Abstract
Echocardiography is the primary imaging modality for diagnosing cardiac disease in children, with quantitation largely based on nomograms. Over the past decade, significant efforts have been made to address the numerical and methodological limitations of earlier nomograms. As a result, robust and reliable pediatric echocardiographic nomograms are now available for most two-dimensional anatomical measurements, three-dimensional volumes, and strain parameters. These more recent nomograms are based on adequate sample sizes, strict inclusion and exclusion criteria, and rigorous statistical methodologies. They have demonstrated good reproducibility with minimal differences across different authors, establishing them as reliable diagnostic tools. Despite these advances, some limitations persist. Certain ethnic groups remain underrepresented, and data for preterm and low-weight infants are still limited. Most existing nomograms are derived from European and North American populations, with sparse data from Asia and very limited data from Africa and South America. Nomograms for preterm and low-weight infants are few and cover only selected cardiac structures. Although diastolic parameter nomograms are available, the data remain heterogeneous due to challenges in normalizing functional parameters according to age and body size. The accessibility of current nomograms has greatly improved with the development of online calculators and mobile applications. Ideally, integration of nomograms into echocardiographic machines and reporting systems should be pursued. Future studies are needed to develop broader, more comprehensive, and multi-ethnic nomograms, with better representation of preterm and low-weight populations, and to validate new parameters derived from emerging three- and four-dimensional echocardiographic techniques.
1. Introduction
Echocardiography is the first imaging modality for the assessment of congenital and acquired cardiac diseases at all ages, and quantification is an essential part of this modality [1,2]. In the pediatric age, as for any other body size measures, cardiac measures need to be compared to standards of normality according to age and body size [1,2,3,4]. Thus, pediatric echocardiographic nomograms are essential tools to understand whether a given cardiac measure of a given child with a given age, weight, and height is within the range of normality or not, and if not, how far it is from normality [1,2,3,4]. This process is essential to discriminate between healthy and pathological and to grade disease severity. Within the last 15 years, there has been a great amount of attention on pediatric echocardiographic nomograms, and great advances have been made [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19]. Thanks to this improvement pediatric echocardiographic nomograms for most two-dimensional [2,3,4,5,6,7,8,9,10,11,12] and functional [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32] parameters are currently available. Pediatric echocardiographic nomograms on myocardial strain analysis [33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57], 3D [58,59,60,61,62,63,64], and newer myocardial work parameters [65,66,67,68,69,70,71] are also recently becoming available. Despite advances, however, nomograms are not perfect tools, and limitations persist [72]. Despite advances in electronic tools [73,74,75], many current nomograms are still difficult to find and not present in online z-score calculators (e.g., parameterz.com) [74]. A few nomograms furthermore still present numerical and methodical limitations that may hamper their clinical utility [72]. Knowledge of the existence of current nomograms is essential for their use, and knowledge of their strengths and limitations is essential for their correct utilization [72].
The aim of the present study is to provide an updated and systematic evaluation of the reliability, comprehensiveness, and accessibility of pediatric echocardiographic nomograms published over the past two decades.
1.1. Literature Search Criteria
In April 2025, a research review was conducted utilizing three medical search engines: the National Library of Medicine, Science Direct, and the Cochrane Library. This review focused on Medical Subject Headings (MeSH) and the free-text terms “echocardiography”, and “normal values in children”.
The search parameters were further refined by incorporating the keywords “nomograms, Z-scores, neonates and infants, preterm, low-weight, three-dimensional, speckle tracking echocardiography, ventricular strain, atrial strain, diastolic”. Additionally, we identified other potentially relevant publications through a manual examination of references from all eligible studies and review articles, as well as the Science Citation Index Expanded available on Web of Science. The titles and abstracts of all articles identified through this search strategy were thoroughly evaluated. Manuscripts were excluded if they (a) utilized imaging techniques that differed from echocardiography, (b) contained a mixed population of adults and children, or (c) were written in a language other than English. This review was executed by the PRISMA 2020 statement [76].
All articles were evaluated independently by two specialists in pediatric echocardiography (M.C. and P.M.), and they were included in this study after reaching a consensus.
1.2. Search Results
Out of the 180 publications identified for potential inclusion in this study, 72 (40%) were excluded based on the above criteria, while 108 (60%) were ultimately selected for analysis and systematic review (Figure 1).

Figure 1.
Selection diagram according to PRISMA guidelines.
2. General Aspects
- 1.
- Accuracy of a nomogram
Ideally, before trusting the use of a specific nomogram, one should verify its accuracy [2,72]. Accurate nomograms should be calculated by using the following: (i) the standardized method for cardiac measurements by echocardiography, (ii) a healthy population (with clear definition of inclusion and exclusion criteria), (iii) adequate sample size, (iv) consistent methods for standardization and expression of data, (v) rigorous statistical approach, and (vi) attention to confounders [2,72].
- (a)
- Measurement Standardization
The establishment of pediatric echocardiographic nomograms necessitates addressing multiple methodological issues. Foremost is the standardization of measurement techniques. Older nomograms presented inconsistencies regarding the timing of measurements within the cardiac cycle and the anatomical landmarks employed [2,72]. This generated confusion in the range of values proposed and difficulties in comparison among different nomogram sources. Guidelines for two-dimensional measurements in pediatric echocardiography were published in 2010 and recently renewed [1,7]. Protocols for imaging acquisition and basic measurements calculation have also been standardized for myocardial strain speckle tracking echocardiography and three-dimensional echocardiography [1,56,57,77,78]. These documents allowed a more standardized way to measure [1,56,57,77,78]. As a consequence, more recent nomograms have been calculated by using uniform methods of measurement, thus allowing comparison among them.
- (b)
- Inclusion and Exclusion Criteria
The careful definition of inclusion and exclusion criteria is essential to ensure the validity of normative datasets [2,72]. Only healthy pediatric subjects should be enrolled; however, the term “healthy” lacks a uniform operational definition and may differ across studies [2,78,79]. Recent investigations have generally converged on the exclusion of individuals with genetic syndromes, neuromuscular disorders, systemic or pulmonary hypertension, arrhythmias, connective tissue diseases, a family history of congenital heart disease, or suboptimal image quality [2,72]. Conversely, neonates with minor cardiac anomalies—such as a small patent ductus arteriosus or a restrictive patent foramen ovale in the early postnatal period—are frequently included [2,72]. Unresolved issues remain regarding the inclusion of preterm infants and the treatment of data from obese children [2,72].
- (c)
- Sample Size Considerations
The determination of appropriate sample size is a critical methodological component [2,72]. Contemporary guidelines recommend stratifying the pediatric population into six age groups [80,81,82] and enrolling a minimum of 100 subjects per group to achieve a 95% confidence interval with a margin of error of ±0.1, assuming normal distribution and a standard deviation of 0.5 [2,72]. Some authors advocate for at least 120 subjects per group, whereas others suggest a lower limit of 80 [2,72]. Given the reality that not all echocardiographic studies will yield analyzable data for every parameter, a pragmatic approach is to account for a 70–80% data completion rate [2,72]. Accordingly, to meet statistical requirements, each age group should include approximately 140 subjects [2,72]. Furthermore, to allow for sex- and ethnicity-specific analyses, these numbers should be doubled and then multiplied by the number of ethnic groups studied [2,72]. For six age groups, this equates to 840 subjects per sex, 1680 for both sexes, and up to 5040 individuals when considering three ethnic categories [2,72]. Nonetheless, most existing nomograms fail to meet these thresholds, which may limit their generalizability [2,72].
- (d)
- Normalization and Expression of Data
Normalization of echocardiographic variables relative to body size is fundamental [2,72,73]. Among the various body size metrics, body surface area (BSA), particularly when calculated using the Haycock formula [79], is the most widely adopted [2,72]. However, age, weight, height, heart rate, stroke volume, and left ventricular dimensions have also been used, particularly for diastolic and strain parameters [33,72]. The choice of indexing variable remains controversial, especially in the context of obesity, where BSA may obscure the influence of excess adiposity [79,83]. For left ventricular mass (LVM), indexing to height raised to an exponential power (most commonly height^2.7) is considered standard due to its superior approximation of lean body mass [79,84]. This approach has been endorsed by major guidelines and used to define left ventricular hypertrophy (LVH) in pediatric populations. However, age-specific considerations may be necessary, as fixed cut-offs are not universally applicable to younger children [79,83]. Recent findings suggest that indexing LVM to height^2.16 may reduce false positives in younger cohorts [79,83]. Additionally, bias introduced by body mass index (BMI) has been reported in Z-score models that rely on a single normalization variable [79,83]. Multivariable models incorporating both weight and height may mitigate this effect and improve the robustness of normative data [79,84].
