Pellucid Marginal Degeneration: A Comprehensive Review of Pathophysiology, Diagnosis, and Management Strategies
Abstract
1. Introduction
2. Pathophysiology and Epidemiology
3. Clinical Signs
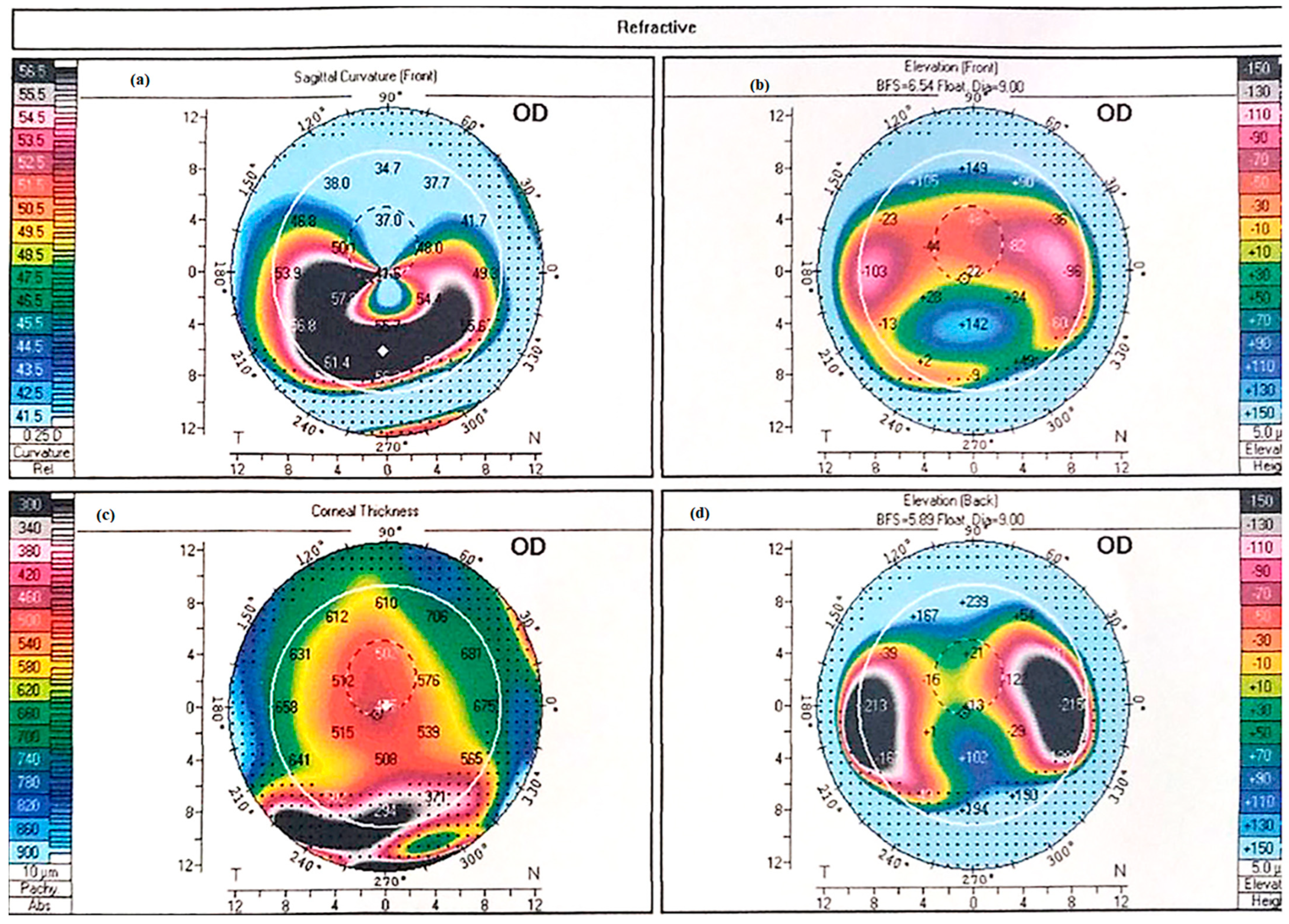
4. Diagnosis
5. Differential Diagnosis
6. Management
7. Conservative Management
8. Surgical Management
- (1)
- Alterations of corneal biomechanics
- (2)
- Toric intraocular lens implantation
- (3)
- Full-thickness surgery
- (4)
- Partial-thickness surgery
8.1. Alterations of Corneal Biomechanics
8.1.1. Collagen Cross Linking
8.1.2. Intrastromal Corneal Rings
8.2. Toric Intraocular Lens Implantation
8.3. Full-Thickness Surgery
8.3.1. Penetrating Keratoplasty
Penetrating Keratoplasty (PK) in PMD
8.3.2. PK Surgical Alternatives
Full-Thickness Crescentic Wedge Resection
8.4. Partial-Thickness Procedures
8.4.1. Crescentic Lamellar Keratoplasty (CLK)
8.4.2. Deep Anterior Lamellar Keratoplasty (DALK)
8.4.3. Historical Techniques
9. Conclusions
Pellucid Marginal Degeneration: Diagnostic and Therapeutic Challenges
Funding
Conflicts of Interest
References
- Sridhar, M.; Mahesh, S.; Bansal, A.K.; Nutheti, R.; Rao, G. Pellucid marginal corneal degeneration. Ophthalmology 2004, 111, 1102–1107. [Google Scholar] [CrossRef]
- Kompella, V.B.; Aasuri, M.K.; Rao, G.N. Management of pellucid marginal corneal degeneration with rigid gas permeable contact lenses. CLAO J. 2002, 28, 140–145. [Google Scholar] [PubMed]
- Sahu, J.; Raizada, K. Pellucid marginal corneal degeneration. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Cameron, J.A.; Mahmood, M.A. Superior corneal thinning with pellucid marginal degeneration. Am. J. Ophthalmol. 1990, 109, 486–487. [Google Scholar] [CrossRef] [PubMed]
- Karabatsas, C.H.; Cook, S.D. Topographic analysis in pellucid marginal corneal degeneration and keratoglobus. Eye 1996, 10, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.A.P.; Rodrigues, P.F.; Lamazales, L.L. Keratoconus epidemiology: A review. Saudi J. Ophthalmol. 2022, 36, 3–6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Das, A.V.; Pillutla, L.N.; Chaurasia, S. Clinical profile and demographic distribution of pellucid marginal corneal degeneration in India: A study of 559 patients. Indian J. Ophthalmol. 2021, 69, 3488–3493. [Google Scholar] [CrossRef] [PubMed]
- Krachmer, J.H.; Feder, R.S.; Belin, M.W. Keratoconus and related noninflammatory corneal thinning disorders. Surv. Ophthalmol. 1984, 28, 232–293. [Google Scholar] [CrossRef] [PubMed]
- Robin, J.B.; Schanzlin, D.J.; Verity, S.M.; Barron, B.A.; Arffa, R.C.; Suarez, E.; Kaufman, H.E. Peripheral corneal disorders. Surv. Ophthalmol. 1986, 31, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Jinabhai, A.; Radhakrishnan, H.; O’Donnell, C. Pellucid corneal marginal degeneration: A review. Contact Lens Anterior Eye 2011, 34, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.K.; Fogla, R.; Padmanabhan, P.; Prema, M.; Sitalakshmi, G. Corneal topography in atypical pellucid marginal degeneration. Cornea 1999, 18, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Wagenhorst, B.B. Unilateral pellucid marginal degeneration in an elderly patient. Br. J. Ophthalmol. 1996, 80, 927–928. [Google Scholar] [CrossRef] [PubMed]
