Effectiveness of an Essential Oil Mouthwash on Halitosis in Obese Patients with Periodontitis: A Short-Term Clinical Evaluation
Abstract
1. Introduction
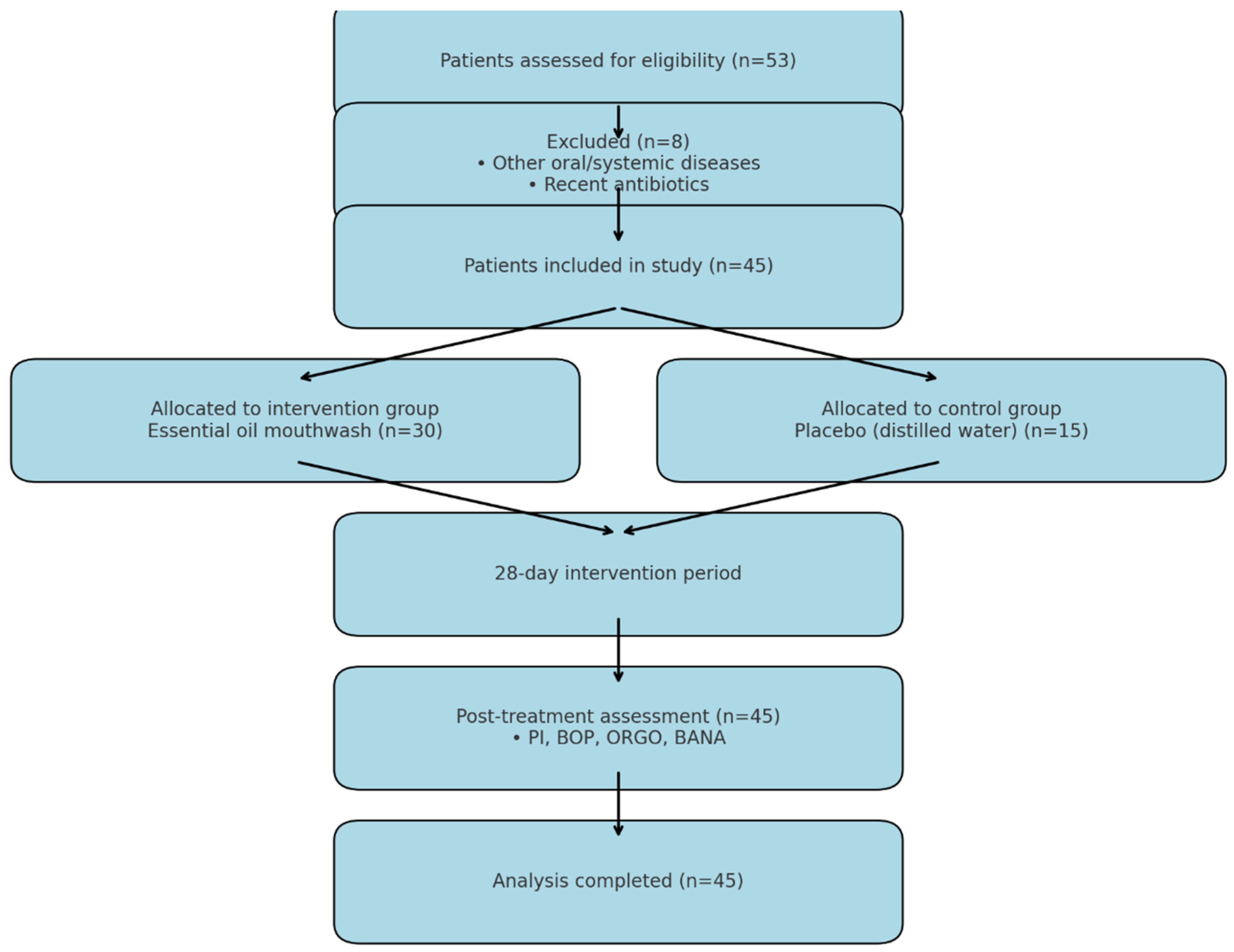
2. Materials and Methods
2.1. Participants and Eligibility Criteria
- Obesity, defined as a body mass index (BMI) ≥ 30 kg/m2, classified according to World Health Organization (WHO) thresholds (Class I: 30–34.9 kg/m2; Class II: 35–39.9 kg/m2; Class III: ≥40 kg/m2).
- Diagnosis of chronic periodontitis, confirmed by a periodontist and classified according to the EFP 2017 guidelines.
