Mechanical Versus Biological Bentall Procedure: A Propensity-Score Matching Analysis of 548 Consecutive Patients
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
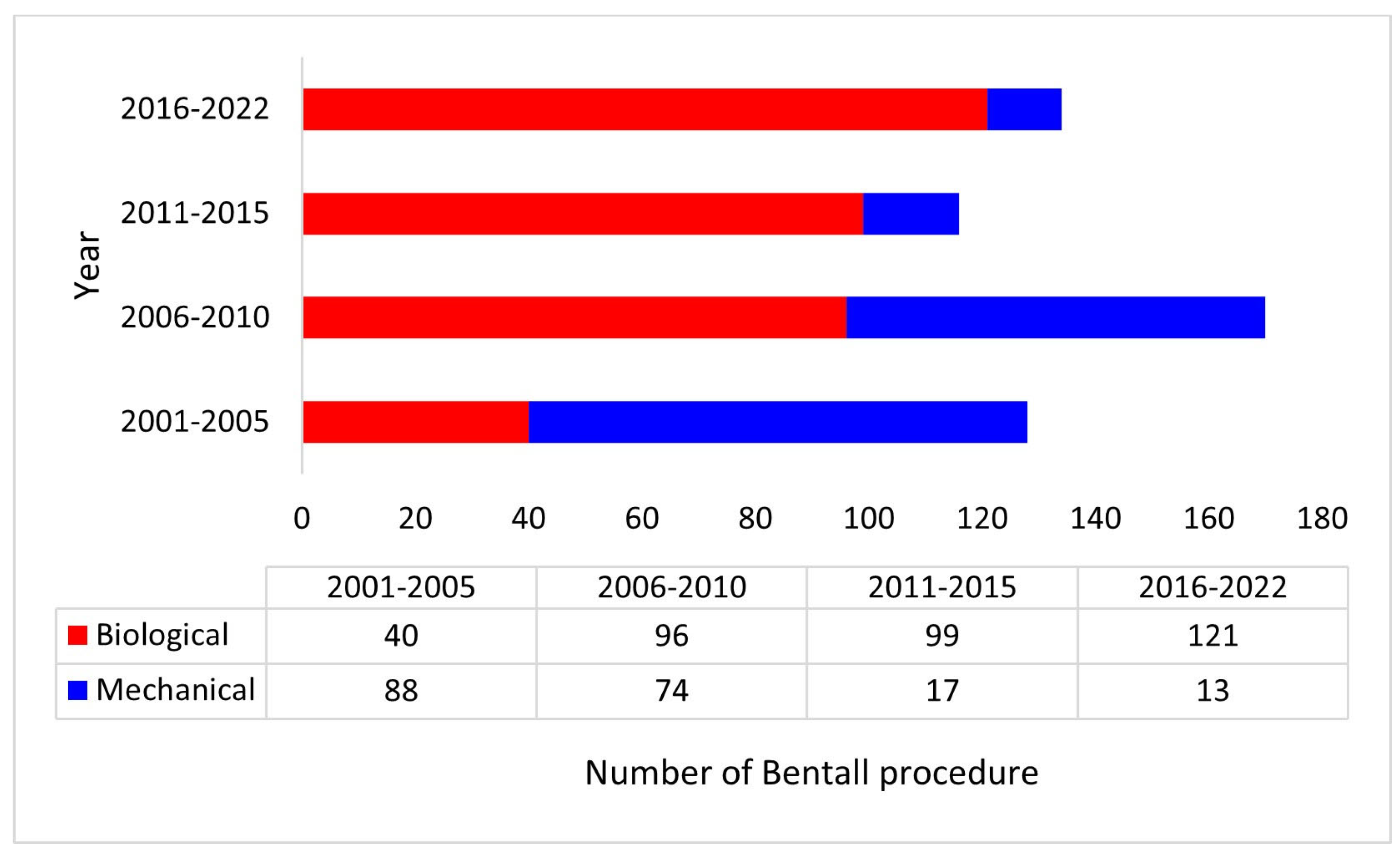
3. Results
3.1. Demography
3.2. Subgroup Analysis
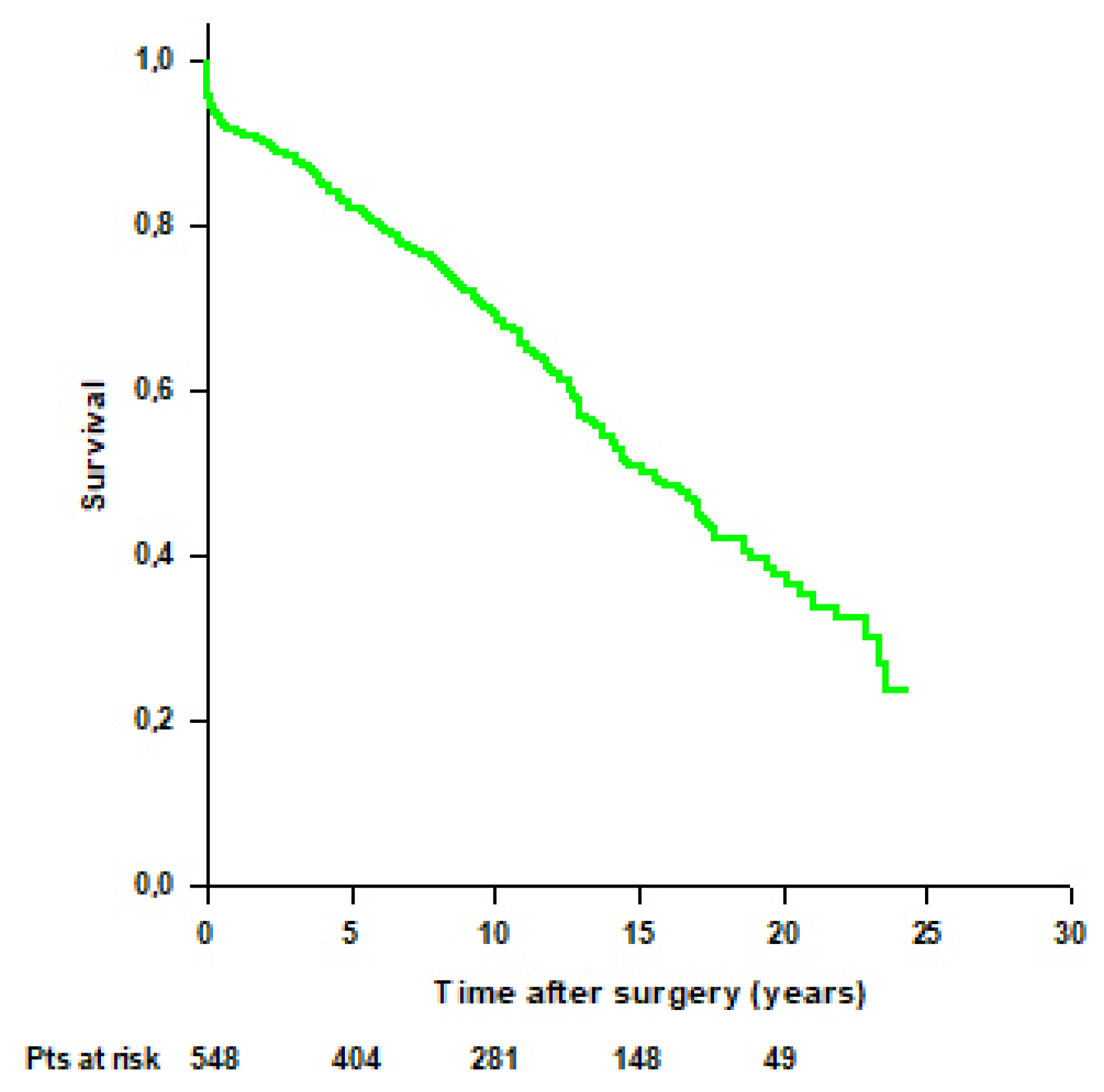
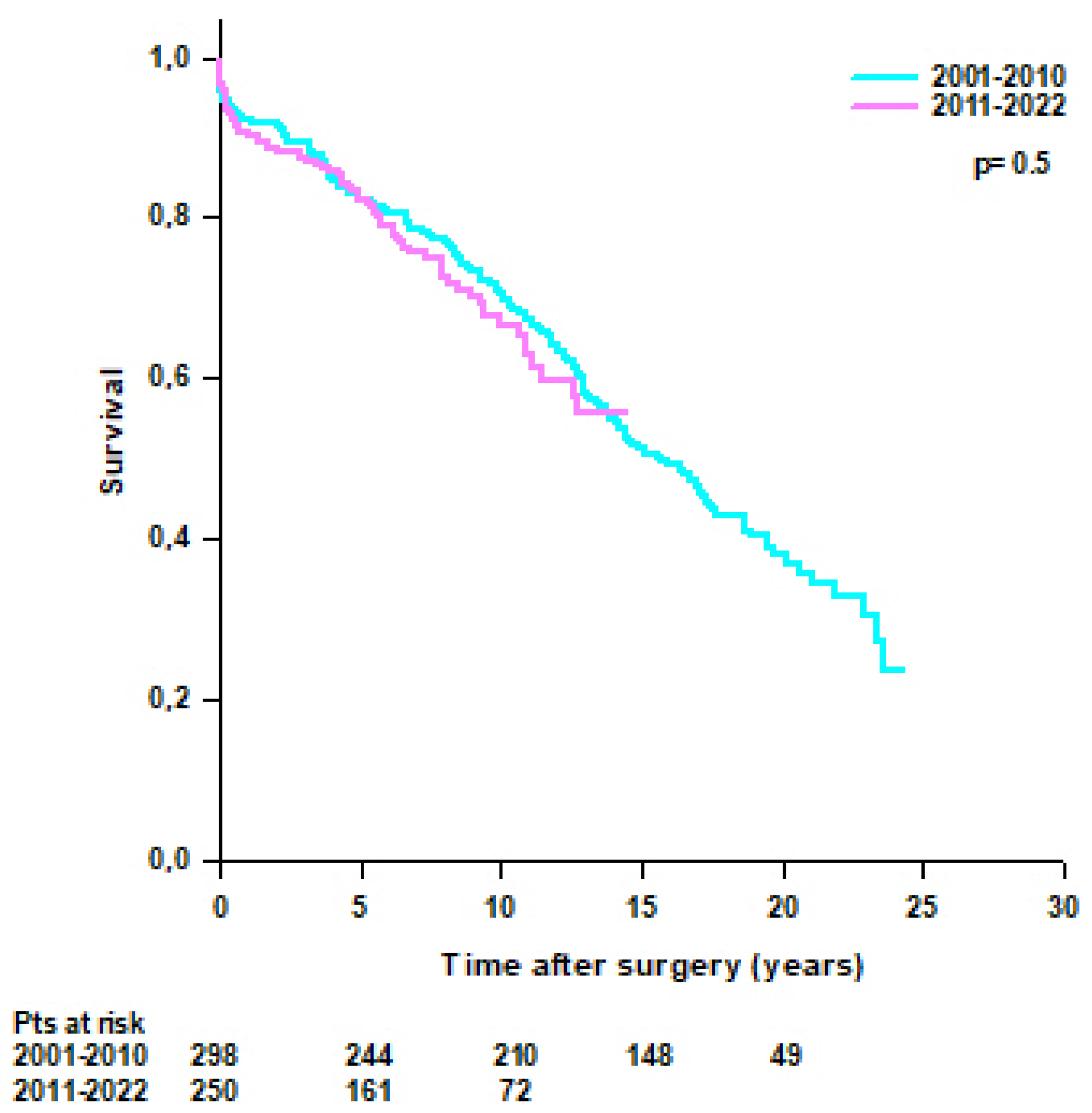
3.3. Survival
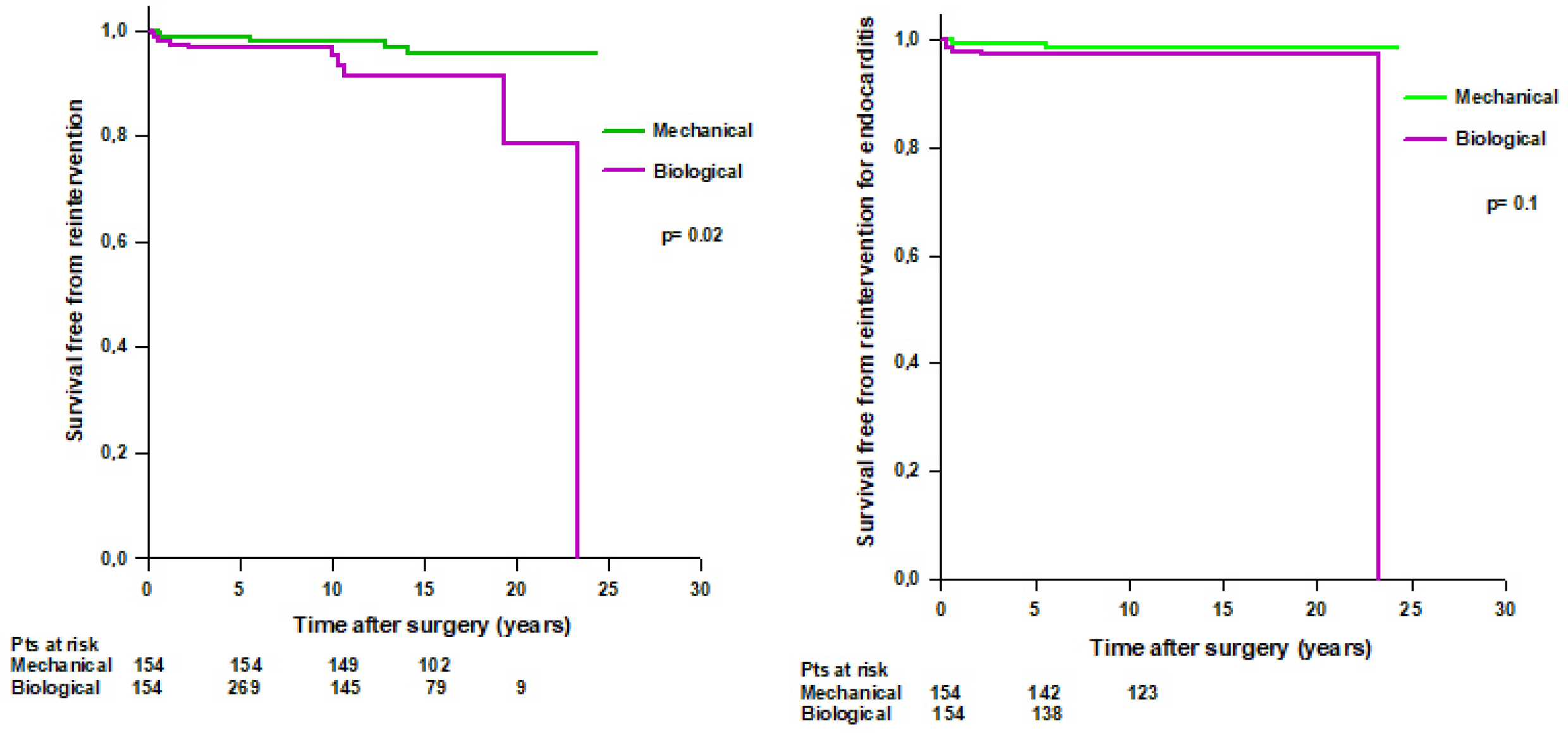
3.4. Reintervention
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AVR | Aortic valve replacement |
| BMI | Body mass index |
| BSA | Body surface area |
| BAV | Bicuspid aortic valve |
| CABG | Coronary artery bypass-grafting |
| CAH | Cryopreserved aortic homograft |
| CAV | Cerebrovascular accident |
| CI | Confidence Interval |
| CPB | Cardiopulmonary bypass |
| CRRT | Continuous renal replacement therapy |
| DAH | Decellularized aortic homograft |
| ECMO | Extra-corporeal membrane oxygenation |
| IABP | Intra-aortic ballon pump |
| INR | International normalized ratio |
| LCOS | Low cardiac output syndrome |
| LVEF | Left ventricular ejection fraction |
| MI | Myocardial infarction |
| MR | Mitral regurgitation |
| MV | Mitral valve |
| PM | Pacemaker |
| PSM | Propensity score matching |
| SMD | Standardized mean difference |
| SVD | Structural valve degeneration |
References
- Bentall, H.A.; De Bono, A. A technique for complete replacement of the ascending aorta. Thorax 1968, 23, 338–339. [Google Scholar] [CrossRef]
- Kouchoukos, N.T.; Marshall, W.G., Jr.; Wedige-Stecher, T.A. Eleven-year experience with composite graft replacement of the ascending aorta and aortic valve. J. Thorac. Cardiovasc. Surg. 1986, 92, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Beyersdorf, F.; Vahanian, A.; Milojevic, M.; Praz, F.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. J. Cardiothorac. Surg. 2021, 60, 727–800. [Google Scholar] [CrossRef]
- Chiang, Y.P.; Chikwe, J.; Moskowitz, A.J.; Itagaki, S.; Adams, D.H.; Egorova, N.N. Survival and long-term outcomes following bioprosthetic vs mechanical aortic valve replacement in patients aged 50 to 69 years. JAMA 2014, 312, 1323–1329. [Google Scholar] [CrossRef]
- Glaser, N.; Jackson, V.; Holzmann, M.J.; Franco-Cereceda, A.; Sartipy, U. Aortic valve replacement with mechanical vs. biological prostheses in patients aged 50–69 years. Eur. Heart J. 2016, 37, 2658–2667. [Google Scholar] [CrossRef]