- Taglia, D.P.; Sugar, J. Superior pellucid marginal corneal degeneration with hydrops. Arch. Ophthalmol. 1997, 115, 274–275. [Google Scholar] [CrossRef] [PubMed]
- Varley, G.A.; Macsai, M.S.; Krachmer, J.H. The results of penetrating keratoplasty for pellucid marginal corneal degeneration. Am. J. Ophthalmol. 1990, 110, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Kayazawa, F.; Nishimura, K.; Kodama, Y.; Tsuji, T.; Itoi, M. Keratoconus with pellucid marginal corneal degeneration. Arch. Ophthalmol. 1984, 102, 895–896. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Paulus, Y.M.; Cockerham, G.C.; Kenyon, K.R. Keratoconus and Other Non-inflammatory Thinning Conditions. In Duane’s Foundations of Clinical Ophthalmology; Tasman, W., Jaeger, E.A., Eds.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2008; Volume 4, Chapter 16C. [Google Scholar]
- McKay, T.B.; Hjortdal, J.; Sejersen, H.; Asara, J.M.; Wu, J.; Karamichos, D. Endocrine and metabolic pathways linked to keratoconus: Implications for the role of hormones in the stromal microenvironment. Sci. Rep. 2016, 6, 25534. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yuan, Y.; Sun, T.; Zhang, Y.; Chen, Y. Associations between keratoconus and the level of sex hormones: A cross-sectional study. Front. Med. 2022, 9, 828233. [Google Scholar] [CrossRef] [PubMed]
- Krachmer, J.H. Pellucid marginal corneal degeneration. Arch. Ophthalmol. 1978, 96, 1217–1221. [Google Scholar] [CrossRef] [PubMed]
- Ueji, N.; Kato, K.; Yonekawa, Y.; Takeuchi, M.; Takashima, Y.; Hirano, K.; Kondo, M. Case of unilateral pellucid marginal corneal degeneration progressing to corneal perforation with keratoconus in contralateral eye. Am. J. Ophthalmol Case Rep. 2022, 25, 101293. [Google Scholar] [CrossRef] [PubMed]
- Mounir, A.; Abdellah, M.M.; Awny, I.; Aldghaimy, A.H.; Mostafa, E.M. Demographic, clinical and tomographic characteristics of pellucid marginal degeneration patients in South Egyptian population Keratoconus with pellucid marginal corneal degeneration. Int. Ophthalmol. 2022, 42, 3237–3242. [Google Scholar] [CrossRef] [PubMed]
- Iselin, K.C.; Mrochen, M. Evaluation of ectatic corneal disorders using swept-source anterior segment OCT: Current role and limitations. Clin. Ophthalmol. 2021, 15, 1499–1509. [Google Scholar]
- Moshirfar, M.; Edmonds, N.J.; Behunin, L.N.; Christiansen, M.S. Current options in the management of Pellucid Marginal Degeneration. J. Refract. Surg. 2014, 30, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.W.; Jurkunas, U.V.; Harissi-Dagher, M.; Poothullil, A.M.; Tobaigy, F.M.; Azar, D.T. Ectatic disorders associated with a claw-shaped pattern on corneal topography. Am. J. Ophthalmol. 2007, 144, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Murri, M.S.; Birdsong, C.O.; Ronquillo, Y.; Morshifar, M. Terrien marginal degeneration. Surv. Ophthalmol. 2019, 64, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.A.; Ulate, R.; Goldich, Y.; Rootman, S.D.; Chan, C.C. Terrien marginal degeneration: Clinical characteristics and outcomes. Am. J. Ophthalmol. 2015, 160, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.B.; Jones, D.B.; Wilhelmus, K.R. Acute hydrops in pellucid marginal corneal degeneration. Am. J. Ophthalmol. 