- Poor oral hygiene, indicated by a plaque index ≥10% and gingival index ≥ 10%.
- Self-reported halitosis.
2.2. Intervention Protocol
2.3. Clinical Assessment
- Plaque Index (PI): Assessed using the O’Leary plaque control record, examining representative vestibular and lingual surfaces of molars and incisors.
- Gingival Index (GI): Evaluated using the Löe and Silness index.
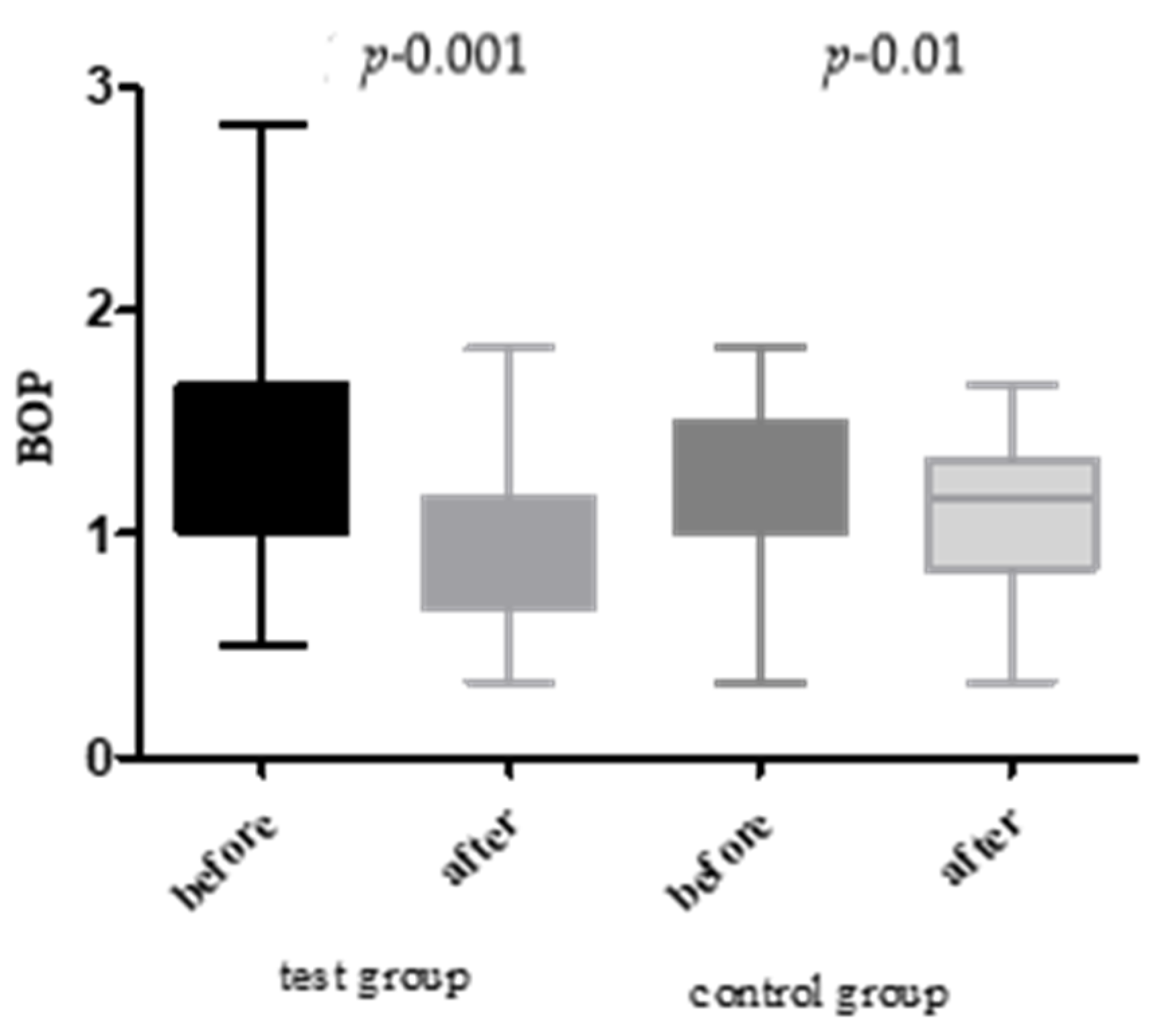
- Bleeding on Probing (BOP): Recorded as the percentage of sites exhibiting bleeding upon gentle probing.