- (e)
- Statistical Modeling and Z-Score Calculation
Z-scores are the most employed method for expressing normalized echocardiographic data [2,72]. Various regression models—including linear, polynomial, exponential, logarithmic, and square root functions—are used to generate Z-score equations [2,72]. The Lambda–Mu–Sigma (LMS) method has recently been utilized [9,85]. Despite its importance, heteroscedasticity—the non-constant variance of residuals—remains an underreported issue in many nomographic studies [86,87,88,89]. Residual distribution and mean square error (MSE) should be routinely evaluated to ensure model validity [2,72,83,86,87,88]. The model with the highest coefficient of determination (R2) that also satisfies assumptions of homoscedasticity is preferred [2,72]. There is no universally accepted R2 threshold, although values > 0.75 are generally considered substantial, and >0.6 acceptable [2,72]. In structural measurements, R2 values typically range from 0.5 to 0.9, whereas functional parameters show weaker correlations with body size, often with R2 values < 0.5 [2,72]. In such cases, descriptive statistics (mean ± standard deviation) or centile curves may serve as alternative approaches [72].
- (f)
- Confounding Factors and Inherent Limitations
The influence of confounding variables—including sex, ethnicity, prematurity, mode of delivery, and intra/inter-observer variability—must be carefully considered [2,72]. In neonates and infants, rapid physiological changes and growth during the first months of life can significantly affect echocardiographic measurements [2,72]. These dynamic changes, combined with external factors such as patient cooperation and operator expertise, may introduce unpredictable bias [2,72]. Certain imaging planes, such as apical four- and two-chamber views, are particularly susceptible to variability from minor probe angulation. Despite attempts at standardization, operator-dependent variability remains a significant limitation in echocardiographic quantification [2,72].
- 2.
- Nomograms for specific cardiac measurements
- a.
- Two-Dimensional Measurements
Over the last decade comprehensive two-dimensional pediatric echocardiographic nomograms covering a broad range of parameters across valvular, arterial, chamber, and coronary artery dimensions have been published [2,3,4,5,6,7]. These nomograms cover the whole pediatric age, including neonatal age, and are characterized by a rigorous statistical approach [2,3,4,5]. All data were normalized by BSA and expressed with Z-score, obtained with coefficient of determination > 0.66 [3,4,10,12,18,90,91], with limited exceptions [6,11]. Current nomograms, including more recent ones, mostly derive from North America and Europe, while data from Asia [6,7] and Africa and South America [92,93] are limited. Echocardiographic nomograms by Lopez et al. (2020) [2] were developed from a large multi-ethnic cohort of 3566 healthy American children aged 0–18 years, including Caucasian, Black, and Hispanic subjects. These nomograms encompass a broad range of echocardiographic parameters (e.g., coronary arteries, valve areas, and ventricular volumes). Cantinotti et al. [3,4] proposed updated nomograms based on a homogeneous population of 1151 healthy Caucasian Italian children aged 0–18 years. These nomograms were derived from prospectively collected data at a single center with standardized imaging protocols and centralized analysis, improving internal consistency and reproducibility [3,4]. They include measurements of atrioventricular valves, the aorta (at multiple levels), pulmonary artery and branches, aortic arch segments, and left ventricular dimensions by M-mode [3,4].
Comparison among nomograms [3,4,72,90,91,94,95] revealed that the nomograms by Cantinotti [3,4] and Lopez [2] were the most statistically similar, particularly across a wide range of body surface areas (BSAs). Z-score differences between these two datasets were mostly below 0.5, suggesting limited clinical discordance. In contrast, older nomograms by Sluysmans and Colan and Pettersen et al. [90,91], based on smaller or less diverse cohorts, showed greater divergence from Lopez et al., particularly at the extremes of BSA. Figure 2 illustrates an example of the differences between older and more recent nomograms. Percentile curves for mitral and tricuspid valve dimensions are shown, along with a comparison of predicted values expressed as Z-scores, using the nomograms by Lopez et al. [2] as the reference standard. For a few specific parameters such as aortic isthmus and ascending aorta diameters, however, even among nomograms by Lopez and Cantinotti [2,3,4], significant differences were noted. For example, at a BSA of 0.21 m2, the aortic isthmus diameter was significantly smaller in Cantinotti’s nomograms [3,4] (mean 4.47 mm) compared to Lopez’s (mean 5.73 mm) [2], with a Z-score difference of −0.70. These discrepancies, especially in the infant population, may result in clinically relevant differences in diagnosis and management of congenital heart disease, such as the classification of valve hypoplasia or aortic arch hypoplasia.
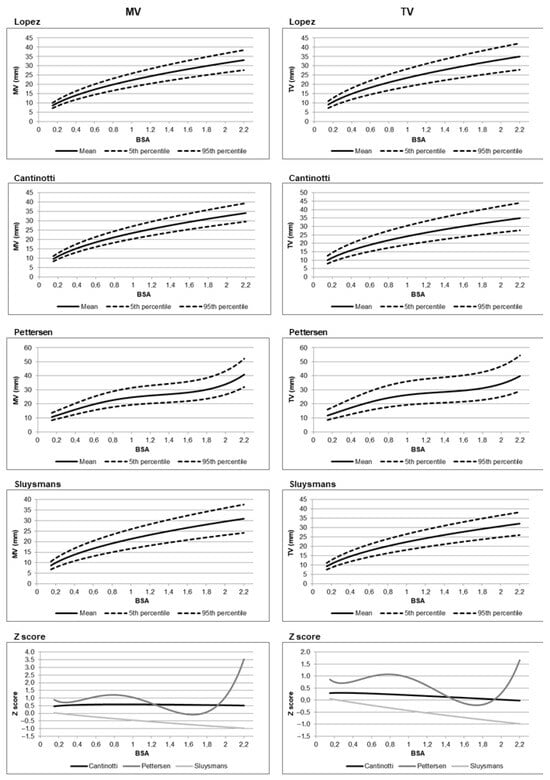
Figure 2.
Percentile charts of predicted values for the mitral valve (MV) and tricuspid valve (TV) are shown for the nomograms by Lopez et al. [2], Cantinotti et al. [96], Pettersen et al. [91], and Sluysmans et al [90]. In addition, Z-score-based comparisons of predicted values from Cantinotti et al. and Pettersen et al. versus those from Lopez et al. (used as the reference standard) are presented. For example, with body surface area as the independent variable, the graphs illustrate the Z-score deviations of the mean predicted values from Cantinotti and Pettersen relative to the mean values reported by Lopez et al. Z-score calculation is performed as follows: Z-score (Cantinotti) = (predicted mean from Cantinotti et al.—predicted mean from Lopez et al.)/root mean square error (RMSE) of Lopez et al. Z-score (Pettersen) = (predicted mean from Pettersen et al.—predicted mean from Lopez et al.)/RMSE of Lopez et al.
Also more recent nomograms, furthermore, present a few limitations. While the Lopez [2] nomograms benefit from a large, multi-ethnic sample, they also suffer from methodological limitations, such as retrospective data collection from 19 centers and broad inclusion criteria for ethnic subgroups, potentially reducing statistical power to detect meaningful inter-ethnic differences. In contrast, Cantinotti et al.’s nomograms [3,4] offer higher standardization but are limited in ethnic diversity.
Despite more recent nomograms covering a broad range of 2D parameters, gaps for a few measurements remain. Relatively limited data are present for a few cardiac structures including cardiac chamber areas in apical four- and two-chamber views [96], left ventricular volume using subxiphoid imaging [2], left atrial volume via the biplane area-length method [91], and right ventricular dimension using sub-xyphoid view [20].
In Table 1 major pediatric echocardiographic nomograms for 2D parameters are summarized, while, in Table 2, major pediatric echocardiographic nomograms for coronary arteries are reported.

Table 1.
Major pediatric echocardiographic nomograms for 2D measures.

Table 2.
Major pediatric echocardiographic coronary artery nomograms.
- b.
- Diastolic Function and Other Functional Parameters
Assessment of diastolic function is critical in the management of both congenital and acquired heart disease in children [13,14,15,16,17,18,19,20,21,97]. However, the interpretation of diastolic indices remains challenging, especially in neonates and infants, due to the rapid maturational changes that occur in early life [13,14,15,16,17,18,19,20,21,97]. Age-specific normative values are essential to differentiate physiological variation (such as the common inversion of mitral E and A wave velocities in neonates) from pathological findings [13,14,15,16,17,18,19,20,21].
Numerous nomograms for pulsed and tissue Doppler-derived diastolic parameters have been published [13,14,15,16,17,18,19,20,21], but many suffer from methodological limitations, including heterogeneous measurement techniques and inconsistent age stratification [94]. The scarce correlation of diastolic parameters with most body size parameters and age hampered the possibility of performing z-score equations with sufficient statistical power [95]. Thus, most studies are limited to providing data as percentiles or mean ± standard deviation [95]. Compared to Z-scores, the use of percentiles or mean values is limited in assessing the severity of cardiac abnormalities in pediatric echocardiography. Percentiles only indicate a relative rank without quantifying deviation from normal, while mean values do not account for individual variability [2,72]. Z-scores offer a standardized, continuous measure of how far a value deviates from the mean, adjusting for body size and age [2,72]. This enables a more precise assessment of abnormality severity and supports clinical decision making more effectively [2,72].
Recent investigations [14,17,18] have sought to overcome these issues. Dallaire et al. reported z-scores for a broad set of diastolic indices derived from pulsed, tissue, and color Doppler in a cohort of 233 healthy children aged 1–18 years [14]. However, the majority of indices demonstrated a nonlinear relationship with somatic growth and significant heteroscedasticity, and the correlation coefficients (R2), although not reported, appeared low based on the scatterplots. Additionally, although heart rate was found to influence some parameters, it was not incorporated into the final analysis.