1989, 107, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Tzelikis, P.; Cohen, E.J.; Rapuano, C.; Hammersmith, K.; Laibson, P.R. Management of pellucid marginal corneal degeneration. Cornea 2005, 24, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Jinabhai, A.; Radhakrishnan, H.; Tromans, C.; O’Donnell, C. Visual performance and optical quality with soft lenses in keratoconus patients. Ophthalmic Physiol. Opt. 2012, 32, 100–116. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, C.E.; Shah, A.; Weissman, B.A. Bitoric gas-permeable contact lens application in pellucid marginal corneal degeneration. Eye Contact Lens 2005, 31, 241–243. [Google Scholar] [CrossRef] [PubMed]
- Kastl, P.R.; Kirby, R.G. Bitoric rigid gas permeable lens fitting in highly astigmatic patients. Eye Contact Lens 1987, 13, 215–216. [Google Scholar]
- Visser, E.S.; Visser, R.; van Lier, H.J.; Otten, H.M. Modern scleral lenses part I: Clinical features. Eye Contact Lens 2007, 33, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.T.; Pullum, K.W.; Buckley, R.J. Medical applications of scleral contact lenses: 2. Gas-permeable scleral contact lenses. Cornea 1995, 14, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Levit, A.; Benwell, M.; Evans, B.J.W. Randomised controlled trial of corneal vs. scleral rigid gas permeable contact lenses for keratoconus and other ectatic corneal disorders. Contact Lens Anterior Eye 2020, 43, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, H.; Shaygan, N.; Asgari, S.; Rezvan, F.; Asgari, S. ClearKone-Synergeyes or rigid gas-permeable contact lens in keratoconic patients. Eye Contact Lens 2014, 40, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Akcay, B.I.S.; Kockar, A.; Limon, U.; Kardes, E.; Dursun, A.D. Comparison of Clinical and Topographic Outcomes of Hybrid and Scleral Lenses in Advanced Keratoconus. Beyoglu Eye J. 2021, 7, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Snibson, G.R. Collagen cross-linking: A new treatment paradigm in corneal disease—A review. Clin. Exp. Ophthalmol. 2010, 38, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Kymionis, G.D.; Karavitaki, A.E.; Kounis, G.A.; Portaliou, D.M.; Yoo, S.H.; Pallikaris, I.G. Management of pellucid marginal corneal degeneration with simultaneous customized photorefractive keratectomy and collagen crosslinking. J. Cataract Refract. Surg. 2009, 35, 1298–1301. [Google Scholar] [CrossRef] [PubMed]
- Irajpour, M.; Noorshargh, P.; Peyman, A. Corneal Cross-Linking in Pellucid Marginal Degeneration: Evaluation after Five Years. J. Curr. Ophthalmol. 2022, 34, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Mamoosa, B.; Razmjoo, H.; Peyman, A.; Ashtari, A.; Ghafouri, I.; Moghaddam, A.G. Short-term result of collagen crosslinking in pellucid marginal degeneration. Adv. Biomed. Res. 2016, 5, 194. [Google Scholar] [CrossRef] [PubMed]
- Kymionis, G.D.; Grentzelos, M.A.; Portaliou, D.M.; Karavitaki, A.E.; Krasia, M.S.; Dranidis, G.K.; Kozobolis, V.P. Photorefractive keratectomy followed by same-day corneal collagen crosslinking after intrastromal corneal ring segment implantation for pellucid marginal degeneration. J. Cataract Refract. Surg. 2010, 36, 1783–1785. [Google Scholar] [CrossRef] [PubMed]
