Scrotal Migration of the Ventriculoperitoneal Shunt in a 1-Year-Old Pediatric Patient: A Case Report and Systematic Literature Review
Abstract
1. Introduction
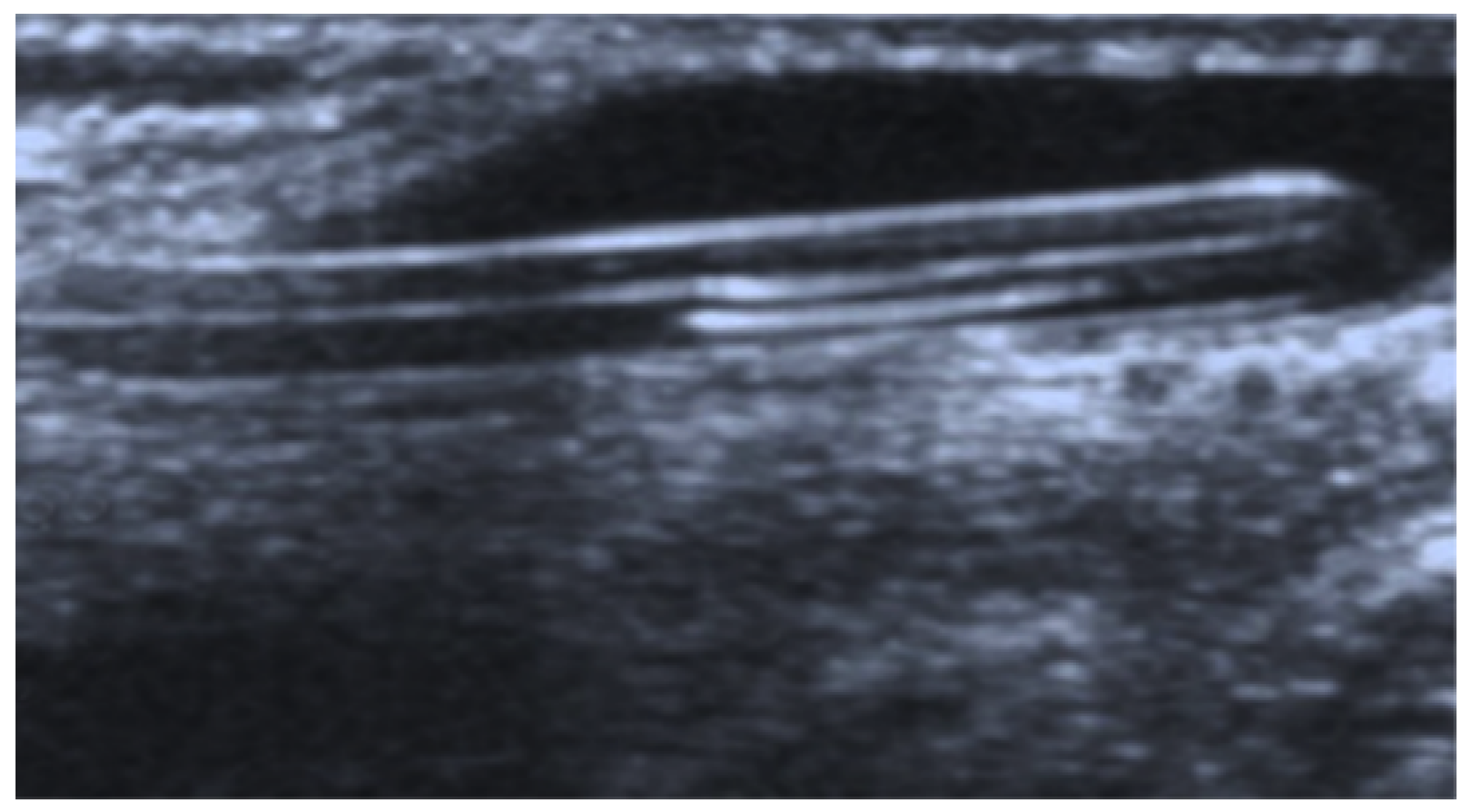
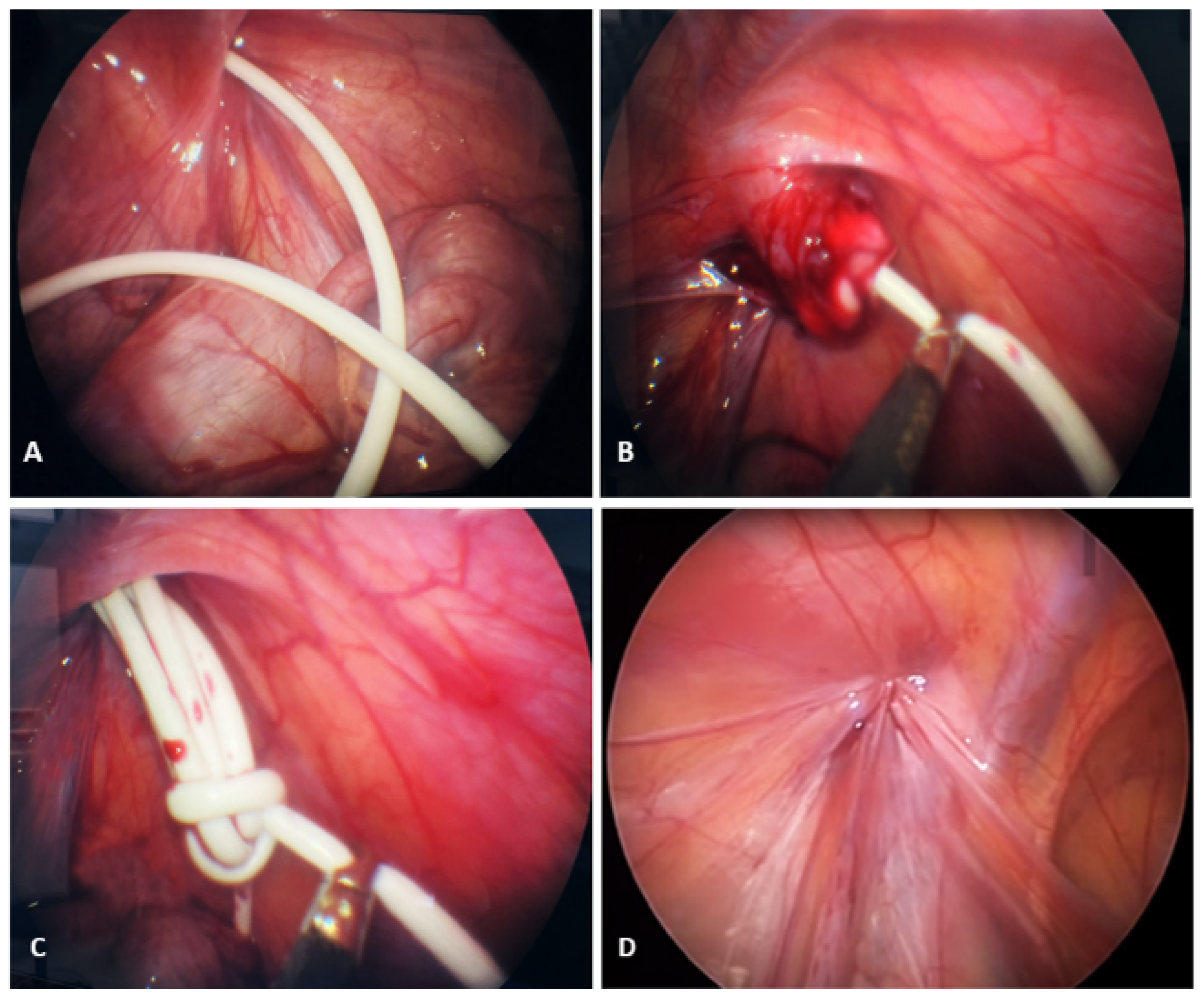
2. Case Presentation
3. Methods
3.1. Inclusion and Exclusion Criteria
3.2. Data Sources and Search Strategy
3.3. Study Selection and Data Collection Process
3.4. Risk of Bias Assessment of Included Studies
3.5. Statistical Analysis
4. Results
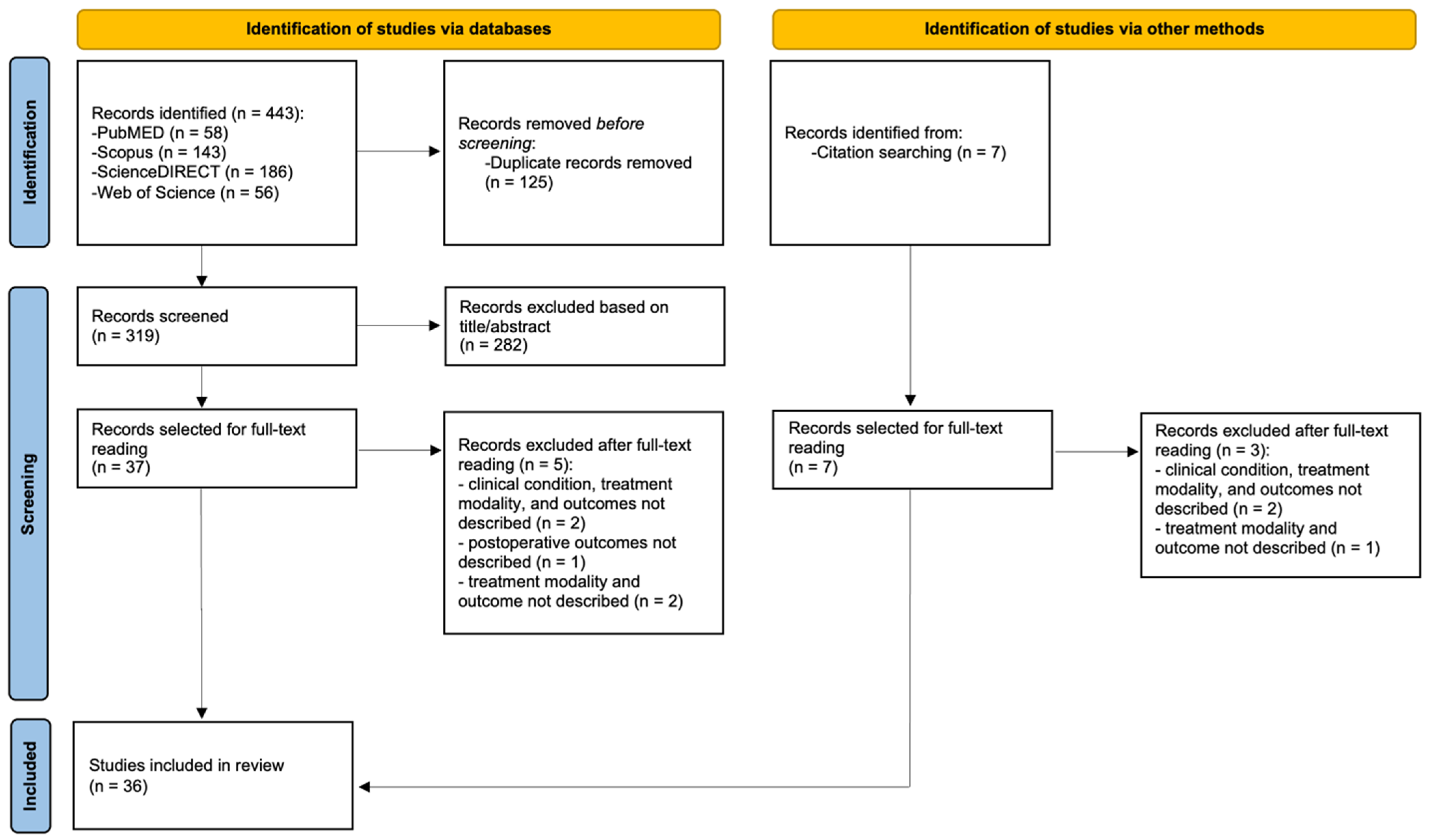
4.1. Study Selection
4.2. Study Characteristics
4.3. Risk of Bias in Studies
4.4. Summary of the Included Studies
5. Discussion
Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anwar, F.; Zhang, K.; Sun, C.; Pang, M.; Zhou, W.; Li, H.; He, R.; Liu, X.; Ming, D. Hydrocephalus: An Update on Latest Progress in Pathophysiological and Therapeutic Research. Biomed. Pharmacother. 2024, 181, 117702. [Google Scholar] [CrossRef]
- Kahle, K.T.; Klinge, P.M.; Koschnitzky, J.E.; Kulkarni, A.V.; MacAulay, N.; Robinson, S.; Schiff, S.J.; Strahle, J.M. Paediatric Hydrocephalus. Nat. Rev. Dis. Primer 2024, 10, 35. [Google Scholar] [CrossRef]
- Alkhudari, A.; Galal, M.; Wagley, Z.; Sabbah, B.N.; Houdane, A.; Aljabr, A. A Case of Spontaneous Resolution of a Scrotal Ventriculoperitoneal Shunt Migration. Radiol. Case Rep. 2022, 17, 3620–3623. [Google Scholar] [CrossRef]
- Ferras, M.; McCauley, N.; Stead, T.; Ganti, L.; Desai, B. Ventriculoperitoneal Shunts in the Emergency Department: A Review. Cureus 2020, 12, e6857. [Google Scholar] [CrossRef] [PubMed]
- Dowlati, E.; Shashaty, M.; Carroll, A.H.; Pivazyan, G.; Briscoe, J.; Patel, N.; Shahjouie, S.; Anaizi, A.N.; Jackson, P.G.; Nair, M.N. General Surgery Involvement with Ventriculoperitoneal Shunt Insertions Reduces Revision Rates. Clin. Neurol. Neurosurg. 2020, 199, 106263. [Google Scholar] [CrossRef] [PubMed]
- Bakal, Ü.; Poyraz, A.K.; Tartar, T.; Akdeniz, İ.; Sürme, M.B.; Çelik, S.; Saraç, M. A Rare Complication of Ventriculoperitoneal Shunt: Asymptomatic Small Bowel Perforation. Istanb. Med. J. 2019, 20, 571–573. [Google Scholar] [CrossRef]
- Lee, B.S.; Vadera, S.; Gonzalez-Martinez, J.A. Rare Complication of Ventriculoperitoneal Shunt. Early Onset of Distal Catheter Migration into Scrotum in an Adult Male: Case Report and Literature Review. Int. J. Surg. Case Rep. 2015, 6, 198–202. [Google Scholar] [CrossRef]
- Khoudir, M.; Harris, L.; Toescu, S.M.; Vaqas, B. Scrotal Migration of a Ventriculoperitoneal Shunt in an Adult. A Case Report and Literature Review. Brain Spine 2022, 2, 100898. [Google Scholar] [CrossRef]
- Ghritlaharey, R.K. Management of Ventriculoperitoneal Shunt Complications in Children: A Review of 34 Cases. Afr. J. Paediatr. Surg. 2023, 20, 109–115. [Google Scholar] [CrossRef]
- Javeed, F.; Tariq, M.; Butt, H.; Rehman, L. Scrotal Migration of the Ventriculoperitoneal Shunt: A Case Report and Review of the Literature. Cureus 2024, 16, e63384. [Google Scholar] [CrossRef]
- Notarianni, C.; Vannemreddy, P.; Caldito, G.; Bollam, P.; Wylen, E.; Willis, B.; Nanda, A. Congenital Hydrocephalus and Ventriculoperitoneal Shunts: Influence of Etiology and Programmable Shunts on Revisions: Clinical Article. J. Neurosurg. Pediatr. 2009, 4, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Paff, M.; Alexandru-Abrams, D.; Muhonen, M.; Loudon, W. Ventriculoperitoneal Shunt Complications: A Review. Interdiscip. Neurosurg. 2018, 13, 66–70. [Google Scholar] [CrossRef]
- Taha, M.M.; Almenshawy, H.A.; Ezzat, M.; Elbadawy, M.K. Migration of Distal End of VP Shunt into the Scrotum: A Management Review. Surg. J. 2022, 08, e245–e248. [Google Scholar] [CrossRef]
- Pogorelić, Z.; Rikalo, M.; Jukić, M.; Katić, J.; Jurić, I.; Furlan, D.; Budimir, D.; Biočić, M. Modified Marcy repair for indirect inguinal hernia in children: A 24-year single-center experience of 6826 pediatric patients. Surg. Today 2017, 47, 108–113. [Google Scholar] [CrossRef]
- Pogorelić, Z. Advances and Future Challenges of Minimally Invasive Surgery in Children. Children 2022, 9, 1959. [Google Scholar] [CrossRef]