Roberson et al. [17] provided normative data on tissue Doppler-derived mitral and tricuspid velocities from 634 healthy children aged 1 day to 18 years, normalized by age and heart rate. However, z-scores were only provided for a limited subset of variables, and regression coefficients (R2) were not reported. The most extensive study to date, involving 904 healthy Italian subjects aged 0–17 years [18], reported very weak correlations (R2 ranging from 0.18 to 0.53) between mitral Doppler indices and body surface area, and no significant associations with heart rate or blood pressure. Due to the low R2 values, z-score computation was not feasible, and normative data were presented as percentiles and mean ± standard deviation stratified by age and body surface area [18].
Despite these limitations, recent nomograms present reproducible patterns that can assist clinicians in routine practice. Normal ranges published in recent studies are consistent in older children and comparable to adult reference values [14,17,18]. Nonetheless, discrepancies remain. For instance, the late diastolic flow velocity or A wave at the mitral valve for a 3-year-old child varies from 42 cm/s [13] to 50 cm/s [19] to 61 cm/s [16], depending on the nomogram used.
Data on neonates and infants remain sparse and inconsistent, complicating classification of diastolic dysfunction in this age group [94]. Adult standards may be applicable in children over 3 years old but are unreliable in younger children, especially due to high neonatal heart rates [1,95].
In Table 3 and Supplemental Table S1 major pediatric echocardiographic nomograms for functional parameters are reported.

Table 3.
Major pediatric echocardiographic nomograms for functional diastolic parameters.
- c.
- Strain Analysis
Non-invasive evaluation of left ventricular myocardial strain and strain rate is gaining widespread acceptance for the follow-up and management of children with congenital heart disease (CHD) [33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55]. Over the past decade, several nomograms for left ventricular strain and strain rate in the pediatric population have been proposed [33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55]. As with other functional parameters, correlations of speckle tracking echocardiography (STE) values with body size parameters and age were weak; thus, most studies normalized values by age [9,21,33,34,35,36,37,40,42,43,44,45,47,52], reporting data as means with standard deviations [9,33,35,40,42,43,44,45,47,50,52]. Although the heart rate was typically assessed, Boettler et al. [98] were the only group to apply formal normalization for heart rate. Dallaire et al. [46] proposed z-score equations normalized by body surface area (BSA), but the correlation between strain parameters and BSA was extremely weak (R2 ranging from 0.03 to 0.002), introducing substantial bias in the resulting equations [46].
Some of these limitations may be partially overcome. For instance, three-dimensional echocardiography offers potential advantages by enabling more comprehensive spatial assessment and avoiding loss of speckle due to out-of-plane motion [41]. Similarly, newer studies have begun to implement more standardized methodologies and wider sample cohorts [47,52]. Nevertheless, certain intrinsic limitations—such as high heart rate and rapid physiological changes—make strain analysis particularly unreliable in neonates and infants [99]. Beyond infancy (age > 1 year), strain values tend to be more stable and reproducible across age groups [100]. For example, in a 3-year-old boy, global longitudinal strain varied from −23.6% [45] to −20.7% [44] to −21.3% [101], while, in a 14-year-old girl, reported values ranged from −21.8% [45] and −21.8% [44] to −22.7% [99].
Due to the relatively small variation in strain values with age, two recent meta-analyses proposed a single reference range for pediatric left and right ventricular strain [l49]. However, this approach oversimplifies the problem and runs counter to the foundational principle of nomograms [72,73,100]. Larger, adequately stratified studies are needed to clarify maturational trends in strain parameters across the pediatric age spectrum [72,73,100].
Data on basal–apical rotation, torsion, twist, and untwist in children remain limited [100,101,102]. Kim et al. [102], using 2D speckle tracking echocardiography in 80 children (3 months–15 years), found that both apical and basal rotation and twist (when corrected for left ventricular length) decreased with age, whereas untwisting recoil was unaffected by age. Takahashi et al. [102], in a larger cohort (111 subjects, aged 3–40 years), similarly demonstrated that twist, untwist, and deformation rates were higher in younger individuals. Notably, most of these parameters correlated more strongly with heart rate than with age [102]. A 3DSTE study on 311 subjects from childhood to adulthood reported the lowest torsion values in children and adolescents, with progressive increases reaching a plateau in adulthood [103].
- (c1) Ventricular Strain
a. Relationship with Age and Body Size
Several studies have demonstrated a mild inverse correlation between strain parameters and age, with correlation coefficients ranging from −0.8 to −0.007 [99]. However, these relationships are nonlinear, as shown in large pediatric cohorts [99]. For example, left ventricle global longitudinal strain (LV GLS) values are higher in infants and toddlers (31 days–24 months) compared to older age groups, while significantly lower values are observed in adolescents (11–18 years) [99]. In contrast, LV circumferential strain (CS) shows minimal variation across age groups, with slightly higher values in older children [98]. No significant age-related differences were found for right ventricle (RV) GLS [104].
In a cohort of over 1000 subjects under 21 years, both LV longitudinal and circumferential strain peaked around 4–5 years of age and declined thereafter [52]. Similarly, RV longitudinal strain followed a similar pattern [52]. Mild inverse correlations were also observed between strain values and body surface area (BSA), although these were generally weak [52].
b. Sex Differences
Most studies reported no significant sex-related differences in strain parameters [100]. When present, differences were minimal and primarily observed in adolescents, likely due to pubertal timing differences [99]. Some data showed slightly higher LV and RV strain values in females, though not consistently across studies [104].
c. Inter-Vendor Variability
Vendor-related variability is a known limitation in STE [53,54,55]. Good agreement was found between GE and TomTec platforms for LV GLS, with intraclass correlation coefficients (ICCs) ranging from 0.88 to 0.9 [53,54,55]. Variability, however, increased for LV CS and in children under 3 years, where ICCs were notably lower. Comparisons between Philips QLAB (versions 10.5, 10.8, AutoSTRAIN) and TomTec software version TTA2.00.03 revealed significant discrepancies in strain values, especially for older QLAB versions [53,54,55].
Agreement between GE and Philips platforms was poor, with interclass correlation coefficients (ICCs) as low as 0.34 for longitudinal and 0.12 for circumferential strain. Intra-vendor software version differences were also notable; QLAB 10.5 tended to yield higher strain values than QLAB 10.8 and AutoSTRAIN, especially in older children [53,54,55].
d. Inter- and Intra-Observer Variability
Observer variability depends on both the software and frame rate. For Philips QLAB 10.8, intra-observer agreement for LV strain was moderate (ICC: 0.5–0.79), while inter-observer agreement ranged from mild to good (ICC: 0.8–0.96) [53,54,55,99]. AutoSTRAIN showed excellent intra- and inter-observer agreement, especially for LV strain [53,54,55,98]. TomTec and GE also demonstrated good reproducibility, with ICCs > 0.85 for LV GLS and slightly lower values for CS. Higher frame rates were associated with better reproducibility [53,54,55,99].
- (c2) Atrial Strain
a. Maturational Variation
Atrial strain parameters show significant age-dependent variations [33,36,43,49,50]. Nomograms based on 2D speckle-tracking echocardiography (STE) demonstrate a nonlinear positive correlation between left atrial (LA) reservoir strain (LASr) and age, as well as a nonlinear negative correlation between LA contractile strain and age, with the most rapid changes occurring in infancy [33,36,43,49,50]. Both LA and right atrial (RA) conduit strain values are reduced in younger children, whereas contractile strain values are higher during early life [33,36,43,49,50].
Using 3D STE [49], all LA strain components show a declining trend with age, although the correlations are weak (r = 0.14 for global longitudinal strain; r = 0.31 for global 3D strain).
b. P-Gating vs. R-Gating and Software Differences
Strain values derived from P-wave gating are consistently lower than those from R-wave gating for both LA and RA, across all pediatric age groups [33,100]. Furthermore, significant differences are observed between atrial strain values obtained using atrial-specific software (QLAB 10) versus older ventricular-specific software (QLAB 9) adapted for atrial analysis [33,98].
In particular, LASr values are significantly lower when assessed with atrial-specific software across all age groups (p < 0.001), while RA Sr values differ only in certain age groups. LA conduit strain (LASct) values are higher in younger children and lower in older ones when measured with QLAB 10 compared to QLAB 9 [33,99].
In Table 4 and Table 5, major pediatric echocardiographic nomograms for STE strain parameters are reported.

Table 4.
Major pediatric STE nomograms for ventricular strain values.

Table 5.
Major pediatric STE nomograms for atrial strain values.
- d.