- Kymionis, G.D.; Kontadakis, G.A.; Kounis, G.A.; Portaliou, D.M.; Karavitaki, A.E.; Pallikaris, I.G. Corneal collagen cross-linking with riboflavin and ultraviolet-A irradiation in patients with thin corneas. J. Cataract Refract. Surg. 2010, 36, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Cagil, N.; Sarac, O.; Kocaturk, T.; Can, I. Combined transepithelial phototherapeutic keratectomy and corneal collagen cross-linking for pellucid marginal degeneration. J. Cataract Refract. Surg. 2015, 41, 1215–1222. [Google Scholar]
- Hashemian, S.J.; Abdolalizadeh, P.; Ghiasian, L.; Aghaei, H.; Hadavandkhani, A.; Semnani, F.N.; Jafari, M.E.; Hashemian, S.M.; Hashemian, M.S. Outcomes of a Single-Segment Intrastromal Corneal Ring in Early Keratoconus and Early Pellucid Marginal Degeneration. Int. Ophthalmol. 2022, 42, 2987–2996. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Kumar, D.A.; Agarwal, A.; Rajaraman, R.; Saijimol, A.I. Corneal allogenic intrastromal ring segments (CAIRS) combined with corneal crosslinking for keratoconus: Initial clinical results. J. Refract. Surg. 2018, 34, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Kubaloglu, A.; Sari, E.S.; Cinar, Y.; Koytak, A.; Kurnaz, E.; Piñero, D.P.; Ozerturk, Y. A single 210-degree arc length intrastromal corneal ring implantation for the management of pellucid marginal corneal degeneration. Am. J. Ophthalmol. 2010, 150, 185–192.e1. [Google Scholar] [CrossRef] [PubMed]
- Mularoni, A.; Torreggiani, A.; di Biase, A.; Laffi, G.L.; Tassinari, G. Conservative treatment of early and moderate pellucid marginal degeneration: A new refractive approach with intracorneal rings. Ophthalmology 2005, 112, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Kugler, L.J.; Hill, S.; Sztipanovits, D.; Boerman, H.; Swartz, T.S.; Wang, M.X. Corneal melt of incisions overlying corneal ring segments: Case series and literature review. Cornea 2011, 30, 968–971. [Google Scholar] [CrossRef] [PubMed]
- Balestrazzi, A.; Baiocchi, S.; Cartocci, G.; Tosi, G.M.; Martone, G.; Michieletto, P. Mini-incision cataract surgery and toric lens implantation for the reduction of high myopic astigmatism in patients with pellucid marginal degeneration. Eye 2015, 29, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Matalia, H.; Nandini, C.; Matalia, J. Long-term outcome of custom toric intraocular lens for treating high astigmatism in case of cataract associated with pellucid marginal corneal degeneration. Indian J. Ophthalmol. 2020, 68, 3082–3084. [Google Scholar] [CrossRef] [PubMed]
- Bahar, I.; Bialer, O. Cataract extraction and toric intraocular lens implantation for the management of pellucid marginal degeneration and cataract. Int. J. Keratoconus Ectatic Corneal Dis. 2012, 1, 66–67. [Google Scholar] [CrossRef]
- Han, D.C.Y.; Lim, L. Implantation of toric intraocular lens in pellucid marginal degeneration: A case report on ocular aberrometry outcome. J. Clin. Exp. Ophthalmol. 2012, 3, 204. [Google Scholar]
- Luck, J. Customized ultra-high-power toric intraocular lens implantation for pellucid marginal degeneration and cataract. J. Cataract Refract. Surg. 2010, 36, 1235–1238. [Google Scholar] [CrossRef] [PubMed]
