049 |
| Valvular heart disease | 3.15 (2.17–4.58) | <0.001 | 2.14 (1.39–3.29) | 0.001 |
| Atrial fibrillation | 1.43 (1.009–2.03) | 0.044 | 1.51 (1.05–2.17) | 0.024 |
| Cerebral vascular accident | 1.29 (0.84–1.98) | 0.234 | ||
| Chronic kidney disease | 2.34 (1.60–3.42) | <0.001 | 1.96 (1.30–2.95) | 0.001 |
| End-stage renal disease | 3.28 (1.96–5.50) | <0.001 | 2.22 (1.19–4.12) | 0.012 |
| Peripheral artery disease | 6.78 (3.30–13.9) | <0.001 | 2.94 (1.32–6.52) | 0.008 |
| LBBAP | 0.58 (0.38–0.88) | 0.012 | 0.56 (0.37–0.84) | 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, P.-W.; Chen, U.; Wu, P.-J.; Chong, S.-Z.; Fang, Y.-N.; Chen, Y.-L.; Chen, M.-C.; Chen, H.-C. Sex Differences and Long-Term Outcomes in Patients with Left Bundle Branch Area Pacing Compared with Right Ventricular Pacing. J. Clin. Med. 2025, 14, 5256. https://doi.org/10.3390/jcm14155256
Yang P-W, Chen U, Wu P-J, Chong S-Z, Fang Y-N, Chen Y-L, Chen M-C, Chen H-C. Sex Differences and Long-Term Outcomes in Patients with Left Bundle Branch Area Pacing Compared with Right Ventricular Pacing. Journal of Clinical Medicine. 2025; 14(15):5256. https://doi.org/10.3390/jcm14155256
Chicago/Turabian StyleYang, Po-Wei, Uei Chen, Po-Jui Wu, Shaur-Zheng Chong, Yen-Nan Fang, Yung-Lung Chen, Mien-Cheng Chen, and Huang-Chung Chen. 2025. "Sex Differences and Long-Term Outcomes in Patients with Left Bundle Branch Area Pacing Compared with Right Ventricular Pacing" Journal of Clinical Medicine 14, no. 15: 5256. https://doi.org/10.3390/jcm14155256
APA StyleYang, P.-W., Chen, U., Wu, P.-J., Chong, S.-Z., Fang, Y.-N., Chen, Y.-L., Chen, M.-C., & Chen, H.-C. (2025). Sex Differences and Long-Term Outcomes in Patients with Left Bundle Branch Area Pacing Compared with Right Ventricular Pacing. Journal of Clinical Medicine, 14(15), 5256. https://doi.org/10.3390/jcm14155256