- Three-Dimensional Measurements
The potential of three-dimensional (3D) echocardiography for the diagnosis and management of CHD—particularly valvular abnormalities—has been increasingly recognized [80]. Compared to 2D echocardiography, 3D imaging provides superior accuracy in assessing valve areas and chamber volumes, as well as a more realistic evaluation of right ventricular size [80]. However, pediatric data remain limited [58,59,60,61,62,63,64]. Aside from functional parameters [49,58], few nomograms exist for 3D-derived anatomical measures, and they all pertain to ventricular [58,59,60,61,62] or atrial volumes [64]. Either Z-score [49,58,59,62] has been used for normalization, and R2 when reported [49,62] were good (e.g., ≥0.79). The Finnish group has published a few studies based on a cohort of 169 healthy subjects aged 2–27 years, including data on atrial and ventricular function, aortic and mitral annular areas, and left ventricular mass [103,105,106]. These studies showed that 3D echocardiography yielded larger valvular area estimates than 2D and Doppler-based methods [106], while left ventricular mass calculated by 3D methods was lower than estimates from 2D and M-mode echocardiography [103].
In Table 6 major pediatric echocardiographic nomograms for 3D parameters are reported.

Table 6.
Major pediatric echocardiographic nomograms for 3D measures.
3. Limitations of Current Nomograms
Despite the availability of pediatric echocardiographic nomograms covering a broad range of parameters and age groups, significant gaps in knowledge remain. Data on premature and low-birth-weight infants are still limited [107,108,109,110,111,112,113], although this population presents unique challenges for clinical decision making due to differences in cardiopulmonary transition and cardiac development compared to term and normal-weight neonates. Consequently, the application of nomograms derived from term, normal-weight infants to this subgroup may lead to suboptimal assessments.
The limited data available for preterm infants primarily include measurements of left ventricular diameters and wall thickness [107,108,109,110,111,112], as well as aortic and left atrial dimensions assessed by M-mode [107,108,109,110,111,112,113]. Only a few studies have reported M-mode data on right ventricular end-diastolic diameter [112], pulmonary artery dimensions [112], and Doppler-derived velocities of the valves and great vessels, such as the aorta and pulmonary artery [111].
Most currently available nomograms originate from Europe and North America, while data from Asia are scarce [6,7,9,11,107,109,112], and those from Africa [38,42,89,92] and South America [112] are extremely limited. Interestingly, among the limited neonatal datasets available, only a few originate from Europe [111] and North America [113], with the majority coming from Asia [107,108,109,112] and Australia [110]. Table 7 summarizes the main pediatric echocardiographic nomograms currently available for preterm and low-birth-weight infants.

Table 7.
Major pediatric echocardiographic nomograms for preterm low-weight babies are reported.
Data for Junior Athletes is also extremely limited [114,115,116,117,118] (Supplemental Table S2). Nomograms calculated in the general population cannot be applied to athletes, whose cardiac dimensions are physiologically increased. Lastly, a few CHD-specific nomograms are lacking.
4. Future Directions
As echocardiography continues to evolve—particularly with the emergence of advanced functional and 3D modalities—nomograms must also be updated to reflect these developments [72]. New parameters are continually being introduced, requiring the creation of new normal reference ranges. Ideally, nomograms should be easily accessible to clinicians and integrated into digital platforms. Free online z-score calculators (e.g., parameterz.com, CardioZ, BabyNorm Calculator) allow for the combination of multiple nomograms and help clinicians assess whether measurements fall within normal limits [73,74,75]. While there is sufficient coverage for 2D measures, not all data, however, are currently available on these tools [73,74,75]. Unfortunately, new normative data deriving from 3D and strain analysis remain difficult to find, as well as the ranges of normality for most of the 2D functional parameters. Artificial intelligence (AI) [119,120] may facilitate a more efficient, comprehensive, and rapid application of available Z-scores, enabling easier comparison across different datasets. Furthermore, AI could support the identification of strengths and limitations inherent to the specific Z-score references employed.
The integration of z-scores into echocardiographic reporting software will be a crucial step toward standardization and clinical utility. Ultimately, embedding nomogram-based tools directly into echocardiographic machines could enable the real-time detection of abnormal values. Furthermore, the development of software supporting automated or semi-automated measurements will help reduce inter- and intra-observer variability [72].
Collaborative development with echocardiographic vendors aimed at integrating AI-based tools into ultrasound systems for semi-automated measurements and automatic Z-score computation would offer substantial clinical benefits.
Multicenter collaborations and the establishment of standardized data-sharing platforms are strongly recommended to overcome current data gaps in specific subpopulations, such as preterm and low-birth-weight neonates, junior athletes, and individuals from African, South American, and Asian regions.
5. Conclusions
The use of nomograms is strongly recommended for quantitative pediatric echocardiography. Although earlier nomograms were limited by methodological and numerical flaws [72], recent efforts have led to substantial improvements and the development of more robust tools. While reliable nomograms now exist for many 2D anatomical measurements, 3D volumes, and strain parameters, data for diastolic parameters remain heterogeneous due to the difficulty in normalizing these data by age and body size parameters. Future works should focus on comparison among different ethnic groups, including African and South American (which are currently lacking), and on premature low-weight babies, whose data are currently lacking.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/jcm14155215/s1, Supplemental Table S1: Major pediatric nomograms for functional assessment of the right ventricle by echocardiography; Supplemental Table S2: Major echocardiographic nomograms for junior athletes.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by G.C., E.F., G.S., A.P., N.A., and R.G. The first draft of the manuscript was written by M.C. with supervision of P.M., and all authors commented on previous versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lopez, L.; Colan, S.D.; Frommelt, P.C.; Ensing, G.J.; Kendall, K.; Younoszai, A.K.; Lai, W.W.; Geva, T. Recommendations for quantification methods during the performance of a pediatric echocardiogram: A report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J. Am. Soc. Echocardiogr. 2010, 23, 465–495. [Google Scholar] [CrossRef]
- Lopez, L.; Colan, S.; Stylianous, M.; Granger, S.; Trachtenberg, F.; Frommelt, P.; Pearson, G.; Camarda, J.; Cnota, J.; Cohen, M.; et al. Relationship of Echocardiographic Z Scores Adjusted for Body Surface Area to Age, Sex, Race, and Ethnicity: The Pediatric Heart Network Normal Echocardiogram Database. Circ. Cardiovasc. Imaging 2017, 10, e006979. [Google Scholar] [CrossRef]
- Cantinotti, M.; Scalese, M.; Murzi, B.; Assanta, N.; Spadoni, I.; Festa, P.; De Lucia, V.; Crocetti, M.; Marotta, M.; Molinaro, S.; et al. Echocardiographic nomograms for ventricular, valvular and arterial dimensions in Caucasian children with a special focus on neonates, infants and toddlers. J. Am. Soc. Echocardiogr. 2014, 27, 179–191.e2. [Google Scholar] [CrossRef]