- Camoriano, G.D.; Aman, S.; Gimbel, H.V. Toric collagen copolymer phakic intraocular lens to correct myopic astigmatism in eyes with pellucid marginal degeneration. J. Cataract Refract. Surg. 2012, 38, 256–262. [Google Scholar] [CrossRef] [PubMed]
- de Vries, E.; de Vries, D.; de Vries, L. Use of Verisyse/Artisan phakic intraocular lens for the correction of high myopia in a patient with early-stage pellucid marginal degeneration. J. Cataract Refract. Surg. 2008, 34, 1700–1702. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; He, W.; Zhang, K.; Lu, Y.; Zhu, Y. Toric intraocular lens implantation in eyes with pellucid marginal degeneration. BMC Ophthalmol. 2017, 17, 22. [Google Scholar]
- Cherry, P.M.; Diggory, P.; Ficker, L.A. Corneal graft size and endothelial cell loss. Br. J. Ophthalmol. 1979, 63, 815–820. [Google Scholar]
- Tuberville, A.W.; Wood, T.O.; McLaughlin, B.J. The effects of eccentric donor recipient grafts on the survival of corneal allografts. Am. J. Ophthalmol. 1983, 95, 177–182. [Google Scholar]
- Tzelikis, P.F.; Cohen, E.J.; Rapuano, C.J. Long-term visual outcome of penetrating keratoplasty for pellucid marginal degeneration. Ophthalmology 1999, 106, 776–781. [Google Scholar]
- Speaker, M.G.; Guerriero, P.N.; Met, J.; Freilich, D.B. A study of penetrating keratoplasty using frozen and fresh donor tissue. Am. J. Ophthalmol. 1983, 96, 729–735. [Google Scholar]
- Varley, G.A.; Meisler, D.M.; Parks, M.M. Penetrating keratoplasty in pellucid marginal corneal degeneration. Ophthalmology 1995, 102, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Durand, M.; Hersh, P.S. Corneal wedge resection for pellucid marginal corneal degeneration. Ophthalmic Surg. Lasers Imaging Retin. 1991, 22, 442–447. [Google Scholar]
- MacLean, H.; Khan, S.; Muhtaseb, M. The results of femtosecond laser-assisted wedge resection for the treatment of pellucid marginal degeneration. J. Refract. Surg. 2012, 28, 884–888. [Google Scholar]
- Busin, M.; Scorcia, V.; Zambianchi, L.; Ponzin, D. Combined use of crescentic resection and relaxing incisions for the treatment of pellucid marginal corneal degeneration. Am. J. Ophthalmol. 2008, 145, 675–681. [Google Scholar]
- Genç, S.; Çakir, H.; Güler, E.; Çalli, Ü. Refractive and corneal aberrometric changes after crescentic lamellar wedge resection in pellucid marginal degeneration. Eye Contact Lens 2018, 44 (Suppl. S2), S76–S80. [Google Scholar] [CrossRef] [PubMed]
- Michael, T.; Ioannis, G.; Ferdinardo, M.; Ioannis, A. Endothelium sparing—Air assisted wedge resection for the treatment of pellucid marginal degeneration. Indian J. Ophthalmol. 2024, 72 (Suppl. S2), S314–S318. [Google Scholar] [CrossRef] [PubMed]
- Tsatsos, M.; Giachos, I.; Martini, F. Femto second laser assisted wedge resection for the treatment of PMD. Indian J. Ophthalmol. 2024, 72 (Suppl. S5), S923–S924. [Google Scholar] [CrossRef] [PubMed]
- Kymionis, G.; Voulgari, N.; Samutelela, E.; Kontadakis, G.; Tabibian, D. Combined corneal wedge resection and corneal cross-linking for pellucid marginal degeneration: A first report. Ther. Clin. Risk Manag. 2019, 15, 1319–1324. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schanzlin, D.J.; Sarno, E.M.; Robin, J.B. Crescentic lamellar keratoplasty for pellucid marginal degeneration. Am. J. Ophthalmol. 1983, 96, 253–254. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer, J.I. Crescentic lamellar keratoplasty for pellucid marginal degeneration. Am. J. Ophthalmol. 1984, 97, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Al-Torbak, A.A.; Al-Motowa, S.; Al-Assiri, A.; Al-Kharashi, S.; Al-Shahwan, S.; Al-Mezaine, H.; Teichmann, K. Deep anterior lamellar keratoplasty for management of keratoconus in Saudi Arabia. Br. J. Ophthalmol. 2007, 91, 1212–1216. [Google Scholar]