2.4. Halitosis Evaluation
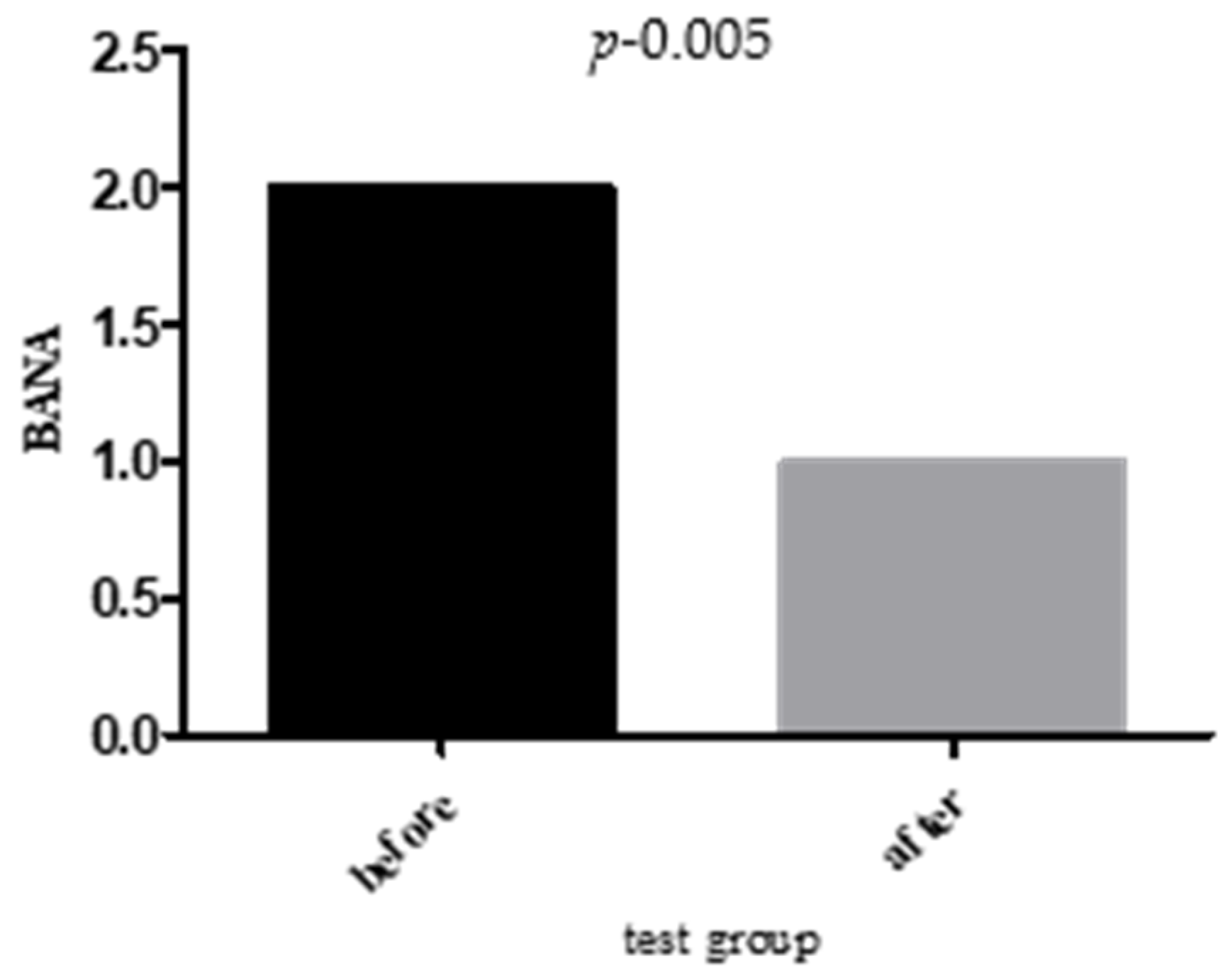
2.5. Microbiological Analysis
- Negative: No color change.
- Slight Positive: Light blue speckling.
- Positive: Uniform strong blue color.