- Etz, C.D.; Bischoff, M.S.; Bodian, C.; Roder, F.; Brenner, R.; Griepp, R.B.; Di Luozzo, G. The Bentall procedure: Is it the gold standard? A series of 597 consecutive cases. J. Thorac. Cardiovasc. Surg. 2010, 140, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Pantaleo, A.; Murana, G.; Di Marco, L.; Jafrancesco, G.; Barberio, G.; Berretta, P.; Leone, A.; Di Bartolomeo, R.; Pacini, D. Biological versus mechanical Bentall procedure for aortic root replacement: A propensity score analysis of a consecutive series of 1112 patients. Eur. J. Cardiothorac. Surg. 2017, 52, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Lechiancole, A.; Celiento, M.; Isola, M.; Gatti, G.; Melina, G.; Vendramin, I.; Battistella, C.; Pappalardo, A.; Sinatra, R.; Bortolotti, U.; et al. Modified Bentall procedure: Mechanical vs biological valved conduits in patients older than 65 years. Int. J. Cardiol. 2019, 296, 38–42. [Google Scholar] [CrossRef]
- Werner, P.; Gritsch, J.; Kaider, A.; Coti, I.; Osorio, E.; Mahr, S.; Stelzmueller, M.-E.; Kocher, A.; Laufer, G.; Andreas, M.; et al. Long Term Results of the Modified Bentall Procedure with Mechanical and Biological Composite Valve Grafts. Front. Cardiovasc. Med. 2022, 9, 867732. [Google Scholar] [CrossRef]
- Perezgrovas-Olaria, R.; Soletti, G.J.; Rahouma, M.; Dimagli, A.; Harik, L.; Cancelli, G.; Yaghmour, M.; Polk, H.; Closkey, B.; Wright, J.; et al. Mortality and reoperation rate of biological versus mechanical Bentall-De Bono operation: A propensity-matched study. Vessel. Plus 2023, 7, 10. [Google Scholar] [CrossRef]
- Chen, C.; Chang, F.; Cheng, Y.; Wu, V.C.; Lin, C.; Chan, Y.; Hung, K.; Chu, P.; Chen, S. Mechanical Versus Bioprosthetic Aortic Valve Replacement in Patients Undergoing Bentall Procedure. J. Am. Heart Assoc. 2024, 13, e030328. [Google Scholar] [CrossRef]
- Erbel, R.; Aboyans, V.; Boileaul, C.; Bossone, E.; Bartolomeo, R.D.; Eggebrecht, H.; Evangelista, A.; Falk, V.; Frank, H.; Gaemperli, O.; et al. ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2873–2926. [Google Scholar] [CrossRef]
- Kouchoukos, N.T.; Haynes, M.; Baker, J.N. The Button Bentall Technique. Oper. Tech. Thorac. Cardiovasc. Surg. 2018, 23, P50–P61. [Google Scholar] [CrossRef]
- VARC-3 WRITING COMMITTEE; Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; et al. Valve Academic Research Consortium 3: Updated Endpoint Definitions for Aortic Valve Clinical Research. J. Am. Coll. Cardiol. 2021, 77, 2717–2746. [Google Scholar] [CrossRef]