- Kodavoor, K.S.; Deb, B.; Ramamurthy, D. Outcome of deep anterior lamellar keratoplasty patients with intraoperative Descemet's membrane perforation: A retrospective cross-sectional study. Indian J. Ophthalmol. 2018, 66, 1574–1579. [Google Scholar] [CrossRef] [PubMed]
- Millar, M.J.; Maloof, A. Deep lamellar keratoplasty for pellucid marginal degeneration: Review of management options for corneal perforation. Cornea 2008, 27, 953–956. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Brahma, A.; Tromans, C.; Ridgway, A. Management of pellucid marginal corneal degeneration. Eye 2000, 14, 629–634. [Google Scholar] [CrossRef] [PubMed]

| Disease | Key Characteristics | Differentiating Features |
|---|---|---|
| Pellucid Marginal Degeneration (PMD) | - Inferior peripheral corneal thinning (crescent-shaped). - Νon-inflammatory. - Irregular astigmatism. - “Beer-belly” appearance on tomography. | - Thinning is localized to the inferior periphery. - No lipid deposits or vascularization. - Crab-claw pattern on tomography. |
| Keratoconus (KC) | - Central or paracentral corneal thinning. - Cone-shaped protrusion. - Irregular astigmatism. | - Thinning and protrusion are central or paracentral. - No inferior crescent-shaped thinning. - Different tomography pattern (not usually crab-claw). |
| Keratoglobus | - Congenital, diffuse corneal thinning. - Globular protrusion of the entire cornea. | - Thinning and protrusion are global, not localized. - Onset is congenital, unlike PMD. |
| Terrien’s Marginal Degeneration | - Peripheral circumferential thinning. - Lipid deposits and pseudopterygium. - Non-inflammatory. | - Thinning is circumferential, not inferior. - Lipid deposits and pseudopterygium are common. - No crab-claw tomography. |
| Peripheral Ulcerative Keratitis (PUK) | - Inflammatory peripheral corneal thinning. - Associated with systemic autoimmune diseases. | - Painful, inflammatory, and often associated with systemic disease. - Epithelial defect and vascularization. |
| Mooren’s Ulcer | - Painful, inflammatory peripheral ulcer. - Epithelial defect. - No systemic association. | - Severe pain and inflammation. - Epithelial defect present. - No lipid deposits or pseudopterygium. |
| Acute Hydrops | - Fluid accumulation in the cornea due to Descemet’s membrane rupture. - Associated with advanced ectatic diseases [28]. | - More common in keratoconus but higher risk in PMD. - Sudden onset of corneal edema and vision loss. |
| Treatment Modality | Indications | Benefits | Limitations |
|---|---|---|---|
| Glasses | Early stages of PMD with mild refractive errors | - Simple and non-invasive - First-line option for mild astigmatism | - Limited effectiveness in advanced stages - Cannot correct irregular astigmatism |