- Cantinotti, M.; Giordano, R.; Scalese, M.; Murzi, B.; Assanta, N.; Spadoni, I.; Maura, C.; Marco, M.; Molinaro, S.; Kutty, S.; et al. Nomograms for two-dimensional echocardiography derived valvular and arterial dimensions in Caucasian children. J. Cardiol. 2017, 69, 208–215. [Google Scholar] [CrossRef]
- Cantinotti, M.; Scalese, M.; Giordano, R.; Franchi, E.; Marchese, P.; Vicava, C.; Assanta, N.; Iervasi, G.; Kutty, S.; Koestenberger, M. Pediatric nomograms for left ventricle biplane 2D volumes in healthy Caucasian children. Echocardiography 2020, 37, 971–975. [Google Scholar] [CrossRef]
- Gokhroo, R.K.; Anantharaj, A.; Bisht, D.; Kishor, K.; Plakkal, N.; Aghoram, R.; Mondal, N.; Pandey, S.K.; Roy, R. A pediatric echocardiographic Z-score nomogram for a developing country: Indian pediatric echocardiography study—The Z-score. Ann. Pediatr. Cardiol. 2017, 10, 31–38. [Google Scholar] [CrossRef]
- Singh, V.; Satheesh, S.; Ganapathy, S.; Nair, N.-P.S.; Mondal, N.; Selvaraj, R.; Mishra, N.; Anantharaj, A. Echocardiographic nomograms and Z-scores for term Indian neonates. Ann. Pediatr. Cardiol. 2023, 16, 11–17. [Google Scholar] [CrossRef]
- Cantinotti, M.; Scalese, M.; Contini, F.V.; Franchi, E.; Viacava, C.; Corana, G.; Pizzuto, A.; Pietro, M.; Santoro, G.; Assanta, N. Comprehensive Two-Dimensional Pediatric Echocardiographic Nomograms for Coronary Artery Sizes in Caucasian Children and Comparison among Major Nomograms. Diagnostics 2024, 14, 1029. [Google Scholar] [CrossRef]
- Kobayashi, T.; Fuse, S.; Sakamoto, N.; Mikami, M.; Ogawa, S.; Hamaoka, K.; Arakaki, Y.; Nakamura, T.; Nagasawa, H.; Kato, T.; et al. A New Z Score Curve of the Coronary Arterial Internal Diameter Using the Lambda-Mu-Sigma Method in a Pediatric Population. J. Am. Soc. Echocardiogr. 2016, 29, 794–801.e29. [Google Scholar] [CrossRef]
- Dallaire, F.; Dahdah, N. New equations and a critical appraisal of coronary artery Z scores in healthy children. J. Am. Soc. Echocardiogr. 2011, 24, 60–74. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Chen, S.B.; Huang, G.Y.; Zhang, H.Y.; Huang, M.R.; Wang, S.S.; Wu, L.; Hong, W.; Shen, R.; Liu, Y.; et al. Coronary artery indexed diameter and z score regression equations in healthy Chinese Han children. J. Clin. Ultrasound 2015, 43, 39–46. [Google Scholar] [CrossRef]
- Olivieri, L.; Arling, B.; Friberg, M.; Sable, C. Coronary artery Z score regression equations and calculators derived from a large heterogeneous population of children undergoing echocardiography. J. Am. Soc. Echocardiogr. 2009, 22, 159–164. [Google Scholar] [CrossRef]
- O’Leary, P.W. Pediatric diastology: Use and limitations of Doppler echocardiography in the evaluation of ventricular diastolic function in children. Prog. Pediatr. Cardiol. 1999, 10, 83–93. [Google Scholar] [CrossRef]
- Dallaire, F.; Slorach, C.; Hui, W.; Sarkola, T.; Friedberg, M.K.; Bradley, T.J.; Jaeggi, E.; Dragulescu, A.; Har, R.L.; Cherney, D.Z.; et al. Reference values for pulse wave Doppler and tissue Doppler imaging in pediatric echocardiography. Circ. Cardiovasc. Imaging 2015, 8, e002167. [Google Scholar] [CrossRef]
- Eidem, B.W.; McMahon, C.J.; Cohen, R.R.; Wu, J.; Finkelshteyn, I.; Kovalchin, J.P.; Ayres, N.A.; Bezold, L.I.; O’Brian Smith, E.; Pignatelli, R.H. Impact of cardiac growth on Doppler tissue imaging velocities: A study in healthy children. J. Am. Soc. Echocardiogr. 2004, 17, 212–221. [Google Scholar] [CrossRef]
- Cui, W.; Roberson, D.A. Left ventricular Tei index in children: Comparison of tissue Doppler imaging, pulsed wave Doppler and M-mode echocardiography normal values. J. Am. Soc. Echocardiogr. 2006, 19, 1438–1445. [Google Scholar] [CrossRef]
- Roberson, D.A.; Cui, W.; Chen, Z.; Madronero, L.F.; Cuneo, B.F. Annular and septal Doppler tissue imaging in children: Normal z-score tables and effects of age, heart rate, and body surface area. J. Am. Soc. Echocardiogr. 2007, 20, 1276–1284. [Google Scholar] [CrossRef]
- Cantinotti, M.; Giordano, R.; Scalese, M.; Murzi, B.; Assanta, N.; Spadoni, I.; Crocetti, M.; Marotta, M.; Molinaro, S.; Kutty, S.; et al. Nomograms for mitral inflow Doppler and tissue Doppler velocities in Caucasian children. J. Cardiol. 2016, 68, 288–299. [Google Scholar] [CrossRef]
- Schmitz, L.; Stiller, B.; Pees, C.; Koch, H.; Xanthopoulos, A.; Lange, P. Doppler-derived parameters of diastolic left ventricular function in preterm infants with a birth weight <1500 g: Reference values and differences to term infants. Early Hum. Dev. 2004, 76, 101–114. [Google Scholar] [CrossRef]
- Cantinotti, M.; Giordano, R.; Scalese, M.; Franchi, E.; Corana, G.; Assanta, N.; Maura, C.; Marco, M.; Molinaro, S.; Koestenberger, M.; et al. Nomograms for echocardiographic right ventricular sub-costal view dimensions in healthy Caucasian children: A new approach to measure the right ventricle. J. Cardiol. 2018, 71, 181–186. [Google Scholar] [CrossRef]
- Cantinotti, M.; Giordano, R.; Scalese, M.; Franchi, E.; Assanta, N.; Molinaro, S.; Marchese, P.; Paterni, M.; Iervasi, G.; Kutty, S.; et al. Nomograms of pulsed Doppler velocities, times, and velocity time integrals for semilunar valves and great arteries in healthy Caucasian children. Int. J. Cardiol. 2019, 285, 133–139. [Google Scholar] [CrossRef]
- Koestenberger, M.; Nage, B.; Ravekes, W.; Avian, A.; Burmas, A.; Grangl, G.; Cvirn, G.; Gamillscheg, A. Right Ventricular Outflow Tract Velocity Time Integral Determination in 570 Healthy Children and in 52 Pediatric Atrial Septal Defect Patients. Pediatr. Cardiol. 2015, 36, 1129–1134. [Google Scholar] [CrossRef]
- Núñez-Gil, I.J.; Rubio, M.D.; Cartón, A.J.; López-Romero, P.; Deiros, L.; García-Guereta, L.; Labrandero, C.; Gutiérrez-Larraya, F. Determination of normalized values of the tricuspid annular plane systolic excursion (TAPSE) in 405 Spanish children and adolescents. Rev. Esp. Cardiol. 2011, 64, 674–680. [Google Scholar] [CrossRef]
- Hashimoto, I.; Watanabe, K.; Kaneda, H. Z-values of tricuspid annular plane systolic excursion in Japanese children. Pediatr. Int. 2015, 57, 199–204. [Google Scholar] [CrossRef]
- Uysal, F.; Bostan, Ö.M.; Çil, E. Determination of reference values for tricuspid annular plane systolic excursion in healthy Turkish children. Anatol. J. Cardiol. 2016, 16, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Weismann, C.G.; Bamdad, M.C.; Abraham, S.; Ghiroli, S.; Dziura, J.; Hellenbrand, W.E. Normal pediatric data for isovolumic acceleration at the lateral tricuspid valve annulus-a heart rate—Dependent measure of right ventricular contractility. Echocardiography 2015, 32, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Koestenberger, M.; Nagel, B.; Ravekes, W.; Avian, A.; Heinzl, B.; Cvirn, G.; Fritsch, P.; Fandl, A.; Rehak, T.; Gamillscheg, A. Reference values of tricuspid annular peak systolic velocity in healthy pediatric patients, calculation of z score, and comparison to tricuspid annular plane systolic excursion. Am. J. Cardiol. 2012, 109, 116–121. [Google Scholar] [CrossRef]
- Terada, T.; Mori, K.; Inoue, M.; Yasunobu, H. Mitral annular plane systolic excursion/left ventricular length (MAPSE/L) as a simple index for assessing left ventricular longitudinal function in children. Echocardiography 2016, 33, 1703–1709. [Google Scholar] [CrossRef]
- Koestenberger, M.; Ravekes, W.; Nagel, B.; Avian, A.; Heinzl, B.; Cvirn, G.; Fritsch, P.; Fandl, A.; Rehak, T.; Gamillscheg, A. Reference values of the right ventricular outflow tract systolic excursion in 711 healthy children and calculation of z-score values. Eur. Heart J.-Cardiovasc. Imaging 2014, 15, 980–986. [Google Scholar] [CrossRef]
- Maffessanti, F.; Muraru, D.; Esposito, R.; Gripari, P.; Ermacora, D.; Santoro, C.; Tamborini, G.; Galderisi, M.; Pepi, M.; Badano, L.P. Age-, Body Size- and Gender-Specific Reference Values for Right Ventricular Volumes and Ejection Fraction by Three-Dimensional Echocardiography: A Multicenter Echocardiographic Study in 507 Healthy Volunteers. Circ. Cardiovasc. Imaging 2013, 6, 700–710. [Google Scholar] [CrossRef]