- Pogorelić, Z.; Stanić, P.; Bašković, M. Comparison of Percutaneous Internal Ring Suturing (PIRS) versus Open Ligation of the Patent Processus Vaginalis for the Treatment of Communicating Pediatric Hydrocele. Children 2024, 11, 437. [Google Scholar] [CrossRef]
- Pogorelić, Z.; Čohadžić, T.; Jukić, M.; Nevešćanin Biliškov, A. Percutaneous Internal Ring Suturing for the Minimal Invasive Treatment of Pediatric Inguinal Hernia: A 5-Year Single Surgeon Experience. Surg. Laparosc. Endosc. Percutan. Tech. 2021, 31, 150–154. [Google Scholar] [CrossRef]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological Quality of Case Series Studies: An Introduction to the JBI Critical Appraisal Tool. JBI Database Syst. Rev. Implement. Rep. 2019, 18, 2127–2133. [Google Scholar] [CrossRef]
- Muhajir, A.S.; Suryaningtyas, W.; Parenrengi, M.A. Scrotal Migration of the Peritoneal Catheter of a Ventriculoperitoneal Shunt: A Case Series in a Single Center. Surg. Neurol. Int. 2025, 16, 106. [Google Scholar] [CrossRef]
- Topp, G.; Entezami, P.; Ambati, S.; Szewczyk, B.; Adamo, M.A. Cerebrospinal Fluid Leakage from Scrotum Secondary to Ventriculoperitoneal Shunt Migration. Asian J. Neurosurg. 2023, 18, 333–335. [Google Scholar] [CrossRef]
- Chanchlani, R.; Sharma, P.K.; Gunasekaran, V.; Kasundra, A. Scrotal Migration of Peritoneal End of Ventriculoperitoneal Shunt in an Infant—A Rare Entity. J. Neurosci. Rural Pract. 2023, 14, 365–367. [Google Scholar] [CrossRef]
- Ahmed, F.; Derwish, W.; Al-wageeh, S.; Ghabisha, S.; Al-shami, E.; Al-naggar, K.; Obaid, G. Migration of a Ventriculo-Peritoneal Shunt into the Scrotum. J. Pediatr. Surg. Case Rep. 2021, 73, 102010. [Google Scholar] [CrossRef]
- Hauser, T.; Auer, C.; Ludwiczek, J.; Senker, W.; Rauch, P.-R.; Kargl, S.; Gruber, A. Treatment Options for Scrotal Migration of Ventriculoperitoneal Shunts: Case Illustration and Systematic Review of 48 Cases. Oper. Neurosurg. 2021, 21, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Abdoli, A.; Ghorbanpour, M.; Fayyazi, A.; Saatian, M.R. Distal Catheter Migration into Scrotum as a Rare Complication of Ventriculoperitoneal Shunt in Pediatrics; A Case Series. Adv. J. Emerg. Med. 2019; in press. [Google Scholar] [CrossRef]
- Agarwal, T.; Pandey, S.; Niranjan, A.; Jain, V.; Mishra, S.; Agarwal, V. Unusual Complication of Ventriculoperitoneal Shunt Surgery. J. Pediatr. Neurosci. 2009, 4, 122–123. [Google Scholar] [CrossRef]
- Dharmajaya, R. Scrotal Migration of Ventriculoperitoneal Shunt: A Case Report at Haji Adam Malik Hospital, Medan. IOP Conf. Ser. Earth Environ. Sci. 2018, 125, 012223. [Google Scholar] [CrossRef]
- Ezzat, A.A.M.; Soliman, M.A.R.; Hasanain, A.A.; Thabit, M.A.; Elshitany, H.; Kandel, H.; Abdel-Bari, S.H.; Ghoul, A.M.F.; Abdullah, A.; Alsawy, M.F.M.; et al. Migration of the Distal Catheter of Ventriculoperitoneal Shunts in Pediatric Age Group: Case Series. World Neurosurg. 2018, 119, e131–e137. [Google Scholar] [CrossRef]
- Paterson, A.; Ferch, R. Infant with Recurrent Ventriculoperitoneal Shunt Migration to Right Scrotum. J. Clin. Neurosci. 2018, 51, 65–66. [Google Scholar] [CrossRef]
- Nawaz, A.; Chaudhry, M.B.H.; Mirza, W.A. Cerebrospinal Fluid Hydrocele Caused by Scrotal Migration of a Ventriculoperitoneal Shunt. BMJ Case Rep. 2018, 2018, bcr-2018-224698. [Google Scholar] [CrossRef]
- Bawa, M.; Garge, S.; Garg, R.; Narasimha Rao, K.L. Scrotal Migration of Tubing: An Unusual Complication after Ventriculo-Peritoneal Shunt. Asian J. Neurosurg. 2017, 12, 738–740. [Google Scholar] [CrossRef]
- Hung, S.-W.; Chen, K.-C.; Wu, C.-C.; Wang, T.-L.; Chor-Ming Lin, A. A 5-Month-Old Infant with Right Scrotum Swelling; a Case Report. Emergency 2017, 5, e48. [Google Scholar]
- Ricci, C.; Velimirovic, B.M.; Fitzgerald, T.N. Case Report of Migration of 2 Ventriculoperitoneal Shunt Catheters to the Scrotum: Use of an Inguinal Incision for Retrieval, Diagnostic Laparoscopy and Hernia Repair. Int. J. Surg. Case Rep. 2016, 29, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Shankar, B.; Narayanan, R.; Paruthikunnan, S.M.; Kulkarni, C.D. Scrotal Migration of Ventriculoperitoneal Shunt. BMJ Case Rep. 2014, 2014, bcr2014204404. [Google Scholar] [CrossRef]