| Soft Contact Lenses | Early stages of PMD with regular astigmatism | - Easy to use and comfortable - Effective for regular astigmatism | - Less effective as irregular astigmatism progresses - Limited in advanced PMD |
| Corneal Rigid Gas Permeable (RGP) Lenses | Moderate to advanced PMD with irregular astigmatism | - Provide better visual acuity in irregular astigmatism - Improved stability | - Fitting can be challenging - Require gradual adjustment of lens diameter |
| Limbal/Mini-Scleral RGP Lenses | Advanced PMD with significant corneal irregularity | - Better centration and stability compared to corneal RGP lenses | - Require expertise in fitting - May still dislodge in severe cases |
| Scleral RGP Lenses | Advanced PMD with inferior corneal protrusion and severe irregularity | - Excellent stability and centration - Vault over the cornea, reducing irregularity impact | - Limited availability of trained practitioners - Reduced tear turnover and debris buildup |
| Hybrid Contact Lenses | Patient intolerance to RGP lenses or needing combined comfort and visual acuity | - Combines RGP vision correction with soft lens comfort | - Prone to tearing at the RGP-soft lens junction - Risk of oxygen permeability issues |
| Third-Generation Hybrid Lenses | Patients with sensitivity to RGP lenses | - Improved visual acuity (up to 20/30) - Better comfort for sensitive patients | - Higher complication rate (e.g., conjunctivitis, edema, allergic reactions) |
| Emerging Hybrid Lenses (e.g., AirFlex) | Emerging option for PMD management | - Promising early results - Combines comfort and visual correction | - Limited long-term data and research |
| Procedure | Mechanism | Indications | Key Benefits | Limitations/Risks |
|---|---|---|---|---|
| Collagen Cross-Linking (CXL) | UV-A-induced photochemical bonding of collagen fibrils to increase corneal rigidity | Progressive PMD with sufficient corneal thickness | - May halt progression of ectasia - Improved corneal biomechanics - Minimally invasive | - Variable visual outcomes - Limited efficacy in highly irregular astigmatism - Risk to limbal stem cells in inferiorly thinned areas |
| CXL-Plus (PRK + CXL) | Surface ablation followed by CXL for refractive and structural improvement | Select PMD cases with central astigmatism and adequate stromal thickness | - May reduce irregular astigmatism - Potential for better uncorrected visual acuity | - Higher biomechanical risk, especially in peripherally thinned corneas - Risk of ectasia progression if poorly selected |
| Intrastromal Corneal Ring Segments (ICRS) | Synthetic PMMA ring segments reshaping the corneal curvature | Mild to moderate PMD with contact lens intolerance | - Reduces astigmatism (2–4 D) - Improves BCVA - Reversible and minimally invasive | - Unpredictable long-term outcomes - Possible complications (e.g., extrusion, infection) - Limited effect in advanced PMD |
| Toric Intraocular Lenses (IOLs) | Astigmatism correction via toric optics during cataract or refractive lens surgery | PMD with coexisting cataract or stable ectasia | - Reduces refractive astigmatism - Good BCVA in selected cases - Single-step solution in cataract surgery | - Does not treat underlying ectasia - Risk of misalignment - IOL power calculation can be inaccurate in irregular corneas |
| Procedure | Benefits | Negatives (Limitations) |