- Koestenberger, M.; Grangl, G.; Avian, A.; Gamillscheg, A.; Grillitsch, M.; Cvirn, G.; Burmas, A.; Hansmann, G. Normal Reference Values and z Scores of the Pulmonary Artery Acceleration Time in Children and Its Importance for the Assessment of Pulmonary Hypertension. Circ. Cardiovasc. Imaging 2017, 10, e005336. [Google Scholar] [CrossRef]
- Koestenberger, M.; Ravekes, W.; Everett, A.D.; Stueger, H.P.; Heinzl, B.; Gamillscheg, A.; Cvirn, G.; Boysen, A.; Fandl, A.; Nagel, B. Right ventricular function in infants, children and adolescents: Reference values of the tricuspid annular plane systolic excursion (TAPSE) in 640 healthy patients and calculation of z score values. J. Am. Soc. Echocardiogr. 2009, 22, 715–719. [Google Scholar] [CrossRef]
- Marchese, P.; Scalese, M.; Giordano, R.; Assanta, N.; Franchi, E.; Koestenberger, M.; Ravaglioli, A.; Kutty, S.; Cantinotti, M. Pediatric ranges of normality for 2D speckle-tracking echocardiography atrial strain: Differences between p- and r-gating and among new (Atrial Designed) and conventional (Ventricular Specific) software’s. Echocardiography 2021, 38, 2025–2031. [Google Scholar] [CrossRef]
- Acheampong, B.; Parra, D.; Havens, C.; Jantzen, D.; Godown, J.; Soslow, J. Vendor independent myocardial strain values in children. Echocardiography 2023, 40, 30–36. [Google Scholar] [CrossRef]
- Adar, A.; Ghelani, S.J.; Sleeper, L.A.; Lu, M.; Marcus, E.; Ferraro, A.M.; Colan, S.D.; Banka, P.; Powell, A.J.; Harrild, D.M. Normal Values for Left Ventricular Strain and Synchrony in Children Based on Speckle Tracking Echocardiography. Am. J. Cardiol. 2019, 123, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Jimbo, S.; Noto, N.; Okuma, H.; Kato, M.; Komori, A.; Ayusawa, M.; Morioka, I. Normal reference values for left atrial strains and strain rates in school children assessed using two-dimensional speckle-tracking echocardiography. Heart Vessels 2020, 35, 1270–1280. [Google Scholar] [CrossRef] [PubMed]
- Harrington, J.K.; Ferraro, A.M.; Colan, S.D.; Sleeper, L.A.; Lu, M.; Adar, A.; Powell, A.J.; Levy, P.T.; Harrild, D.M. Normal Left Ventricular Systolic and Diastolic Strain Rate Values in Children Derived from Two-Dimensional Speckle-Tracking Echocardiography. J. Am. Soc. Echocardiogr. 2021, 34, 1303–1315.e3. [Google Scholar] [CrossRef] [PubMed]
- Kotby, A.A.; Ebrahim, S.O.S.; Al-Fahham, M.M. Reference centiles for left ventricular longitudinal global and regional systolic strain by automated functional imaging in healthy Egyptian children. Cardiol. Young 2023, 33, 26–34. [Google Scholar] [CrossRef]
- Davarpasand, T.; Jalali, A.; Mohseni-Badalabadi, R.; Toofaninejad, N.; Hali, R.; Fallah, F.; Seilani, P.; Hosseinsabet, A. Normal ranges of left atrial phasic strains and strain rates by 2D speckle-tracking echocardiography in pediatrics: A systematic review and meta-analysis. Sci. Rep. 2024, 14, 25888. [Google Scholar] [CrossRef]
- Koopman, L.P.; Rebel, B.; Gnanam, D.; Menting, M.E.; Helbing, W.A.; Boersma, E. Reference values for two-dimensional myocardial strain echocardiography of the left ventricle in healthy children. Cardiol. Young 2019, 29, 325–337. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, J.; Xie, M.; Yin, P.; Liu, W.; Li, Y.; Klas, B.; Sun, J.; Balluz, R.; Ge, S. Left ventricular three-dimensional global systolic strain by real-time three-dimensional speckle-tracking in children: Feasibility, reproducibility, maturational changes, and normal ranges. J. Am. Soc. Echocardiogr. 2013, 26, 853–859. [Google Scholar] [CrossRef]
- Kamel, H.; Elsayegh, A.T.; Nazmi, H.; Attia, H.M. Assessment of left ventricular systolic function using two- and three-dimensional speckle tracking echocardiography among healthy preschool-age pediatric children. Egypt. Heart J. 2022, 74, 21. [Google Scholar] [CrossRef]
- Aristizábal-Duque, C.H.; Fernández Cabeza, J.; Blancas Sánchez, I.M.; Delgado Ortega, M.; Aparicio Martinez, P.; Romero-Saldaña, M.; del Pozo, F.J.F.; Pan, M.; Ruiz Ortiz, M.; Mesa-Rubio, M.D. The Assessment of Myocardial Longitudinal Strain in a Paediatric Spanish Population Using a New Software Analysis. J. Clin. Med. 2022, 11, 3272. [Google Scholar] [CrossRef]
- Marcus, K.A.; Mavinkurve-Groothuis, A.M.; Barends, M.; van Dijk, A.; Feuth, T.; de Korte, C.; Kapusta, L. Reference values for myocardial two-dimensional strain echocardiography in a healthy pediatric and young adult cohort. J. Am. Soc. Echocardiogr. 2011, 24, 625–636. [Google Scholar] [CrossRef]
- Klitsie, L.M.; Roest, A.A.; van der Hulst, A.E.; Stijnen, T.; Blom, N.A.; Ten Harkel, A.D. Assessment of intraventricular time differences in healthy children using two-dimensional speckle tracking echocardiography. J. Am. Soc. Echocardiogr. 2013, 26, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Dallaire, F.; Slorach, C.; Bradley, T.; Hui, W.; Sarkola, T.; Friedberg, M.K.; Jaeggi, E.; Dragulescu, A.; Mahmud, F.H.; Daneman, D.; et al. Pediatric Reference Values and Z Score Equations for Left Ventricular Systolic Strain Measured by Two-Dimensional Speckle-Tracking Echocardiography. J. Am. Soc. Echocardiogr. 2016, 29, 786–793.e8. [Google Scholar] [CrossRef] [PubMed]
- Cantinotti, M.; Scalese, M.; Giordano, R.; Franchi, E.; Assanta, N.; Marotta, M.; Viacava, C.; Molinaro, S.; Iervasi, G.; Santoro, G.; et al. Normative Data for Left and Right Ventricular Systolic Strain in Healthy Caucasian Italian Children by Two-Dimensional Speckle-Tracking Echocardiography. J. Am. Soc. Echocardiogr. 2018, 31, 712–720.e6. [Google Scholar] [CrossRef] [PubMed]
- Levy, P.T.; Mejia, A.A.S.; Machefsky, A.; Fowler, S.; Holland, M.R.; Singh, G.K. Normal ranges of right ventricular systolic and diastolic strain measures in children: A systematic review and meta-analysis. J. Am. Soc. Echocardiogr. 2014, 27, 549–560.e3. [Google Scholar] [CrossRef]
- Ghelani, S.J.; Brown, D.W.; Kuebler, J.D.; Perrin, D.; Shakti, D.; Williams, D.N.; Marx, G.R.; Colan, S.D.; Geva, T.; Harrild, D.M. Left atrial volumes and strain in healthy children measured by three-dimensional echocardiography: Normal values and maturational changes. J. Am. Soc. Echocardiogr. 2018, 31, 187–193. [Google Scholar] [CrossRef]
- Kutty, S.; Padiyath, A.; Li, L.; Peng, Q.; Rangamani, S.; Schuster, A.; Danford, D.A. Functional Maturation of left and right atrial systolic and diastolic performance in infants, children, and adolescents. J. Am. Soc. Echocardiogr. 2013, 26, 398–4092. [Google Scholar] [CrossRef]
- Ramlogan, S.; Aly, D.; France, R.; Schmidt, S.; Hinzman, J.; Sherman, A.; Goudar, S.P.; Forsha, D. Reproducibility and Intervendor Agreement of Left Ventricular Global Systolic Strain in Children Using a Layer-Specific Analysis. J. Am. Soc. Echocardiogr. 2020, 33, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Romanowicz, J.; Ferraro, A.M.; Harrington, J.K.; Sleeper, L.A.; Adar, A.; Levy, P.T.; Powell, A.J.; Harrild, D.M. Pediatric Normal Values and Z Score Equations for Left and Right Ventricular Strain by Two-Dimensional Speckle-Tracking Echocardiography Derived from a Large Cohort of Healthy Children. J. Am. Soc. Echocardiogr. 2023, 36, 310–323. [Google Scholar] [CrossRef] [PubMed]
- Amedro, P.; Bredy, C.; Guillaumont, S.; De La Villeon, G.; Gamon, L.; Lavastre, K.; Meli, A.C.; Richard, S.; Cazorla, O.; Lacampagne, A.; et al. Speckle tracking echocardiography in healthy children: Comparison between the QLAB by Philips and the EchoPAC by General Electric. Int. J. Cardiovasc. Imaging 2019, 35, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, A.M.; Adar, A.; Ghelani, S.J.; Sleeper, L.A.; Levy, P.T.; Rathod, R.H.; Marx, G.R.; Harrild, D.M. Speckle tracking echocardiographically-based analysis of ventricular strain in children: An intervendor comparison. Cardiovasc. Ultrasound 2020, 18, 15. [Google Scholar] [CrossRef]
- Palmer, C.; Truong, V.T.; Klas, B.; Wolking, S.; Ornella, A.; Young, M.; Ngo, T.N.M.; Tretter, J.T.; Nagueh, S.F.; Mazur, W. Left and right atrial speckle tracking: Comparison of three methods of time reference gating. Echocardiography 2020, 37, 1021–1029. [Google Scholar] [CrossRef]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef]