- Erikci, V.; Ganiüsmen, O.; Hoşgör, M. Complications of Ventriculoperitoneal Shunt in Hydrocephalic Children: A Case Report and a Review of the Literature. Ann. Pediatr. Surg. 2014, 10, 50–53. [Google Scholar] [CrossRef]
- Panda, S.S.; Singh, A.; Bajpai, M.; Sharma, N. Shunt in Scrotum: Unusual Complication in Operated Cases of Hydrocephalus. BMJ Case Rep. 2013, 2013, bcr2013201854. [Google Scholar] [CrossRef]
- Shahizon, A.M.M.; Hanafiah, M.; Hing, E.Y.; Julian, M.R. Migration of a Fractured Ventriculoperitoneal Shunt into the Scrotum: A Rare Complication. BMJ Case Rep. 2013, 2013, bcr2013200609. [Google Scholar] [CrossRef]
- Ramareddy, R.; Alladi, A. Scrotal Migration of Ventriculoperitoneal Catheter and Hydrocele Resolving Spontaneously. Indian J. Neurosurg. 2012, 1, 172–174. [Google Scholar] [CrossRef]
- Gupta, M.; Digra, N.C.; Sharma, N.; Goyal, S.; Agrawal, A. Migration of the Peritoneal Catheter of a Ventriculoperitoneal Shunt into the Scrotum. S. Afr. J. Child Health 2012, 6, 93–94. [Google Scholar] [CrossRef]
- Mohammadi, A.; Hedayatiasl, A.; Ghasemi-Rad, M. Scrotal Migration of a Ventriculoperitoneal Shunt: A Case Report and Review of Literature. Med. Ultrason. 2012, 14, 158–160. [Google Scholar] [PubMed]
- Kita, D.; Hayashi, Y.; Kinoshita, M.; Ohama, K.; Hamada, J. Scrotal Migration of the Peritoneal Catheter of a Ventriculoperitoneal Shunt in a 5-Year-Old Male -Case Report-: Case Report. Neurol. Med. Chir. 2010, 50, 1122–1125. [Google Scholar] [CrossRef]
- Lakhoo, K.; Rahman, N. Patent Processus Vaginalis: A Window to the Abdomen. Afr. J. Paediatr. Surg. 2009, 6, 116. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.F.; Moquin, R.R.; Maurer, S.T. Expanding the Differential Diagnosis of the Acute Scrotum: Ventriculoperitoneal Shunt Herniation. Urology 2001, 58, 281. [Google Scholar] [CrossRef] [PubMed]
- Öktem, I.S.; Akdemir, H.; Koc, K.; Menkü, A.; Tucer, B.; Selcuklu, A.; Turan, C. Migration of Abdominal Catheter of Ventriculoperitoneal Shunt into the Scrotum. Acta Neurochir. 1998, 140, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Ammar, A.; Ibrahim, A.W.M.; Nasser, M.; Rashid, M. CSF Hydrocele? Unusual Complication of V-P Shunt. Neurosurg. Rev. 1991, 14, 141–143. [Google Scholar] [CrossRef]
- Calvário, J.S.; Paolioli Neto, E. Hydrocele Following Placement of a Ventriculoperitoneal Shunt Case Report. Arq. Neuropsiquiatr. 1990, 48, 113–115. [Google Scholar] [CrossRef]
- Kwok, C.K.; Yue, C.P.; Wen, H.L. Bilateral Scrotal Migration of Abdominal Catheters: A Rare Complication of Ventriculoperitoneal Shunt. Surg. Neurol. 1989, 31, 330–331. [Google Scholar] [CrossRef]
- Ram, Z.; Findler, G.; Guttman, I.; Cherniak, R.; Knoller, N.; Shacked, I. Ventriculoperitoneal Shunt Malfunction Due to Migration of the Abdominal Catheter into the Scrotum. J. Pediatr. Surg. 1987, 22, 1045–1046. [Google Scholar] [CrossRef]
- Fuwa, I.; Matsukado, Y.; Itoyama, Y.; Yokota, A. Migration of a Dissected Peritoneal Shunt Catheter into the Scrotum. Brain Dev. 1984, 6, 336–338. [Google Scholar] [CrossRef]
- Crofford, M.; Balsam, D. Scrotal Migration of Ventriculoperitoneal Shunts. Am. J. Roentgenol. 1983, 141, 369–371. [Google Scholar] [CrossRef]
- Bristow, D.L.; Buntain, W.L.; James, H.L. Ventriculoperitoneal (VP) Shunt Migration Causing an Acute Scrotum: A Case Report of Doppler Evaluation. J. Pediatr. Surg. 1978, 13, 538–539. [Google Scholar] [CrossRef]
- Levey, S.H.; Cooper, P.; Schiffman, D. Simulated Testicular Torsion in a Neonate Complication of Ventriculoperitoneal Shunt. Urology 1977, 9, 174–176. [Google Scholar] [CrossRef]
- Brainwood, M.; Beirne, G.; Fenech, M. Persistence of the Processus Vaginalis and Its Related Disorders. Australas. J. Ultrasound Med. 2020, 23, 22–29. [Google Scholar] [CrossRef]
- Hammer, G.; Hall, S.; Davis, P.J. Anesthesia for General Abdominal, Thoracic, Urologic, and Bariatric Surgery. In Smith’s Anesthesia for Infants and Children; Elsevier: New York, NY, USA, 2011; pp. 745–785. [Google Scholar] [CrossRef]