- Clark, T.G.; Altman, D.G.; De Stavola, B.L. Quantification of the completeness of follow-up. Lancet 2002, 359, 1309–1310. [Google Scholar] [CrossRef]
- Head, S.J.; Çelik, M.; Kappetein, A.P. Mechanical versus bioprosthetic aortic valve replacement. Eur. Heart J. 2017, 38, 2183–2191. [Google Scholar] [CrossRef]
- BBowdish, M.E.; Mehaffey, J.H.; Chang, S.-C.; O’gAra, P.T.; Mack, M.J.; Goldstone, A.B.; Chikwe, J.; Gillinov, A.M.; Wu, C.; Fontana, G.P.; et al. Bioprosthetic vs Mechanical Aortic Valve Replacement in Patients 40 to 75 Years of Age. J. Am. Coll. Cardiol. 2025, 85, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Mookhoek, A.; Korteland, N.M.; Arabkhani, B.; Di Centa, I.; Lansac, E.; Bekkers, J.A.; Bogers, A.J.; Takkenberg, J.J. Bentall procedure: A systematic review and meta-analysis. Ann. Thorac. Surg. 2016, 101, 1684–1689. [Google Scholar] [CrossRef] [PubMed]
- Glaser, N.; Jackson, V.; Holzmann, M.J.; Franco-Cereceda, A.; Sartipy, U. Prosthetic Valve Endocarditis After Surgical Aortic Valve Replacement. Circulation 2017, 136, 329–331. [Google Scholar] [CrossRef]
- Anantha-Narayanan, M.; Reddy, Y.N.V.; Sundaram, V.; Murad, M.H.; Erwin, P.J.; Baddour, L.M.; Schaff, H.V.; Nishimura, R.A. Endocarditis risk with bioprosthetic and mechanical valves: Systematic review and meta-analysis. Heart 2020, 106, 1413–1419. [Google Scholar] [CrossRef]
- Formica, F.; Gallingani, A.; D’Alessandro, S.; Tuttolomondo, D.; Hernandez-Vaquero, D.; Singh, G.; Grassa, G.; Pattuzzi, C.; Maestri, F.; Nicolini, F. Long-term outcomes comparison of Bentall-De Bono-versus valve-sparing aortic root replacement: An updated systematic review and reconstructed time-to-event meta-analysis. Int. J. Cardiol. 2025, 419, 132728. [Google Scholar] [CrossRef]
- Elbatarny, M.; Tam, D.Y.; Edelman, J.J.; Rocha, R.V.; Chu, M.W.; Peterson, M.D.; El-Hamamsy, I.; Appoo, J.J.; Friedrich, J.O.; Boodhwani, M.; et al. Canadian Thoracic Aortic Collaborative (CTAC) Investigators, Valve-sparing root replacement versus composite valve grafting in aortic root dilation: A metaanalysis. Ann. Thorac. Surg. 2020, 110, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Salmasi, M.Y.; Theodoulou, I.; Iyer, P.; Al-Zubaidy, M.; Naqvi, D.; Snober, M.; Oo, A.; Athanasiou, T. Comparing outcomes between valve-sparing root replacement and the Bentall procedure in proximal aortic aneurysms: Systematic review and meta-analysis. Interact. Cardiovasc. Thorac. Surg. 2019, 29, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Sa, M.P.; Jacquemyn, X.; Van