2.6. Statistical Analysis
3. Results
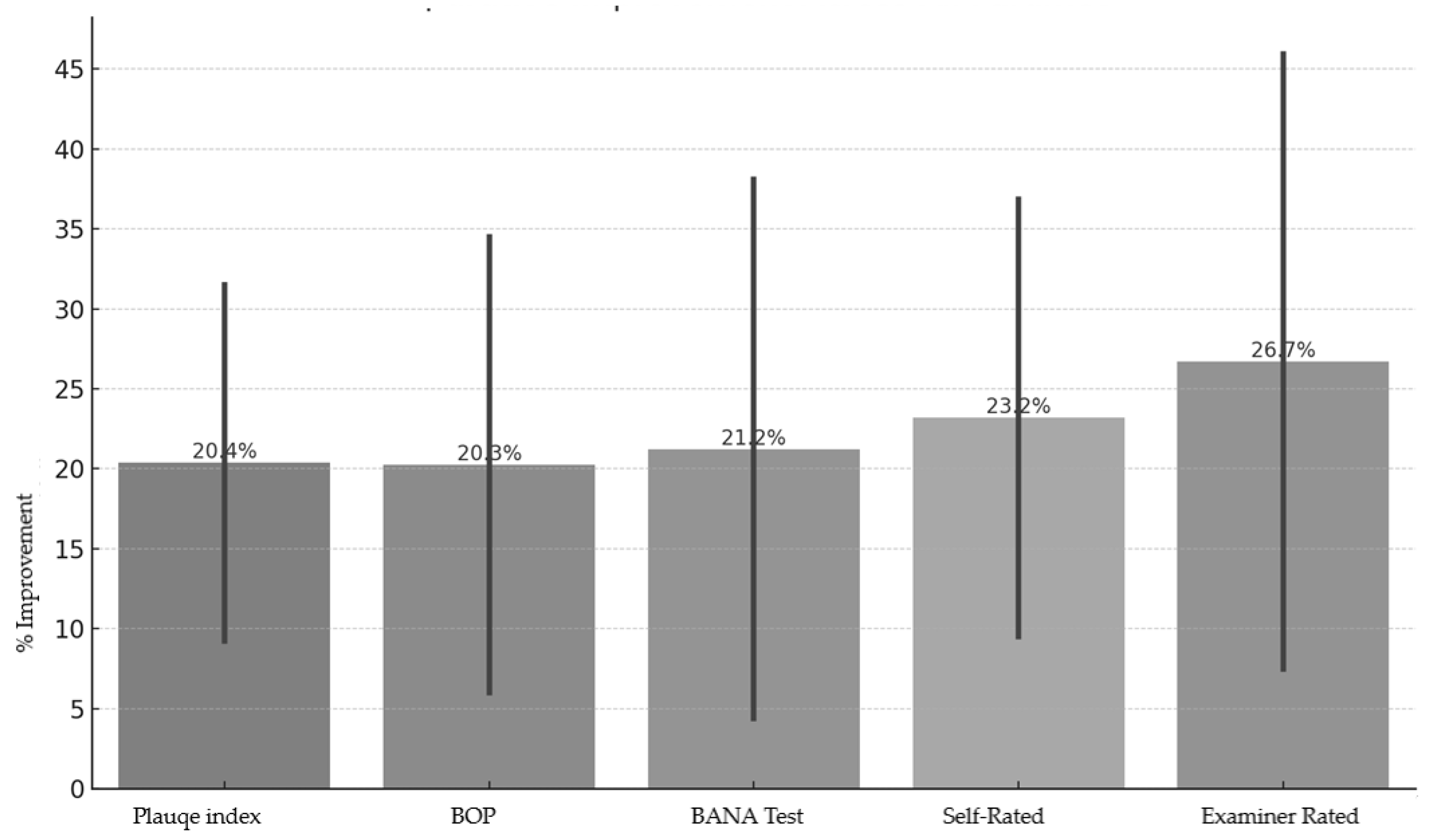
- Plaque Index: ↓ 31.5% (test) vs. ↓ 9.25% (control).
- BOP: ↓ 34.49% (test) vs. ↓ 6.02% (control).
- BANA Positivity: ↓ 38.09% (test) vs. ↓ 4.38% (control).
- Self-reported halitosis: ↓ 36.84% (test) vs. ↓ 9.52% (control).