- Yeap, E.; Nataraja, R.M.; Pacilli, M. Inguinal Hernias in Children. Aust. J. Gen. Pract. 2020, 49, 38–43. [Google Scholar] [CrossRef]
- Kalra, A.; Wehrle, C.J.; Tuma, F. Anatomy, Abdomen and Pelvis, Peritoneum. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Wu, Y.; Chen, Y.; Ng, L.P.; Low, S.Y.Y. Spontaneous Regression of Migrated Ventriculoperitoneal Shunt Catheter from Scrotum to Peritoneum: A Case-Based Review. Childs Nerv. Syst. 2024, 40, 19–25. [Google Scholar] [CrossRef]
- Tzeng, S.-W.; Tsai, M.-C. Dose Hydrocele in Children Caused by Ventriculoperitoneal Shunt Migration Always Need Surgical Intervention? Watch and Wait—A Rare Cause of Hydrocele. Iran. J. Pediatr. 2023, 33, e122323. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| Period of the study | All available literature to date | / |
| Language | English | Languages that are not English |
| Study design | Case report, retrospective study (case–control studies or case series), meta-analysis, and systematic review | Conference abstracts, commentaries, personal communications, discussion, and editorials |
| Participants | <18 years | >18 years |
| Study topic | Ventriculoperitoneal shunt with scrotal migration | Ventriculoperitoneal shunt with non-scrotal migration |
| Author | Year of Publication | Country | Study Design | Sample Size |
|---|---|---|---|---|
| Muhajir et al. [19] | 2025 | Indonesia | Case series | 3 |
| Javeed et al. [10] | 2024 | Pakistan | Case report | 1 |
| Topp et al. [20] | 2023 | Albany | Case report | 1 |
| Chanchlani et al. [21] | 2023 | India | Case report | 1 |
| Taha et al. [13] | 2022 | Egypt | Case report | 1 |
| Alkhudari et al. [3] | 2022 | Saudi Arabia | Case report | 1 |
| Ahmed et al. [22] | 2021 | Yemen | Case report | 1 |
| Hauser et al. [23] | 2020 | Austria | Case report | 1 |
| Abdoli et al. [24] | 2019 | Iran | Case series | 4 |
| Agarwal et al. [25] | 2019 | India | Case report | 1 |
| Dharmajaya [26] | 2018 | Indonesia | Case report | 1 |
| Ezzat et al. [27] | 2018 | Germany | Case series | 3 |
| Paterson et al. [28] | 2018 | Australia | Case report | 1 |
| Nawaz et al. [29] | 2018 | Pakistan | Case report | 1 |
| Bawa et al. [30] | 2017 | India | Case series | 4 |
| Hung et al. [31] | 2017 | Taiwan | Case report | 1 |
| Ricci et al. [32] | 2016 | USA | Case report | 1 |
| Shankar et al. [33] | 2014 | India | Case report | 1 |
| Erikci et al. [34] | 2013 | Turkey | Case report | 1 |
| Panda et al. [35] | 2013 | India | Case report | 1 |
| Shahizon et al. [36] | 2013 | Malaysia | Case report | 1 |
| Ramareddy et al. [37] | 2012 | India | Case report | 1 |
| Gupta et al. [38] | 2012 | India | Case report | 1 |
| Mohammadi et al. [39] | 2012 | Iran | Case report | 1 |
| Kita et al. [40] | 2010 | Japan | Case report | 1 |
| Rahman et al. [41] | 2009 | UK | Case report | 1 |
| Ward et al. [42] | 2001 | Japan | Case report | 1 |
| Öktem et al. [43] | 1998 | Turkey | Case series | 4 |
| Ammar et al. [44] | 1991 | Saudi Arabia | Case report | 1 |
| Calvario et al. [45] | 1991 | Brazil | Case report | 1 |
| Kwok et al. [46] | 1989 | Hong Kong | Case report | 1 |
| Ram et al. [47] | 1987 | Israel | Case report | 1 |
| Fuwa et al. [48] | 1984 | Japan | Case report | 1 |
| Crofford et al. [49] | 1983 | USA | Case series | 4 |
| Bristow et al. [50] | 1978 | USA | Case report | 1 |
| Levey et al. [51] | 1977 | USA | Case report | 1 |
| Methodological Quality of Included Study According to JBI Critical Appraisal Checklist for Case Report | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Overall Score | |
| Muhajir et al. [19] | Yes | Yes | Yes | U | Yes | Yes | Yes | No | Yes | NA | 70% (Medium quality) |
| Abdoli et al. [24] | Yes | No | Yes | U | Yes | No | No | No | Yes | NA | 40% (Low quality) |
| Ezzat et al. [27] | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | NA | 70% (Medium quality) |
| Bawa et al. [30] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | 80% (High quality) |
| Öktem et al. [43] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | NA | 70% (Medium quality) |
| Crofford et al. [49] | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | NA | 70% (Medium quality) |