den Eynde, J.; Chu, D.; Serna-Gallegos, D.; Coselli, J.S.; Sultan, I. Long-term outcomes of valve-sparing aortic root versus composite aortic valve graft replacement for aortic root aneurysm: Meta-analysis of reconstructed time-to-event data. Am. J. Surg. 2023, 226, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Svensson, L.G.; Pillai, S.T.; Rajeswaran, J.; Desai, M.Y.; Griffin, B.; Grimm, R.; Hammer, D.F.; Thamilarasan, M.; Roselli, E.E.; Pettersson, G.B.; et al. Long-term survival, valve durability, and reoperation for 4 aortic root procedures combined with ascending aorta replacement. J. Thorac. Cardiovasc. Surg. 2016, 151, 764–774. [Google Scholar] [CrossRef]
- Galeone, A.; Trojan, D.; Gardellini, J.; Di Gaetano, R.; Faggian, G.; Luciani, G.B. Cryopreserved aortic homografts for complex aortic valve or root endocarditis: A 28-year experience. Eur. J. Cardiothorac. Surg. 2022, 62, ezac193. [Google Scholar] [CrossRef]
- Galeone, A.; Gardellini, J.; Trojan, D.; Di Nicola, V.; Di Gaetano, R.; Faggian, G.; Luciani, G.B. Three Decades of Experience with Aortic Prosthetic Valve Endocarditis. J. Cardiovasc. Dev. Dis. 2023, 10, 338. [Google Scholar] [CrossRef]
- Horke, A.; Tudorache, I.; Laufer, G.; Andreas, M.; Pomar, J.L.; Pereda, D.; Quintana, E.; Sitges, M.; Meyns, B.; Rega, F.; et al. Early results from a prospective, single-arm European trial on decellularized allografts for aortic valve replacement: The ARISE study and ARISE Registry data. Eur. J. Cardiothorac. Surg. 2020, 58, 1045–1053. [Google Scholar] [CrossRef]
- Andreeva, A.; Werner, P.; Coti, I.; Kocher, A.; Laufer, G.; Ehrlich, M.; Zimpfer, D.; Andreas, M. Decellularized aortic homografts versus mechanical composite grafts for aortic root replacement. Eur. J. Cardiothorac. Surg. 2024, 66, ezae314. [Google Scholar] [CrossRef]



| Unmatched Population | PS-Matched Population | ||||||
|---|---|---|---|---|---|---|---|
| Biological Bentall (n = 356) | Mechanical Bentall (n = 192) | p | Biological Bentall (n = 154) | Mechanical Bentall (n = 154) | p | SMD | |
| Preoperative characteristics | |||||||
| Age, years | 68 (61–73) | 59 (50–65) | <0.001 | 62 (55–68) | 62 (53–66) | 0.3 | 0.09 |
| Male sex | 292 (82%) | 168 (88%) | 0.1 | 131 (85%) | 135 (88%) | 0.6 | 0.1 |
| BMI | 26 (24–29) | 27 (24–30) | 0.1 | 26 (24–29) | 27 (24–30) | 0.4 | 0.06 |
| BSA, m2 | 1.93 (1.8–2.06) | 1.98 (1.83–2.09) | 0.01 | 1.97 (1.83–2.1) | 1.97 (1.84–2.08) | 0.8 | 0.01 |