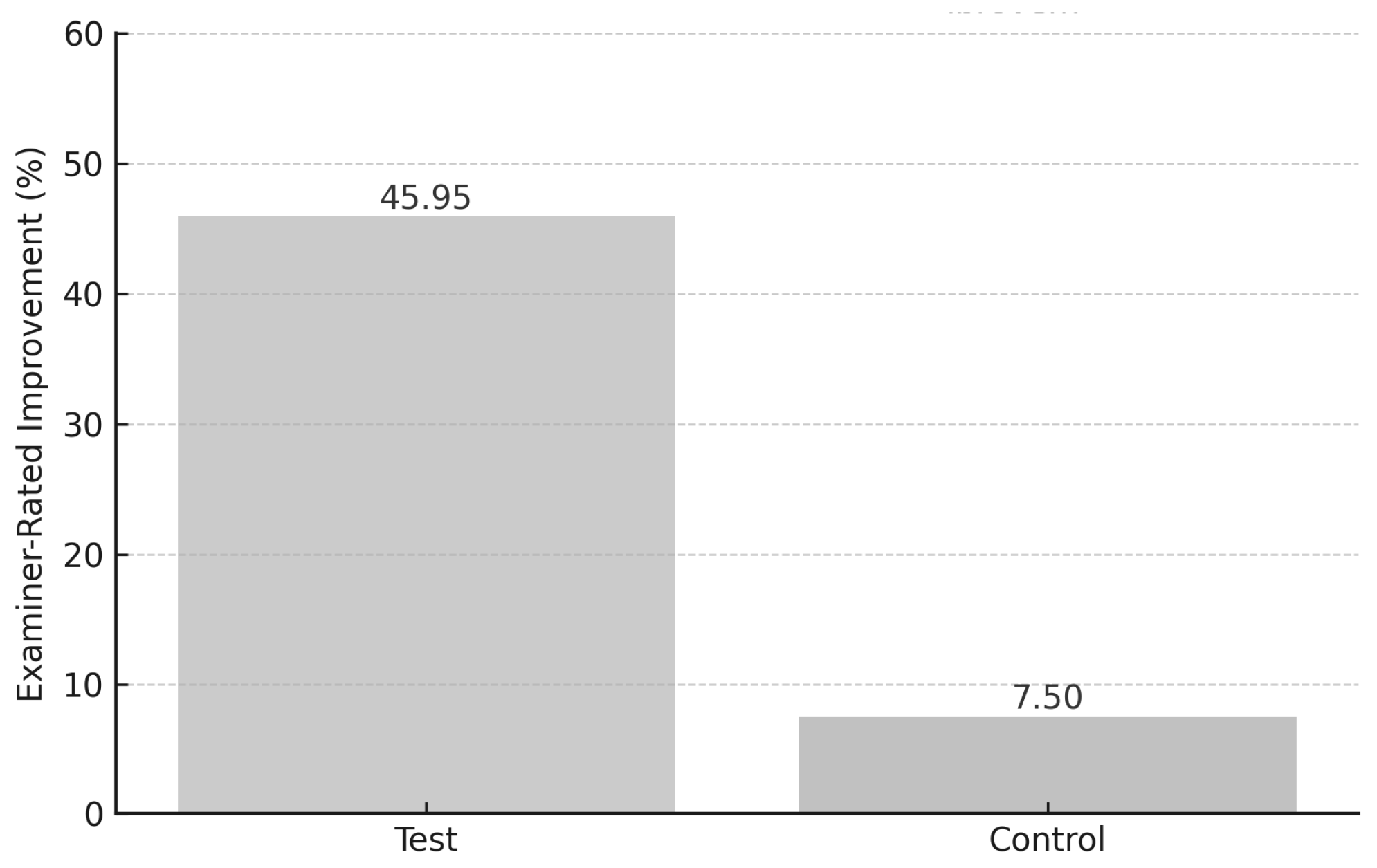
- Examiner-rated halitosis: ↓ 45.95% (test) vs. ↓ 7.5% (control).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Renvert, S.; Noack, M.J.; Lequart, C.; Roldán, S.; Laine, M.L. The Underestimated Problem of Intra-Oral Halitosis in Dental Practice: An Expert Consensus Review. Clin. Cosmet. Investig. Dent. 2020, 12, 251–262. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mento, C.; Lombardo, C.; Milazzo, M.; Whithorn, N.I.; Boronat-Catalá, M.; Almiñana-Pastor, P.J.; Fernàndez, C.S.; Bruno, A.; Muscatello, M.R.A.; Zoccali, R.A. Adolescence, Adulthood and Self-Perceived Halitosis: A Role of Psychological Factors. Medicina 2021, 57, 614. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Du, M.; Li, L.; Jiang, H.; Zheng, Y.; Zhang, J. Prevalence and relevant factors of halitosis in Chinese subjects: A clinical research. BMC Oral Health 2019, 19, 45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Porter, S.R.; Scully, C. Oral malodour (halitosis). BMJ 2006, 333, 632–635. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tonzetich, J. Production and origin of oral malodor: A review of mechanisms and methods of analysis. J. Periodontol. 1977, 48, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Loesche, W.J.; Kazor, C. Microbiology and treatment of halitosis. Periodontology 2000 2002, 28, 256–279. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, S.; Sotozono, M.; Ohkura, N.; Noiri, Y. Evidence on the Use of Mouthwash for the Control of Supragingival Biofilm and Its Potential Adverse Effects. Antibiotics 2022, 11, 727. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amou, T.; Hinode, D.; Yoshioka, M.; Grenier, D. Relationship between halitosis and periodontal disease—Associated oral bacteria in tongue coatings. Int. J. Dent. Hyg. 2014, 12, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D. Periodontal microbial ecology. Periodontology 2000 2005, 38, 135–187. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S149–S161. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. S1), S173–S182. [Google Scholar] [CrossRef] [PubMed]
- Preshaw, P.M.; Alba, A.L.; Herrera, D.; Jepsen, S.; Konstantinidis, A.; Makrilakis, K.; Taylor, R. Periodontitis and diabetes: A two-way relationship. Diabetologia 2012, 55, 21–31. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bhuyan, R.; Bhuyan, S.K.; Mohanty, J.N.; Das, S.; Juliana, N.; Juliana, I.F. Periodontitis and Its Inflammatory Changes Linked to Various Systemic Diseases: A Review of Its Underlying Mechanisms. Biomedicines 2022, 10, 2659. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Struch, F.; Schwahn, C.; Wallaschofski, H.; Grabe, H.J.; Völzke, H.; Lerch, M.M.; Meisel, P.; Kocher, T. Self-reported halitosis and gastro-esophageal reflux disease in the general population. J. Gen. Intern. Med. 2008, 23, 260–266. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barrak, I.; Stájer, A.; Gajdács, M.; Urbán, E. Small, but smelly: The importance of Solobacterium moorei in halitosis and other human infections. Heliyon 2020, 6, e05371. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Genco, R.J.; Grossi, S.G.; Ho, A.; Nishimura, F.; Murayama, Y. A proposed model linking inflammation to obesity, diabetes, and periodontal infections. J. Periodontol. 