| Methodological Quality of the Included Study According to the JBI Critical Appraisal Checklist for Case Report | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Overall Score | |
| Javeed et al. [10] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 100% (High quality) |
| Topp et al. [20] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 100% (High quality) |
| Chanchlani et al. [21] | Yes | Yes | Yes | Yes | No | No | No | Yes | 62.5% (Medium quality) |
| Taha et al. [13] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 100% (High quality) |
| Alkhudari et al. [3] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 100% (High quality) |
| Ahmed et al. [22] | Yes | Yes | Yes | No | No | No | Yes | Yes | 62.5% (Medium quality) |
| Hauser et al. [23] | Yes | Yes | Yes | Yes | Yes | No | No | Yes | 75% (Medium quality) |
| Agarwal et al. [25] | Yes | No | No | Yes | Yes | No | Yes | No | 50% (Low quality) |
| Dharmajaya [26] | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | 87.5% (High quality) |
| Paterson et al. [28] | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | 87.5% (Medium quality) |
| Nawaz et al. [29] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 100% (High quality) |
| Hung et al. [31] | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | 87.5% (High quality) |
| Ricci et al. [32] | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | 87.5% (High quality) |
| Shankar et al. [33] | Yes | Yes | Yes | Yes | Yes | No | No | Yes | 75% (Medium quality) |
| Erikci et al. [34] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 100% (High quality) |
| Panda et al. [35] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 100% (High quality) |
| Shahizon et al. [36] | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | 87.5% (High quality) |
| Ramareddy et al. [37] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 100% (High quality) |
| Gupta et al. [38] | Yes | Yes | Yes | Yes | Yes | No | No | Yes | 75% (Medium quality) |
| Mohammadi et al. [39] | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | 87.5% (High quality) |
| Kita et al. [40] | Yes | Yes | Yes | Yes | Yes | No | No | No | 62.5% (Medium quality) |
| Rahman et al. [41] | Yes | Yes | Yes | Yes | Yes | No | No | Yes | 75% (Medium quality) |
| Ward et al. [42] | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | 87.5% (High quality) |
| Ammar et al. [44] | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | 87.5% (High quality) |
| Calvario et al. [45] | Yes | Yes | Yes | Yes | No | No | No | Yes | 62.5% (Medium quality) |
| Kwok et al. [46] | Yes | Yes | Yes | Yes | Yes | No | No | Yes | 75% (Medium quality) |
| Ram et al. [47] | Yes | Yes | Yes | Yes | Yes | No | No | Yes | 75% (Medium quality) |
| Fuwa et al. [48] | Yes | Yes | Yes | Yes | Yes | No | NA | Yes | 75% (Medium quality) |
| Bristow et al. [50] | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | 87.5% (High quality) |
| Levey et al. [51] | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | 87.5% (High quality) |
| Age (Months) | Indications for VPS | Side | Time to Clinical Presentation After Shunting | Symptoms | Imaging and Laboratory Findings | Inguinal Hernia | |
|---|---|---|---|---|---|---|---|
| Muhajir et al. [19] | 5, 10, 6 | Case 1: Congenital hydrocephalus Case 2: Hydrocephalus secondary to aqueductal stenosis Case 3. Multiloculated hydrocephalus Dandy–Walker variant | L, R, R | 4, 11, and 4 months | Case 1: Vomiting, palpable distal tip catheter in the scrotum Case 2: Seizures, testicular swelling Case 3: Vomiting, enlarged abdomen with a lump, and redness on the scrotum | Cases 1.–3.: Abdominal X-ray | No |
| Javeed et al. [10] | 7 | Hydrocephalus due to myelomeningocele | R | 13 days | Five-day history of scrotal swelling, vomiting, and irritability. | Abdominal X-ray | No |
| Topp et al. [20] | 16 | Hydrocephalus due to myelomeningocele and Chiari II malformation | R | 15 months | Two-day history of scrotal swelling, clear fluid draining from the scrotal sac, emesis, and progressive lethargy. | US, CT Leukocytosis and anion gap acidosis. | R |