| LVEF (%) | 60 (55–60) | 60 (52–60) | 0.2 | 60 (55–60) | 60 (55–60) | 0.5 | 0.06 |
| Bicuspid aortic valve | 81 (23%) | 57 (30%) | 0.07 | 37 (24%) | 43 (28%) | 0.5 | 0.1 |
| Coronary artery disease | 46 (13%) | 21 (11%) | 0.5 | 25 (16%) | 18 (12%) | 0.3 | 0.1 |
| Acute aortic dissection | 43 (12%) | 38 (20%) | 0.02 | 26 (17%) | 26 (17%) | 0.9 | 0 |
| Infective endocarditis | 16 (4%) | 8 (4%) | 0.9 | 5 (3%) | 5 (3%) | 0.9 | 0 |
| Urgent/emergent surgery | 58 (16%) | 45 (23%) | 0.05 | 28 (18%) | 32 (21%) | 0.6 | 0.07 |
| Redo surgery | 43 (12%) | 28 (15%) | 0.4 | 24 (16%) | 23 (15%) | 0.9 | 0.02 |
| Intraoperative characteristics | |||||||
| CPB time, min | 160 (135–203) | 159 (135–207) | 0.9 | 164 (141–224) | 155 (134–201) | 0.1 | |
| Aortic cross-clamping, min | 128 (109–149) | 121 (106–151) | 0.1 | 131 (110–153) | 120 (106–144) | 0.03 | |
| Circulatory arrest | 55 (15%) | 44 (23%) | 0.04 | 29 (19%) | 35 (23%) | 0.4 | |
| Aortic valve size, mm | 25 (25–27) | 25 (25–27) | 0.9 | 25 (25–27) | 25 (25–27) | 0.1 | |
| Aortic graft size, mm | 28 (28–30) | 28 (26–30) | <0.001 | 28 (28–30) | 28 (26–30) | <0.001 | |
| Arch replacement | 11 (3%) | 5 (3%) | 0.9 | 7 (5%) | 4 (3%) | 0.5 | |
| Emiarch replacement | 13 (4%) | 5 (3%) | 0.8 | 5 (3%) | 5 (3%) | 0.9 | |
| CABG | 54 (15%) | 24 (13%) | 0.4 | 31 (20%) | 20 (13%) | 0.1 | |
| MV repair/replacement | 10 (3%) | 6 (3%) | 0.9 | 5 (3%) | 3 (2%) | 0.7 | |
| Perioperative characteristics | |||||||
| IABP/ECMO | 15 (4%) | 6 (3%) | 0.4 | 8 (5%) | 3 (2%) | 0.2 | |
| Revision for bleeding | 23 (6%) | 17 (9%) | 0.4 | 11 (7%) | 15 (10%) | 0.5 | |
| MI | 5 (1%) | 2 (1%) | 0.9 | 2 (1%) | 2 (1%) | 0.9 | |
| Sepsis | 7 (2%) | 5 (3%) | 0.8 | 6 (4%) | 3 (2%) | 0.4 | |
| CVA | 13 (4%) | 8 (4%) | 0.9 | 8 (5%) | 8 (5%) | 0.9 | |
| CRRT | 0 | 1 (1%) | - | 0 | 1 (1%) | - | |
| PM implantation | 1 (0.3%) | 1 (1%) | 0.8 | 0 | 1 (1%) | - | |
| Group 1 2001–2010 (n = 298) | Group 2 2011–2022 (n = 250) | p | |
|---|---|---|---|
| Preoperative characteristics | |||
| Age, years | 65 (57–71) | 65 (55–71) | 0.8 |
| Male sex | 256 (86%) | 204 (82%) | 0.2 |
| BMI | 27 (25–29) | 26 (24–29) | 0.3 |
| BSA, m2 | 1.94 (1.8–2.03) | 1.97 (1.84–2.1) | 0.05 |
| LVEF (%) | 60 (55–60) | 60 (51–60) | 0.004 |
| Bicuspid aortic valve | 75 (25%) | 63 (25%) | 0.9 |
| Coronary artery disease | 40 (13%) | 27 (11%) | 0.6 |
| Acute aortic dissection | 41 (14%) | 40 (16%) | 0.5 |
| Infective endocarditis | 12 (4%) | 12 (5%) | 0.8 |