- Voigt, J.U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S.; et al. Definitions for a common standard for 2D speckle tracking echocardiography: Consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J. Am. Soc. Echocardiogr. 2015, 28, 183–193. [Google Scholar] [CrossRef]
- Kuebler, J.D.; Ghelani, S.; Williams, D.M.; Nathan, M.; Marx, G.; Colan, S.D.; Harrild, D.M. Normal Values and Growth-Related Changes of Left Ventricular Volumes, Stress, and Strain in Healthy Children Measured by 3-Dimensional Echocardiography. Am. J. Cardiol. 2018, 122, 331–339. [Google Scholar] [CrossRef]
- Jone, P.N.; Le, L.; Pan, Z.; Colen, T.; Shigemitsu, S.; Khoo, N.S.; Goot, B.H.; Parthiban, A.; Harrild, D.M.; Ferraro, A.M.; et al. A multicenter study of three-dimensional echocardiographic evaluation of normal pediatric left ventricular volumes and function. Echocardiography 2021, 38, 641–645. [Google Scholar] [CrossRef]
- Krell, K.; Laser, K.T.; Dalla-Pozza, R.; Winkler, C.; Hildebrandt, U.; Kececioglu, D.; Breuer, J.; Herberg, U. Real-time three-dimensional echocardiography of the left ventricle—Pediatric percentiles and head-to-head comparison of different contour finding algorithms: A multicenter study. J. Am. Soc. Echocardiogr. 2018, 31, 702–711.e13. [Google Scholar] [CrossRef]
- Herberg, U.; Smit, F.; Winkler, C.; Dalla-Pozza, R.; Breuer, J.; Laser, K.T. Real-time 3D-echocardiography of the right ventricle—Paediatric reference values for right ventricular volumes using knowledge-based reconstruction: A multicentre study. Quant. Imaging Med. Surg. 2021, 11, 2905–2917. [Google Scholar] [CrossRef] [PubMed]
- Cantinotti, M.; Scalese, M.; Giordano, R.; Franchi, E.; Marchese, P.; Assanta, N.; Molinaro, S.; Paterni, M.; Iervasi, G.; Koestenberger, M.; et al. Three-Dimensional Echocardiography Derived Nomograms for Left Ventricular Volumes in Healthy Caucasian Italian Children. J. Am. Soc. Echocardiogr. 2019, 32, 794–797.e1. [Google Scholar] [CrossRef] [PubMed]
- Jone, P.N.; Schäfer, M.; Pan, Z.; Bremen, C.; Ivy, D.D. 3D echocardiographic evaluation of right ventricular function and strain: A prognostic study in paediatric pulmonary hypertension. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Linden, K.; Goldschmidt, F.; Laser, K.T.; Winkler, C.; Körperich, H.; Dalla-Pozza, R.; Breuer, J.; Herberg, U. Left Atrial Volumes and Phasic Function in Healthy Children: Reference Values Using Real-Time Three-Dimensional Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1036–1045.e9. [Google Scholar] [CrossRef]
- Sabatino, J.; Borrelli, N.; Fraisse, A.; Herberg, J.; Karagadova, E.; Avesani, M.; Bucciarelli, V.; Josen, M.; Paredes, J.; Piccinelli, E.; et al. Abnormal myocardial work in children with Kawasaki disease. Sci. Rep. 2021, 11, 7974. [Google Scholar] [CrossRef]
- Sabatino, J.; Leo, I.; Strangio, A.; La Bella, S.; Borrelli, N.; Avesani, M.; Josen, M.; Paredes, J.; Piccinelli, E.; Sirico, D.; et al. Echocardiographic Normal Reference Ranges for Non-invasive Myocardial Work Parameters in Pediatric Age: Results from an International Multi-Center Study. Front. Cardiovasc. Med. 2022, 9, 792622. [Google Scholar] [CrossRef]
- Cui, C.; Zheng, Q.; Li, Y.; Huang, D.; Hu, Y.; Wang, Y.; Liu, R.; Liu, L.; Zhang, L. Reference Values of Noninvasive Myocardial Work Indices Measured by Echocardiography in Healthy Children. Front. Pediatr. 2022, 10, 792526. [Google Scholar] [CrossRef]
- Pham, T.T.M.; Truong, V.T.; Vu, P.N.; Tran, T.X.; Nguyen, N.N.H.; Nguyen, L.P.T.; Tu, H.N.T.; Palmer, C.; Tretter, J.T.; Levy, P.; et al. Echocardiographic Reference Ranges of Non-invasive Myocardial Work Indices in Children. Pediatr. Cardiol. 2022, 43, 82–91. [Google Scholar] [CrossRef]
- Luo, X.; Ge, Q.; Su, J.; Zhou, N.; Li, P.; Xiao, X.; Chen, Y.; Wang, D.; Ma, Y.; Ma, L.; et al. Normal ranges of non-invasive left ventricular myocardial work indices in healthy young people. Front. Pediatr. 2022, 10, 1000556. [Google Scholar] [CrossRef]
- Tretter, J.T.; Pradhan, S.; Truong, V.T.; Mullikin, A.; Mazur, W.; Hill, G.D.; Redington, A.N.; Taylor, M.D. Non-invasive left ventricular myocardial work indices in healthy adolescents at rest. Int. J. Cardiovasc. Imaging 2021, 37, 2429–2438. [Google Scholar] [CrossRef]
- Marchese, P.; Scalese, M.; Assanta, N.; Franchi, E.; Viacava, C.; Santoro, G.; Corana, G.; Pizzuto, A.; Contini, F.V.; Kutty, S.; et al. Normal Values for Echocardiographic Myocardial Work in a Large Pediatric Population. Diagnostics 2024, 14, 1022. [Google Scholar] [CrossRef]
- Cantinotti, M.; Scalese, M.; Giordano, R.; Assanta, N.; Marchese, P.; Franchi, E.; Viacava, C.; Koestenberger, M.; Jani, V.; Kutty, S. A statistical comparison of reproducibility in current pediatric two-dimensional echocardiographic nomograms. Pediatr. Res. 2021, 89, 579–590. [Google Scholar] [CrossRef]
- Available online: https://zscore.chboston.org/ (accessed on 10 October 2024).
- Available online: https://parameterz.com (accessed on 10 October 2024).
- Available online: https://www.pediatricheartnetwork.org/z-scores-calculator/ (accessed on 10 October 2024).
- Available online: https://www.prisma-statement.org/ (accessed on 10 October 2024).
- Lopez, L.; Saurers, D.L.; Barker, P.C.A.; Cohen, M.S.; Colan, S.D.; Dwyer, J.; Forsha, D.; Friedberg, M.K.; Lai, W.W.; Printz, B.F.; et al. Guidelines for Performing a Comprehensive Pediatric Transthoracic Echocardiogram: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2024, 37, 119–170. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.; Lopez, L.; Acar, P.; Friedberg, M.K.; Khoo, N.S.; Ko, H.H.; Marek, J.; Marx, G.; McGhie, J.S.; Meijboom, F.; et al. Three-dimensional Echocardiography in Congenital Heart Disease: An Expert Consensus Document from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2017, 30, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Mahgerefteh, J.; Lai, W.; Colan, S.; Trachtenberg, F.; Gongwer, R.; Stylianou, M.; Bhat, A.H.; Goldberg, D.; McCrindle, B.; Frommelt, P.; et al. Height Versus Body Surface Area to Normalize Cardiovascular Measurements in Children Using the Pediatric Heart Network Echocardiographic Z-Score Database. Pediatr. Cardiol. 2021, 42, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.; Thomson, D.; Seto, I.; Contopoulos-Ioannidis, D.G.; Ioannidis, J.P.; Curtis, S.; Constantin, E.; Batmanabane, G.; Hartling, L.; Klassen, T. Standard 6: Age groups for pediatric trials. Pediatrics 2012, 129, S153–S160. [Google Scholar] [CrossRef]
- Thompson, W.; Endriss, J. The required sample size when estimating variances. Am. Stat. 1961, 15, 22–23. [Google Scholar] [CrossRef]
- Kish, L. Survey Sampling; John Wiley & Sons, Inc.: New York, NY, USA, 1965. [Google Scholar]
- Plante, V.; Gobeil, L.; Xiong, W.T.; Touré, M.; Dahdah, N.; Greenway, S.C.; Drolet, C.; Wong, K.K.; Mackie, A.S.; Bradley, T.J.; et al. Alternative to Body Surface Area as a Solution to Correct Systematic Bias in Pediatric Echocardiography Z Scores. Can. J. Cardiol. 2021, 37, 1790–1797. [Google Scholar] [CrossRef]
- Haycock, G.B.; Schwartz, G.J.; Wisotsky, D.H. Geometric method for measuring body surface area: A height-weight formula validated in infants, children, and adults. J. Pediatr. 1978, 93, 62–66. [Google Scholar] [CrossRef]
- Bonatto, R.C.; Fioretto, J.R.; Okoshi, K.; Matsubara, B.B.; Padovani, C.R.; Manfrin, T.C.R.; Gobbi, M.; Martino, R.S.; Bregagnollo, E.A. Percentile curves of normal values of echocardiographic measurements in normal children from the central-southern region of the State of Sao Paulo, Brazil. Arq. Bras. Cardiol. 2006, 87, 711–721. [Google Scholar]
- White, H.A. Heteroscedasticity-consistent covariance matrix estimator and a direct test for heteroscedasticity. Econometrica 1980, 48, 817–838. [Google Scholar] [CrossRef]
- Breusch, T.S.; Pagan, A.R. A Simple test for heteroscedasticity and random coefficient variation. Econometrica 1979, 47, 1287–1294. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Lilliefors, H. On the Kolmogorov–Smirnov test for normality with mean and variance unknown. J. Am. Stat. Assoc. 1967, 62, 399–402. [Google Scholar] [CrossRef]