| Chanchlani et al. [21] | 2 | Communicating hydrocephalus | R | 5 days | Scrotal swelling | Abdomen X-ray | R |
| Taha et al. [13] | 3 | Hydrocephalus due to myelomeningocele | R | NA | Seven-day history of scrotal swelling. | US, abdominal X-ray; Positive translumination | No |
| Alkhudari et al. [3] | 6 | Hydrocephalus secondary to intraventricular hemorrhage (gradus III) | R | NA | Right inguinoscrotal swelling, and a 15-day history of vomiting after each feed, constipation for 6 days | Abdominal X-ray | No |
| Ahmed et al. [22] | 8 | Hydrocephalus | L | 7 months | Seven-day history of left scrotal swelling and fever. | Abdomen X-ray, Positive transillumination | L |
| Hauser et al. [23] | 23 | Hydrocephalus due to closed myelomeningocele accompanied by septum pellucidum agenesis, corpus callosum hypoplasia, and Chiari type II malformation | R | NA | 2-day history of painless scrotal swelling | Physical examination (palpable tube inside the scrotum). | R |
| Abdoli et al. [24] | 24, 12, 18, 12 | Case 1: Hydrocephalus Case 2: Hydrocephalus Case 3: Congenital hydrocephalus Case 4: Hydrocephalus | R, R, R, L | 10 days, 10 months, 5 months, and 8 months | Case 1. Right scrotal swelling Case 2: Inguinal herniation Case 3. Right inguinal region swelling Case 4. Inguinoscrotal swelling | Case 1. Abdominal X-ray Case 2: Intraoperative findings Case 3–4. Surgical exploration | R, R, R, L |
| Agarwal et al. [25] | 14 | NA | R | 7 months | Right scrotal swelling | Abdominal X-ray | No |
| Dharmajaya [26] | 12 | Communicating hydrocephalus | R | 7 months | Right scrotal swelling (slowly grown over 3 days) | Abdominal X-ray | No |
| Ezzat et al. [27] | 2, 2, 1 | Case 1.–3.: Hydrocephalus | NA | 1 month, 1 month, and 1 month | Case 1. Scrotal swelling Case 2: Scrotal swelling, bulging anterior fontanel, and vomiting. Case 3. Scrotal swelling and bulging anterior fontanel. | NA | No |
| Paterson and Ferch [28] | 11 | Hydrocephalus and macrocephaly | R | 5 weeks | Right scrotal swelling | Abdominal X-ray | No |
| Nawaz et al. [29] | 6 | Hydrocephalus secondary to neonatal bacterial meningitis and ventriculitis. | R | 4 months | Right scrotal swelling | The US scrotum revealed right-sided hydrocele and VPS end. | No |
| Bawa et al. [30] | 7, 51, 15, 51 | Case 1.–4.: Congenital hydrocephalus | R, R, R, L | 4, 3, 5, and 4 months | Cases 1.–4.: scrotal swelling | Case 1.–4.: Abdominal X-ray | R, L |
| Hung et al. [31] | 5 | Posthemorrhagic hydrocephalus | R | 2 months | Right scrotal swelling | Abdominal X-ray | No |
| Ricci et al. [32] | 120 | Hydrocephalus | L | 72 months | Seven-day history of left scrotal swelling, vomiting, nausea, headache, and fatigue | Abdominal X-ray | L |
| Shankar et al. [33] | 12 | Hydrocephalus due to type II Chiari malformation | R | 11 months | Right scrotal swelling | Abdominal X-ray | Bilateral |
| Erikci et al. [34] | 48 | Hydrocephalus | L | 46 months | Left scrotal swelling | Abdominal X-ray | No |
| Panda et al. [35] | 60 | Hydrocephalus due to congenital aqueducatal stenosis | L | 42 months | Left inguinoscrotal swelling for the last 9 days | Abdominal X-ray | L |
| Shahizon et al. [36] | 168 | Congenital hydrocephalus | L | 19 months | Left scrotal swelling and fever | Abdominal X-ray and scrotal US | L |
| Ramareddy et al. [37] | 20 | Congenital hydrocephalus | L | NA | Scrotal swelling | Abdominal X-ray | No |
| Gupta et al. [38] | 24 | Congenital hydrocephalus | R | 18 months | Right inguinoscrotal swelling for the last 15 days | Abdominal X-ray | R |
| Mohammadi et al. [39] | 7 | Congenital hydrocephalus | R | 1 month | Right scrotal swelling | Abdominal X-ray and scrotal US | No |
| Kita et al. [40] | 60 | Obstructive (brain tumor) hydrocephalus | L | 4 months | Left scrotal swelling | Abdominal X-ray | No |
| Rahman et al. [41] | 48 | Hydrocephalus secondary to a pilocytic astrocytoma | R | 1 month | Right scrotal swelling | Abdominal X-ray | R |
| Ward et al. [42] | 18 | Meningitis resulting in static encephalopathy and hydrocephalus | R | 7 months | Right scrotal swelling | Abdominal X-ray and positive transillumination test | No |