| Urgent/emergent surgery | 53 (18%) | 50 (20%) | 0.5 |
| Redo surgery | 38 (13%) | 33 (13%) | 0.9 |
| Intraoperative characteristics | |||
| CPB time, min | 156 (135–195) | 162 (136–221) | 0.1 |
| Aortic cross-clamping, min | 123 (105–145) | 129 (111–153) | 0.04 |
| Circulatory arrest | 57 (19%) | 42 (17%) | 0.5 |
| Mechanical valved conduit | 162 (54%) | 30 (12%) | <0.001 |
| Aortic valve size, mm | 25 (23–27) | 25 (25–27) | 0.007 |
| Aortic graft size, mm | 28 (26–28) | 28 (28–30) | <0.001 |
| Arch replacement | 8 (3%) | 8 (3%) | 0.9 |
| Emiarch replacement | 7 (2%) | 11 (5%) | 0.2 |
| CABG | 42 (14%) | 36 (14%) | 0.9 |
| MV repair/replacement | 10 (3%) | 6 (2%) | 0.6 |
| Perioperative characteristics | |||
| IABP/ECMO | 6 (2%) | 15 (6%) | 0.02 |
| Revision for bleeding | 19 (6%) | 21 (8%) | 0.4 |
| MI | 3 (1%) | 4 (2%) | 0.8 |
| Sepsis | 3 (1%) | 9 (4%) | 0.07 |
| CVA | 7 (2%) | 14 (6%) | 0.08 |
| CRRT | 0 | 1 (0.4%) | - |
| PM implantation | 0 | 2 (1%) | - |
| Postoperative characteristics | |||
| Overall mortality | 178 (60%) | 72 (29%) | <0.001 |
| Periprocedural mortality | 16 (5%) | 12 (5%) | 0.9 |
| Early mortality | 7 (2%) | 11 (4%) | 0.2 |
| Late mortality | 155 (52%) | 49 (20%) | <0.001 |
| Reintervention | 10 (3%) | 14 (6%) | 0.2 |
| Infective endocarditis | 5 (2%) | 7 (3%) | 0.5 |
| Aortic disease | 1 (0.3%) | 4 (2%) | 0.2 |
| SVD | 2 (1%) | 1 (0.4%) | 0.8 |
| MR | 1 (0.3%) | 2 (1%) | 0.8 |
| Patient’s Age <65 Years (n = 277) | Patient’s Age ≥65 Years (n = 271) | p | |
|---|---|---|---|
| Preoperative characteristics | |||
| Age, years | 56 (49–61) | 71 (68–75) | <0.001 |
| Male sex | 241 (87%) | 219 (80%) | 0.06 |
| BMI | 27 (24–30) | 26 (24–29) | 0.5 |
| BSA, m2 | 1.74 (1.68–1.8) | 1.71 (1.65–1.75) | <0.001 |
| LVEF (%) | 60 (55–60) | 60 (52–60) | 0.7 |
| Bicuspid aortic valve | 84 (30%) | 54 (20%) | 0.008 |
| Coronary artery disease | 22 (8%) | 45 (17%) | 0.003 |
| Acute aortic dissection | 54 (19%) | 27 (10%) | 0.003 |
| Infective endocarditis | 13 (5%) | 11 (4%) | 0.8 |
| Urgent/emergent surgery | 64 (23%) | 39 (14%) | 0.01 |
| Redo surgery | 36 (13%) | 35 (13%) | 0.9 |
| Intraoperative characteristics | |||
| CPB time, min | 162 (134–222) | 158 (136–191) | 0.4 |
| Aortic cross-clamping, min | 124 (106–150) | 127 (109–148) | 0.3 |
| Circulatory arrest | 59 (51%) | 40 (15%) | <0.001 |
| Mechanical valved conduit | 142 (51%) | 50 (18%) | <0.001 |