- Sluysmans, T.; Colan, S.D. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J. Appl. Physiol. 2005, 99, 445–457. [Google Scholar] [CrossRef]
- Pettersen, M.D.; Du, W.; Skeens, M.E.; Humes, R.A. Regression equations for calculation of z scores of cardiac structures in a large cohort of healthy infants, children, and adolescents: An echocardiographic study. J. Am. Soc. Echocardiogr. 2008, 21, 922–934. [Google Scholar] [CrossRef]
- Majonga, E.D.; Rehman, A.M.; McHugh, G.; Mujuru, H.A.; Nathoo, K.; Patel, M.S.; Munyati, S.; Odland, J.O.; Kranzer, K.; Kaski, J.P.; et al. Echocardiographic reference ranges in older children and adolescents in sub-Saharan Africa. Int. J. Cardiol. 2017, 248, 409–413. [Google Scholar] [CrossRef]
- Majonga, E.D.; Norrish, G.; Rehman, A.M.; Kranzer, K.; Mujuru, H.A.; Nathoo, K.; Odland, J.O.; Kaski, J.P.; Ferrand, R.A. Racial Variation in Echocardiographic Reference Ranges for Left Chamber Dimensions in Children and Adolescents: A Systematic Review. Pediatr. Cardiol. 2018, 39, 859–868. [Google Scholar] [CrossRef]
- Zilberman, M.V.; Khoury, P.R.; Kimball, R.T. Two-dimensional echocardiographic valve measurements in healthy children: Gender-specific differences. Pediatr. Cardiol. 2005, 26, 356–360. [Google Scholar] [CrossRef]
- Gautier, M.; Detaint, D.; Fermanian, C.; Aegerter, P.; Delorme, G.; Arnoult, F.; Milleron, O.; Raoux, F.; Stheneur, C.; Boileau, C.; et al. Nomograms for aortic root diameters in children using two-dimensional echocardiography. Am. J. Cardiol. 2010, 105, 888–894. [Google Scholar] [CrossRef]
- Cantinotti, M.; Scalese, M.; Murzi, B.; Assanta, N.; Spadoni, I.; De Lucia, V.; Crocetti, M.; Cresti, A.; Gallotta, M.; Marotta, M.; et al. Echocardiographic nomograms for chamber diameters and areas in Caucasian children. J. Am. Soc. Echocardiogr. 2014, 27, 1279–1292.e2. [Google Scholar] [CrossRef]
- Ciccone, M.M.; Scicchitano, P.; Zito, A.; Gesualdo, M.; Sassara, M.; Calderoni, G.; Di Mauro, F.; Ladisa, G.; Di Mauro, A.; Laforgia, N. Different functional cardiac characteristics observed in term/preterm neonates by echocardiography and tissue Doppler imaging. Early Hum. Dev. 2011, 87, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Lorch, S.M.; Ludomirsky, A.; Singh, G.K. Maturational and growth-related changes in left ventricular longitudinal strain and strain rate measured by two-dimensional speckle tracking echocardiography in healthy pediatric population. J. Am. Soc. Echocardiogr. 2008, 21, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Cantinotti, M.; Kutty, S.; Giordano, R.; Assanta, N.; Murzi, B.; Crocetti, M.; Marotta, M.; Iervasi, G. Review and status report of pediatric left ventricular systolic strain and strain rate nomograms. Heart Fail. Rev. 2015, 20, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Al Naami, G.; Thompson, R.; Inage, A.; Mackie, A.S.; Smallhorn, J.F. Normal rotational, torsion and untwisting data in children, adolescents and young adults. J. Am. Soc. Echocardiogr. 2010, 23, 286–293. [Google Scholar] [CrossRef]
- Kaku, K.; Takeuchi, M.; Tsang, W.; Takigiku, K.; Yasukochi, S.; Patel, A.R.; Mor-Avi, V.; Lang, R.M.; Otsuji, Y. Age-related normal range of left ventricular strain and torsion using three-dimensional speckle-tracking echocardiography. J. Am. Soc. Echocardiogr. 2014, 27, 55–64. [Google Scholar] [CrossRef]
- Kim, H.J.; Yoon, J.H.; Lee, E.J.; Oh, J.H.; Lee, J.Y.; Lee, S.J.; Han, J.W. Normal left ventricular torsion mechanics in healthy children: Age related changes of torsion parameters are closely related to changes in heart rate. Korean Circ. J. 2015, 45, 131–140. [Google Scholar] [CrossRef]
- Poutanen, T.; Jokinen, E. Left ventricular mass in 169 healthy children and young adults assessed by three-dimensional echocardiography. Pediatr. Cardiol. 2007, 28, 201–207. [Google Scholar] [CrossRef]
- Boettler, P.; Hartmann, M.; Watzl, K.; Maroula, E.; Schulte-Moenting, J.; Knirsch, W.; Dittrich, S.; Kececioglu, D. Heart rate effects on strain and strain rate in healthy children. J. Am. Soc. Echocardiogr. 2005, 18, 1121–1130. [Google Scholar] [CrossRef]
- Poutanen, T.; Jokinen, E.; Sairanen, H.; Tikanoja, T. Left atrial and left ventricular function in healthy children and young adults assessed by three dimensional echocardiography. Heart 2003, 89, 544–549. [Google Scholar] [CrossRef]
- Poutanen, T.; Tikanoja, T.; Sairanen, H.; Jokinen, E. Normal mitral and aortic valve areas assessed by three- and two-dimensional echocardiography in 168 children and young adults. Pediatr. Cardiol. 2006, 27, 217–225. [Google Scholar] [CrossRef]
- Lu, D.F.; Tong, X.M.; Liu, Y.F.; Zhang, H. Reference Values for Point-of-Care Echocardiographic Measurements of Preterm Infants in China. Front. Pediatr. 2022, 10, 894152. [Google Scholar] [CrossRef]
- Ashrafi, A.H.; Lai, W.; Gaffar, S.; Renella, P. Normative Echocardiographic Values for Right and Left Ventricular Function in Extremely Premature Neonates. J. Pediatr. 2021, 236, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Fu, J.; Wu, L.; Liu, X.Y.; Zhang, Y. Percentile curves of normal echocardiographic measurements values for left heart structures in 1570 Han Chinese preterm and term infants. J. Clin. Ultrasound 2022, 50, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Calado, C.; Collins, C.; Drew, S.; Holberton, J. Reference echocardiographic measurements in very low birth weight preterm infants. Am. J. Perinatol. 2019, 36, 303–310. [Google Scholar] [PubMed]
- Skelton, R.; Gill, A.B.; Parsons, J.M. Reference ranges for cardiac dimensions and blood flow velocity in preterm infants. Heart 1998, 80, 281–285. [Google Scholar] [CrossRef]
- Abushaban, L.; Vel, M.T.; Rathinasamy, J.; Sharma, P.N. Normal reference ranges for pulmonary artery diameters in preterm infants. Pediatr. Cardiol. 2017, 38, 1377–1384. [Google Scholar] [CrossRef]
- Choudhry, S.; Salter, A.; Cunningham, T.W.; Levy, P.T.; Nguyen, H.H.; Wallendorf, M.; Singh, G.K.; Johnson, M.C. Normative Left Ventricular M-Mode Echocardiographic Values in Preterm Infants up to 2 kg. J. Am. Soc. Echocardiogr. 2017, 30, 781–789.e4. [Google Scholar] [CrossRef]
- Krysztofiak, H.; Młyńczak, M.; Folga, A.; Braksator, W.; Małek, Ł.A. Normal values for left ventricular mass in relation to lean body mass in child and adolescent athletes. Pediatr. Cardiol. 2019, 40, 204–208. [Google Scholar] [CrossRef]
- Cavarretta, E.; Maffessanti, F.; Sperandii, F.; Guerra, E.; Quaranta, F.; Nigro, A.; Minati, M.; Rebecchi, M.; Fossati, C.; Calò, L.; et al. Reference values of left heart echocardiographic dimensions and mass in male peri-pubertal athletes. Eur. J. Prev. Cardiol. 2018, 25, 1204–1215. [Google Scholar] [CrossRef]
- Sharma, S.; Maron, B.J.; Whyte, G.; Firoozi, S.; Elliott, P.M.; McKenna, W.J. Physiologic limits of left ventricular hypertrophy in elite junior athletes: Relevance to differential diagnosis of athlete’s heart and hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2002, 40, 1431–1436. [Google Scholar] [CrossRef]
- Makan, J.; Sharma, S.; Firoozi, S.; Whyte, G.; Jackson, P.G.; McKenna, W.J. Physiological upper limits of ventricular cavity size in highly trained adolescent athletes. Heart 2005, 9, 495–499. [Google Scholar] [CrossRef]
- George, K.; Sharma, S.; Batterham, A.; Whyte, G.; McKenna, W. Allometric analysis of the association between cardiac dimensions and body size variables in 464 junior athletes. Clin. Sci. 2001, 100, 47–54. [Google Scholar] [CrossRef]
- Chen, L.; Chen, W.; Zhu, Y.; Zhang, Z.; Liu, T.; Zhang, L. Global research landscape on artificial intelligence in echocardiography from 1997 to 2024: Bibliometric analysis. Digit. Health 2025, 11, 20552076251351201. [Google Scholar]
- Mayourian, J.; Asztalos, I.B.; El-Bokl, A.; Lukyanenko, P.; Kobayashi, R.L.; La Cava, W.G.; Ghelani, S.J.; Vetter, V.L.; Triedman, J.K. Electrocardiogram-based deep learning to predict left ventricular systolic dysfunction in paediatric and adult congenital heart disease in the USA: A multicentre modelling study. Lancet Digit. Health 2025, 7, e264–e274. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).