|---|---|---|
| 1. Penetrating Keratoplasty | - Removes all irregular corneal tissue, providing a clear optical surface. - Improves visual acuity significantly (e.g., 20/153 to 20/43 in Tzelikis et al. [60]). | - High risk of graft rejection (up to 50%). - Postoperative astigmatism is common and challenging to manage. - Requires large, eccentric grafts, increasing surgical complexity. - Risk of complications like neovascularization, glaucoma, and infection. |
| 2. Full-Thickness Crescentic Wedge Resection | - Does not require donor tissue. - Shorter recovery time compared to PK. - Reduces astigmatism in some cases. | - Limited evidence and small sample sizes in studies. - Risk of astigmatic drift. - Intraoperative challenges and potential for complications like microperforation. |
| 3. Partial-Thickness Crescentic Wedge Resection | - Preserves endothelial layer, reducing rejection risk. - Improves BCVA (e.g., 20/125 to 20/32 in Genc et al. [65]). - No donor tissue required. | - Risk of intraoperative microperforation (14% in some studies). - Suture-related complications (e.g., infiltration in 34% of cases). - Limited long-term data. |
| 4. Crescentic Lamellar Keratoplasty | - Preserves endothelium, reducing rejection risk. - Improves visual outcomes with contact lenses or spectacles. - Maintains structural integrity of the eye. | - Postoperative astigmatism is common. - Risk of graft–host interface opacification. - Technically challenging with a steep learning curve. |
| 5. Deep Anterior Lamellar Keratoplasty | - Preserves recipient endothelium, reducing rejection risk. - Lower postoperative astigmatism compared to PK. - Shorter steroid therapy duration and fewer complications. | - Technically demanding and time-consuming. - High risk of intraoperative perforation, especially in corneas with hydrops. - Limited improvement in BCVA compared to PK. |
| 6. Lamellar Thermokeratoplasty and Epikeratoplasty | - Historical procedures with limited use today. | - High complication rates. - Outdated and replaced by safer, more effective techniques. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsatsos, M.; Koulotsiou, K.; Giachos, I.; Tsinopoulos, I.; Ziakas, N. Pellucid Marginal Degeneration: A Comprehensive Review of Pathophysiology, Diagnosis, and Management Strategies. J. Clin. Med. 2025, 14, 5178. https://doi.org/10.3390/jcm14155178
Tsatsos M, Koulotsiou K, Giachos I, Tsinopoulos I, Ziakas N. Pellucid Marginal Degeneration: A Comprehensive Review of Pathophysiology, Diagnosis, and Management Strategies. Journal of Clinical Medicine. 2025; 14(15):5178. https://doi.org/10.3390/jcm14155178
Chicago/Turabian StyleTsatsos, Michael, Konstantina Koulotsiou, Ioannis Giachos, Ioannis Tsinopoulos, and Nikolaos Ziakas. 2025. "Pellucid Marginal Degeneration: A Comprehensive Review of Pathophysiology, Diagnosis, and Management Strategies" Journal of Clinical Medicine 14, no. 15: 5178. https://doi.org/10.3390/jcm14155178
APA StyleTsatsos, M., Koulotsiou, K., Giachos, I., Tsinopoulos, I., & Ziakas, N. (2025). Pellucid Marginal Degeneration: A Comprehensive Review of Pathophysiology, Diagnosis, and Management Strategies. Journal of Clinical Medicine, 14(15), 5178. https://doi.org/10.3390/jcm14155178