| Aortic valve size, mm | 25 (25–27) | 25 (23–27) | 0.01 |
| Aortic graft size, mm | 28 (26–30) | 28 (28–30) | 0.9 |
| Arch replacement | 11 (4%) | 5 (2%) | 0.2 |
| Emiarch replacement | 8 (3%) | 10 (4%) | 0.7 |
| CABG | 29 (10%) | 49 (18%) | 0.01 |
| MV repair/replacement | 11 (4%) | 5 (2%) | 0.2 |
| Perioperative characteristics | |||
| IABP/ECMO | 12 (4%) | 9 (3%) | 0.6 |
| Revision for bleeding | 26 (9%) | 14 (5%) | 0.08 |
| MI | 2 (1%) | 5 (2%) | 0.4 |
| Sepsis | 7 (3%) | 5 (2%) | 0.8 |
| CVA | 8 (3%) | 13 (5%) | 0.3 |
| CRRT | 1 (0.4%) | 0 | - |
| PM implantation | 1 (0.4%) | 1 (0.4%) | 0.9 |
| Postoperative characteristics | |||
| Overall mortality | 91 (33%) | 159 (59%) | <0.001 |
| Periprocedural mortality | 16 (6%) | 12 (4%) | 0.5 |
| Early mortality | 9 (3%) | 9 (3%) | 0.9 |
| Late mortality | 66 (24%) | 138 (51%) | <0.001 |
| Reintervention | 18 (6%) | 6 (2%) | 0.02 |
| Infective endocarditis | 8 (3%) | 4 (1%) | 0.4 |
| Aortic disease | 4 (1%) | 1 (0.4%) | 0.3 |
| SVD | 3 (1%) | 0 | - |
| MR | 3 (1%) | 1 (0.4%) | 0.6 |
| Unmatched Population | PS-Matched Population | |||||
|---|---|---|---|---|---|---|
| Biological Bentall (n = 356) | Mechanical Bentall (n = 192) | p | Biological Bentall (n = 154) | Mechanical Bentall (n = 154) | p | |
| Overall mortality | 163 (46%) | 87 (45%) | 0.9 | 55 (36%) | 78 (51%) | 0.01 |
| Periprocedural mortality | 15 (4%) | 13 (7%) | 0.2 | 6 (4%) | 9 (6%) | 0.5 |
| Early mortality | 12 (3%) | 6 (3%) | 0.9 | 4 (3%) | 4 (3%) | 0.9 |
| Late mortality | 136 (38%) | 68 (35%) | 0.5 | 45 (29%) | 65 (42%) | 0.02 |
| Unmatched Population | PS-matched Population | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | |||||
| Variable |
Hazard Ratio (95% CI) | p |
Hazard Ratio (95% CI) | p |
Hazard Ratio (95% CI) | p |
Hazard Ratio (95% CI) | p |
| Age >65 years | 2.48 (1.89–3.24) | <0.001 | 2.22 (1.66–2.96) | <0.001 | 1.92 (1.36–2.7) | <0.001 | 1.74 (1.22–2.47) | 0.002 |
| Sex, female | 1.16 (0.84–1.61) | 0.35 | 1.01 (0.62–1.64) | 0.9 | ||||
| BAV | 0.62 (0.46–0.84) | 0.003 | 0.76 (0.56–1.04) | 0.09 | 0.55 (0.36–0.84) | 0.006 | 0.63 (0.41–0.98) | 0.04 |
| Coronary artery disease | 1.36 (0.96–1.93) | 0.08 | 1.1 (0.68–1.8) | 0.68 | ||||
| Aortic dissection | 1.19 (0.84–1.68) | 0.31 | - | 1.22 (0.78–1.9) | 0.36 | |||
| Infective endocarditis | 1.84 (1.10–3.05) | 0.01 | 1.36 (0.76–2.42) | 0.28 | 1.75 (0.77–3.98) | 0.18 | ||
| Redo surgery | 1.24 (0.87–1.77) | 0.22 | 1.58 (1.01–2.47) | 0.04 | 1.39 (0.88–2.18) | 0.14 | ||