2005, 76 (Suppl. S11), 2075–2084. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Liu, X.; Huang, J.; Sun, Y.; Liu, B.; Song, W.; Meng, L.; Du, M.; Feng, Q. Efficacy of a mouthwash containing ε-poly-L-lysine, funme peptides and domiphen in reducing halitosis and supragingival plaque: A randomized clinical trial. BMC Oral Health 2024, 24, 525. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial Properties of Plant Essential Oils against Human Pathogens and Their Mode of Action: An Updated Review. Evid. Based Complement. Alternat. Med. 2016, 2016, 3012462. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Padol, M.V.; Vishwakarma, P.; Dodamani, A.S.; Gore, A.W.; Chachlani, K.S.; Kharkar, S.P. Comparative evaluation of nutmeg mouthwash and 0.2% chlorhexidine gluconate mouthwash on halitosis and plaque control: A randomized clinical trial. J. Indian Soc. Periodontol. 2022, 26, 384–389. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef] [PubMed]
- Van Strydonck, D.A.; Slot, D.E.; Van der Velden, U.; Van der Weijden, F. Effect of a chlorhexidine mouthrinse on plaque, gingival inflammation and staining in gingivitis patients: A systematic review. J. Clin. Periodontol. 2012, 39, 1042–1055. [Google Scholar] [CrossRef] [PubMed]
- Berchier, C.E.; Slot, D.E.; Van der Weijden, G.A. The efficacy of 0.12% chlorhexidine mouthrinse compared with 0.2% on plaque accumulation and periodontal parameters: A systematic review. J. Clin. Periodontol. 2010, 37, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Kim, M.; Nam, S.H.; Kang, M.S.; Lee, S.A. Effects of Oral Probiotics on Subjective Halitosis, Oral Health, and Psychosocial Health of College Students: A Randomized, Double-Blind, Placebo-Controlled Study. Int. J. Environ. Res. Public Health 2021, 18, 1143. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- López-Valverde, N.; López-Valverde, A.; Macedo de Sousa, B.; Rodríguez, C.; Suárez, A.; Aragoneses, J.M. Role of Probiotics in Halitosis of Oral Origin: A Systematic Review and Meta-Analysis of Randomized Clinical Studies. Front. Nutr. 2022, 8, 787908. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takenaka, S.; Ohsumi, T.; Noiri, Y. Evidence-based strategy for dental biofilms: Current evidence of mouthwashes on dental biofilm and gingivitis. Jpn. Dent. Sci. Rev. 2019, 55, 33–40. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khounganian, R.M.; Alasmari, O.N.; Aldosari, M.M.; Alghanemi, N.M. Causes and Management of Halitosis: A Narrative Review. Cureus 2023, 15, e43742. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Varra, F.N.; Varras, M.; Varra, V.K.; Theodosis-Nobelos, P. Molecular and pathophysiological relationship between obesity and chronic inflammation in the manifestation of metabolic dysfunctions and their inflammation-mediating treatment options (Review). Mol. Med. Rep. 2024, 29, 95. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chung, H.S.; Choi, K.M. Organokines in disease. Adv. Clin. Chem. 2020, 94, 261–321. [Google Scholar] [CrossRef] [PubMed]