| Öktem et al. [43] | 10, 2.5, 9, 2.5 | Case 1.–4.: Hydrocephalus | R, R, R, L | 4 months, 2.5 months, 4 months, and 1 day | Case 1.–3. Right scrotal swelling Case 4. Left erythematous scrotal swelling | Case 1.–4.: Abdominal x-ray | No |
| Ammar et al. [44] | 6 | Hydrocephalus | L | 2 months | Scrotal swelling | Abdominal X-ray and positive transillumination test | No |
| Calvario et al. [45] | 24 | Hydrocephalus | R | NA | Right scrotal swelling | Abdominal X-ray | No |
| Kwok et al. [46] | 6 | Hydrocephalus | L | 5 months | Left scrotal swelling | Abdominal X-ray | No |
| Ram et al. [47] | 36 | Hydrocephalus secondary to meningitis | R | 30 months | Right scrotal swelling | Abdominal X-ray | No |
| Fuwa et al. [48] | 12 | Congenital hydrocephalus and holoprosencephaly | L | 11 months | Left scrotal swelling | Abdominal X-ray | No |
| Crofford et al. [49] | 9, 3, 5, 48 | Case 1.: Hydrocehphalus secondary to subarachnoid hemorrhage Case 2: Hydrocephalus Case 3: Hydrocephalus Case 4.: Hydrocephalus associated with posterior fossa ependymoma | R, R, R, L | 8, 2, 1, and 2 months | Case 1. Right scrotal swelling Case 2: Right scrotal swelling Case 3. Right scrotal swelling Case 4. Left scrotal swelling, vomiting, fever, and headache | Case 1.–4.: Abdominal X-ray | No, R, no, no |
| Bristow et al. [50] | 10 | Hydrocephalus secondary to aqueductal stenosis | R | 1 day | Right scrotal swelling, fever, and pain | Abdominal X-ray, positive transillumination | R |
| Levey et al. [51] | 1 | Hydrocephalus secondary to spina bifida and meningomyelocele | R | 6 days | Right inguinoscrotal swelling | Abdominal X-ray | No |
| Author | Treatment | Intraoperative Complication | Postoperative Complication | Length of Hospital Stay (Days) | Mortality | Follow-Up (Months) |
|---|---|---|---|---|---|---|
| Muhajir et al. [19] | Case 1. Shortening the VPS distal tip catheter and repositioning of VPS into the peritoneal cavity. Case 2: Distal exteriorization of the VPS catheter tip and repositioning of VPS into the peritoneal cavity. Case 3: Shortening the distal tip VPS catheter and repositioning of the VPS into the peritoneal cavity. High ligation of the PV. | None | None | 3, 3, 3 | 0 | NA |
| Javeed et al. [10] | Repositioning of VPS into the peritoneal cavity. | None | None | 2 | 0 | 3 |
| Topp et al. [20] | Shunt externalization (re-internalized 11 days later), hernia repair, scrotal dehiscence repair, and PV closure. | None | None | 3 | 0 | 12 |
| Chanchlani et al. [21] | Repositioning of VPS into the peritoneal cavity and hernia repair. | NA | NA | NA | 0 | NA |
| Taha et al. [13] | Repositioning of VPS into the peritoneal cavity and PV closure. | None | None | NA | 0 | 6 |
| Alkhudari et al. [3] | Spontaneous resolution of the VPS without intervention at the day of admission. | Not applicable | Not applicable | 1 | 0 | 1 |
| Ahmed et al. [22] | Repositioning of VPS into the peritoneal cavity. | None | None | 2 | 0 | 2 |
| Hauser et al. [23] | Manual (non-operative) reposition of shunt catheter and hernia repair. | None | None | 1 | 0 | NA |
| Abdoli et al. [24] | All cases: Repositioning of VPS into the peritoneal cavity and hernia repair. | None | None | 2, 2, 3, NA | 0 | NA |
| Agarwal et al. [25] | Repositioning of VPS into the peritoneal cavity. | None | NA | NA | 0 | NA |
| Dharmajaya [26] | Repositioning of VPS into the peritoneal cavity and PV closure. | None | None | 7 | 0 | NA |
| Ezzat et al. [27] | Repositioning of VPS into the peritoneal cavity (1 laparotomy, and 2 laparoscopy). | None | None | NA | 0 | NA |
| Paterson et al. [28] | Repositioning of VPS into the peritoneal cavity. One week later, due to a recurrence of catheter migration in the right scrotum, a revision operation was made in which the distal end of the catheter was shortened. | None | None | NA | 0 | NA |
| Nawaz et al. [29] | Bilateral herniotomy, left-sided orchidopexy, and repositioning of the VPS tip into the peritoneal cavity. | None | None | NA | 0 | 4 |