| Urgent/emergent surgery | 1.44 (1.06–1.94) | 0.01 | 1.43 (1.09–2) | 0.03 | 1.38 (0.91–2.08) | 0.12 | ||
| Mechanical valved conduit | 0.55 (0.41–0.72) | <0.001 | 0.64 (0.48–0.86) | 0.003 | 0.81 (0.56–1.16) | 0.26 | ||
| IABP | 2.49 (1.17–5.3) | 0.01 | 1.45 (0.59–3.55) | 0.4 | 1.96 (0.62–6.17) | 0.24 | ||
| ECMO | 6.58 (3.08–14.02) | <0.001 | 3.72 (1.56–8.86) | 0.003 | 5.68 (2.08–15.47) | <0.001 | 5.03 (1.79–14.15) | 0.002 |
| Postoperative CVA | 3.79 (2.34–6.16) | <0.001 | 3.37 (2.02–5.62) | <0.001 | 3.58 (2–6.4) | <0.001 | 2.9 (1.59–5.26) | <0.001 |
| Reintervention | 0.79 (0.42–1.49) | 0.47 | 0.85 (0.37–1.94) | 0.7 | ||||
| Unmatched Population | PS-Matched Population | |||||
|---|---|---|---|---|---|---|
| Characteristics | Biological Bentall (n = 356) | Mechanical Bentall (n = 192) | p | Biological Bentall (n = 154) | Mechanical Bentall (n = 154) | p |
| Reintervention | 16 (4%) | 8 (4%) | 0.9 | 10 (6%) | 5 (3%) | 0.3 |
| Infective endocarditis | 9 (3%) | 3 (2%) | 0.6 | 5 (3%) | 2 (1%) | 0.4 |
| Aortic disease | 3 (1%) | 2 (1%) | 0.9 | 2 (1%) | 0 | - |
| SVD | 3 (1%) | 0 | - | 3 (2%) | 0 | - |
| MR | 1 (0.3%) | 3 (2%) | 0.2 | 0 | 3 (2%) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galeone, A.; Gardellini, J.; Perrone, F.; Di Nicola, V.; Dian, G.; Di Gaetano, R.; Luciani, G.B. Mechanical Versus Biological Bentall Procedure: A Propensity-Score Matching Analysis of 548 Consecutive Patients. J. Clin. Med. 2025, 14, 5105. https://doi.org/10.3390/jcm14145105
Galeone A, Gardellini J, Perrone F, Di Nicola V, Dian G, Di Gaetano R, Luciani GB. Mechanical Versus Biological Bentall Procedure: A Propensity-Score Matching Analysis of 548 Consecutive Patients. Journal of Clinical Medicine. 2025; 14(14):5105. https://doi.org/10.3390/jcm14145105
Chicago/Turabian StyleGaleone, Antonella, Jacopo Gardellini, Fabiola Perrone, Venanzio Di Nicola, Giovanni Dian, Renato Di Gaetano, and Giovanni Battista Luciani. 2025. "Mechanical Versus Biological Bentall Procedure: A Propensity-Score Matching Analysis of 548 Consecutive Patients" Journal of Clinical Medicine 14, no. 14: 5105. https://doi.org/10.3390/jcm14145105
APA StyleGaleone, A., Gardellini, J., Perrone, F., Di Nicola, V., Dian, G., Di Gaetano, R., & Luciani, G. B. (2025). Mechanical Versus Biological Bentall Procedure: A Propensity-Score Matching Analysis of 548 Consecutive Patients. Journal of Clinical Medicine, 14(14), 5105. https://doi.org/10.3390/jcm14145105