- Krongbaramee, T.; Zhu, M.; Qian, Q.; Zhang, Z.; Eliason, S.; Shu, Y.; Qian, F.; Akkouch, A.; Su, D.; Amendt, B.A.; et al. Plasmid encoding microRNA-200c ameliorates periodontitis and systemic inflammation in obese mice. Mol. Ther. Nucleic Acids 2021, 23, 1204–1216. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alzahrani, H.G.; AlSarhan, M.A.; Aldohayan, A.; Bamehriz, F.; Alzoman, H.A. Effect of sleeve gastrectomy on the levels of oral volatile sulfur compounds and halitosis-related bacteria. Saudi Dent. J. 2024, 36, 940–946. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Toy, V.E.; Ataoglu, T.; Eltas, A.; Otlu, H.G.; Karabulut, A.B. Obesity as a modifying factor of periodontal therapy outcomes: Local and systemic adipocytokines and oxidative stress markers. Clin. Oral Investig. 2023, 27, 2763–2773. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Bano, N.; Ahmad, T.; Sharangi, A.B.; Upadhyay, T.K.; Alraey, Y.; Alabdallah, N.M.; Rauf, M.A.; Saeed, M. Synergistic Role of Plant Extracts and Essential Oils against Multidrug Resistance and Gram-Negative Bacterial Strains Producing Extended-Spectrum β-Lactamases. Antibiotics 2022, 11, 855. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils-Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Romero-Montero, A.; Melgoza-Ramírez, L.J.; Ruíz-Aguirre, J.A.; Chávez-Santoscoy, A.; Magaña, J.J.; Cortés, H.; Leyva-Gómez, G.; Del Prado-Audelo, M.L. Essential-Oils-Loaded Biopolymeric Nanoparticles as Strategies for Microbial and Biofilm Control: A Current Status. Int. J. Mol. Sci. 2023, 25, 82. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dobler, D.; Runkel, F.; Schmidts, T. Effect of essential oils on oral halitosis treatment: A review. Eur. J. Oral Sci. 2020, 128, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Alsaffar, D.; Alzoman, H. Efficacy of antioxidant mouthwash in the reduction of halitosis: A randomized, double blind, controlled crossover clinical trial. J. Dent. Sci. 2021, 16, 621–627. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Szalai, E.; Tajti, P.; Szabó, B.; Hegyi, P.; Czumbel, L.M.; Shojazadeh, S.; Varga, G.; Németh, O.; Keremi, B. Daily use of chlorine dioxide effectively treats halitosis: A meta-analysis of randomised controlled trials. PLoS ONE 2023, 18, e0280377. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, N.; Li, J.; Qiao, X.; Wu, Y.; Liu, Y.; Wu, C.; Li, L. Efficacy of probiotics in the management of halitosis: A systematic review and meta-analysis. BMJ Open 2022, 12, e060753. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Charles, C.H.; Mostler, K.M.; Bartels, L.L.; Mankodi, S.M. Comparative antiplaque and antigingivitis effectiveness of a chlorhexidine and an essential oil mouthrinse: 6-month clinical trial. J. Clin. Periodontol. 2004, 31, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Nagappan, N.; Palaneeswaran, K.; Nalankilli, A.V.; Nyklesh, V.; Mani, M.; Aziz, M.B.A. Antibacterial Efficacy of Herbal and 0.2% Chlorhexidine Mouth Rinse Against Oral Pathogens—An In vitro Study. J. Pharm. Bioallied. Sci. 2024, 16 (Suppl. S2), S1453–S1455. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jansen, P.M.; Abdelbary, M.M.H.; Conrads, G. A concerted probiotic activity to inhibit periodontitis-associated bacteria. PLoS ONE 2021, 16, e0248308. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, J.A.; Lee, G.R.; Lee, J.Y.; Jin, B.H. Oral Probiotics, Streptococcus salivarius K12 and M18, Suppress the Release of Volatile Sulfur Compounds and a Virulent Protease from Oral Bacteria: An In-Vitro Study. Oral Health Prev. Dent. 2023, 21, 259–270. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, G.; Li, N. Efficacy of Probiotic Tablets in the Reduction of Halitosis: A Randomised, Single Blind, Controlled Clinical Trial. Oral Health Prev. Dent. 2024, 22, 639–646. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lai, S.; Lingström, P.; Cagetti, M.G.; Cocco, F.; Meloni, G.; Arrica, M.A.; Campus, G. Effect of Lactobacillus brevis CD2 containing lozenges and plaque pH and cariogenic bacteria in diabetic children: A randomised clinical trial. Clin. Oral Investig. 2021, 25, 115–123. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Homayouni Rad, A.; Pourjafar, H.; Mirzakhani, E. A comprehensive review of the application of probiotics and postbiotics in oral health. Front. Cell. Infect Microbiol. 2023, 13, 1120995. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Briceag, R.; Caraiane, A.; Raftu, G.; Horhat, R.M.; Bogdan, I.; Fericean, R.M.; Shaaban, L.; Popa, M.; Bumbu, B.A.; Bratu, M.L.; et al. Emotional and Social Impact of Halitosis on Adolescents and Young Adults: A Systematic Review. Medicina 2023, 59, 564. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kahraman, E.N.; Erensoy, Ş.; Dikilitaş, A.; Gülşahı, A.; Aydın, E.Ö.; Özalp Ateş, F.S. Awareness and knowledge of halitosis among students at two different dental universities in Turkey: A cross-sectional survey. BMC Oral Health 2025, 25, 194. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


| Parameter | Test Group (n = 30) | Control Group (n = 15) | p-Value |
|---|---|---|---|
| Mean Age (years) | 43.2 ± 9.4 | 42.7 ± 8.8 | 0.81 |
| Gender (M/F) | 16/14 | 8/7 | 0.92 |
| BMI (kg/m2) | 32.1 ± 2.4 | 31.7 ± 2.3 | 0.63 |
| Smokers (%) | 26.7% | 26.6% | 0.98 |
| Baseline PI | 1.39 ± 0.15 | 1.33 ± 0.13 | 0.17 |
| Baseline BOP (%) | 58.2 ± 6.1 | 56.4 ± 5.7 | 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beresescu, G.; Bereczki-Temistocle, D.L.; Beresescu, L.; Ormenisan, A.; Monea, A.; Razvan-Marius, I. Effectiveness of an Essential Oil Mouthwash on Halitosis in Obese Patients with Periodontitis: A Short-Term Clinical Evaluation. J. Clin. Med. 2025, 14, 5225. https://doi.org/10.3390/jcm14155225
Beresescu G, Bereczki-Temistocle DL, Beresescu L, Ormenisan A, Monea A, Razvan-Marius I. Effectiveness of an Essential Oil Mouthwash on Halitosis in Obese Patients with Periodontitis: A Short-Term Clinical Evaluation. Journal of Clinical Medicine. 2025; 14(15):5225. https://doi.org/10.3390/jcm14155225
Chicago/Turabian StyleBeresescu, Gabriela, Despina Luciana Bereczki-Temistocle, Liana Beresescu, Alina Ormenisan, Adriana Monea, and Ion Razvan-Marius. 2025. "Effectiveness of an Essential Oil Mouthwash on Halitosis in Obese Patients with Periodontitis: A Short-Term Clinical Evaluation" Journal of Clinical Medicine 14, no. 15: 5225. https://doi.org/10.3390/jcm14155225
APA StyleBeresescu, G., Bereczki-Temistocle, D. L., Beresescu, L., Ormenisan, A., Monea, A., & Razvan-Marius, I. (2025). Effectiveness of an Essential Oil Mouthwash on Halitosis in Obese Patients with Periodontitis: A Short-Term Clinical Evaluation. Journal of Clinical Medicine, 14(15), 5225. https://doi.org/10.3390/jcm14155225