| Bawa et al. [30] | Case 1.–4.: Repositioning of VPS into the peritoneal cavity and hernia repair. | None | NA | NA | 0 | NA |
| Hung et al. [31] | Repositioning of VPS into the peritoneal cavity and PV closure. | None | None | 4 | 0 | NA |
| Ricci et al. [32] | Repositioning of VPS into the peritoneal cavity and hernia repair. | None | None | NA | 0 | NA |
| Shankar et al. [33] | Repositioning of VPS into the peritoneal cavity and hernia repair. | None | None | NA | 0 | NA |
| Erikci et al. [34] | Repositioning of VPS into the peritoneal cavity. | None | None | NA | 0 | 120 |
| Panda et al. [35] | Repositioning of VPS into the peritoneal cavity and hernia repair. | None | None | 2 | 0 | 24 |
| Shahizon et al. [36] | Repositioning of VPS into the peritoneal cavity and hernia repair. | None | None | NA | 0 | NA |
| Ramareddy et al. [37] | Spontaneous resolution of the VPS without intervention. | Not applicable | Not applicable | NA | 0 | 36 |
| Gupta et al. [38] | Repositioning of VPS into the peritoneal cavity and hernia repair. | None | None | NA | 0 | NA |
| Mohammadi et al. [39] | Repositioning of the shunt into the peritoneal cavity. | None | None | NA | 0 | 6 |
| Kita et al. [40] | Manual (non-operative) reposition of shunt catheter. Prophylactic obliteration of the PV. | NA | NA | NA | 0 | NA |
| Rahman et al. [41] | Repositioning of VPS into the peritoneal cavity and hernia repair. | None | None | NA | 0 | NA |
| Ward et al. [42] | Repositioning of VPS into the peritoneal cavity. The PV was doubly clamped, transected, and highly ligated. | None | None | NA | 0 | NA |
| Öktem et al. [43] | Case 1.–4.: Repositioning of VPS into the peritoneal cavity, and PV closure. | None | None | NA | 0 | NA |
| Ammar et al. [44] | VPS removal and antibiotic therapy. Insertion of VPS a few weeks later. | None | None | NA | 0 | 12 |
| Calvario et al. [45] | Repositioning of the shunt into the peritoneal cavity. | None | None | NA | 0 | NA |
| Kwok et al. [46] | Repositioning of VPS into the peritoneal cavity and PV closure. | None | None | NA | 0 | NA |
| Ram et al. [47] | Repositioning of the shunt into the peritoneal cavity. | None | None | NA | 0 | NA |
| Fuwa et al. [48] | Repositioning of the new shunt into the peritoneal cavity. | None | None | 10 | 0 | NA |
| Crofford et al. [49] | Case 1. Repositioning of the shunt into the peritoneal cavity Case 2: Repositioning of VPS into the peritoneal cavity and hernia repair. Case 3: Repositioning of the shunt into the peritoneal cavity Case 4: Spontaneous resolution of the VPS without intervention | None | None | NA | NA | NA |
| Bristow et al. [50] | Shortening the distal tip catheter and repositioning of shunt into the peritoneal cavity. (Hernia repair 3 months later). | None | None | NA | 0 | 3 |
| Levey et al. [51] | Repositioning of the shunt into the peritoneal cavity and PV closure. | None | None | NA | 0 | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pogorelić, Z.; Ninčević, S.; Babić, V.; Jukić, M.; Vidović, S. Scrotal Migration of the Ventriculoperitoneal Shunt in a 1-Year-Old Pediatric Patient: A Case Report and Systematic Literature Review. J. Clin. Med. 2025, 14, 5183. https://doi.org/10.3390/jcm14155183
Pogorelić Z, Ninčević S, Babić V, Jukić M, Vidović S. Scrotal Migration of the Ventriculoperitoneal Shunt in a 1-Year-Old Pediatric Patient: A Case Report and Systematic Literature Review. Journal of Clinical Medicine. 2025; 14(15):5183. https://doi.org/10.3390/jcm14155183
Chicago/Turabian StylePogorelić, Zenon, Stipe Ninčević, Vlade Babić, Miro Jukić, and Stipe Vidović. 2025. "Scrotal Migration of the Ventriculoperitoneal Shunt in a 1-Year-Old Pediatric Patient: A Case Report and Systematic Literature Review" Journal of Clinical Medicine 14, no. 15: 5183. https://doi.org/10.3390/jcm14155183
APA StylePogorelić, Z., Ninčević, S., Babić, V., Jukić, M., & Vidović, S. (2025). Scrotal Migration of the Ventriculoperitoneal Shunt in a 1-Year-Old Pediatric Patient: A Case Report and Systematic Literature Review. Journal of Clinical Medicine, 14(15), 5183. https://doi.org/10.3390/jcm14155183










