The Effects of a Combined Exercise Intervention on Body Composition, GDF-15, Apelin-12, and IL-15 Among Older Korean Women According to Obesity Status
Abstract
1. Introduction
2. Materials and Methods
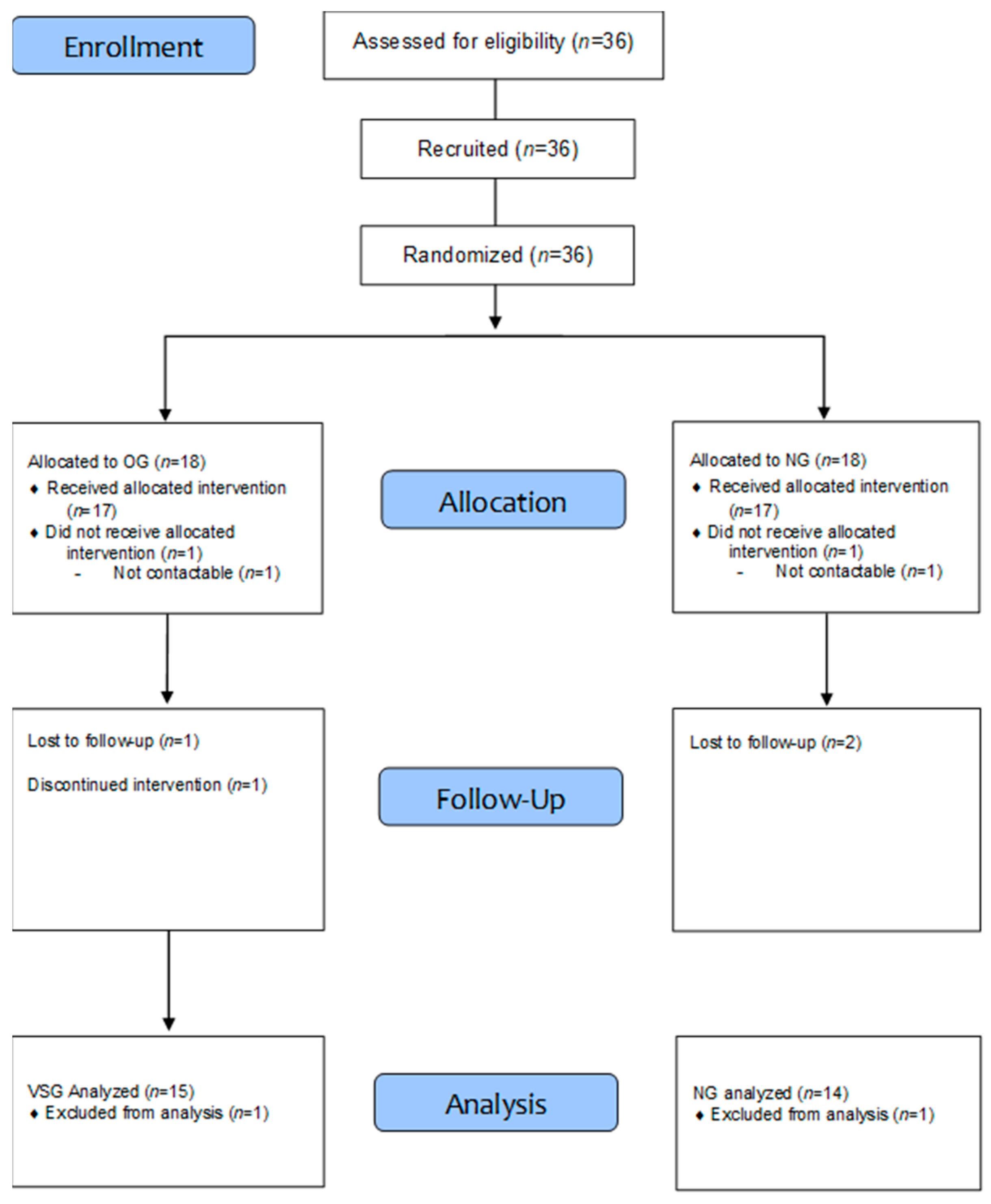
2.1. Study Participants
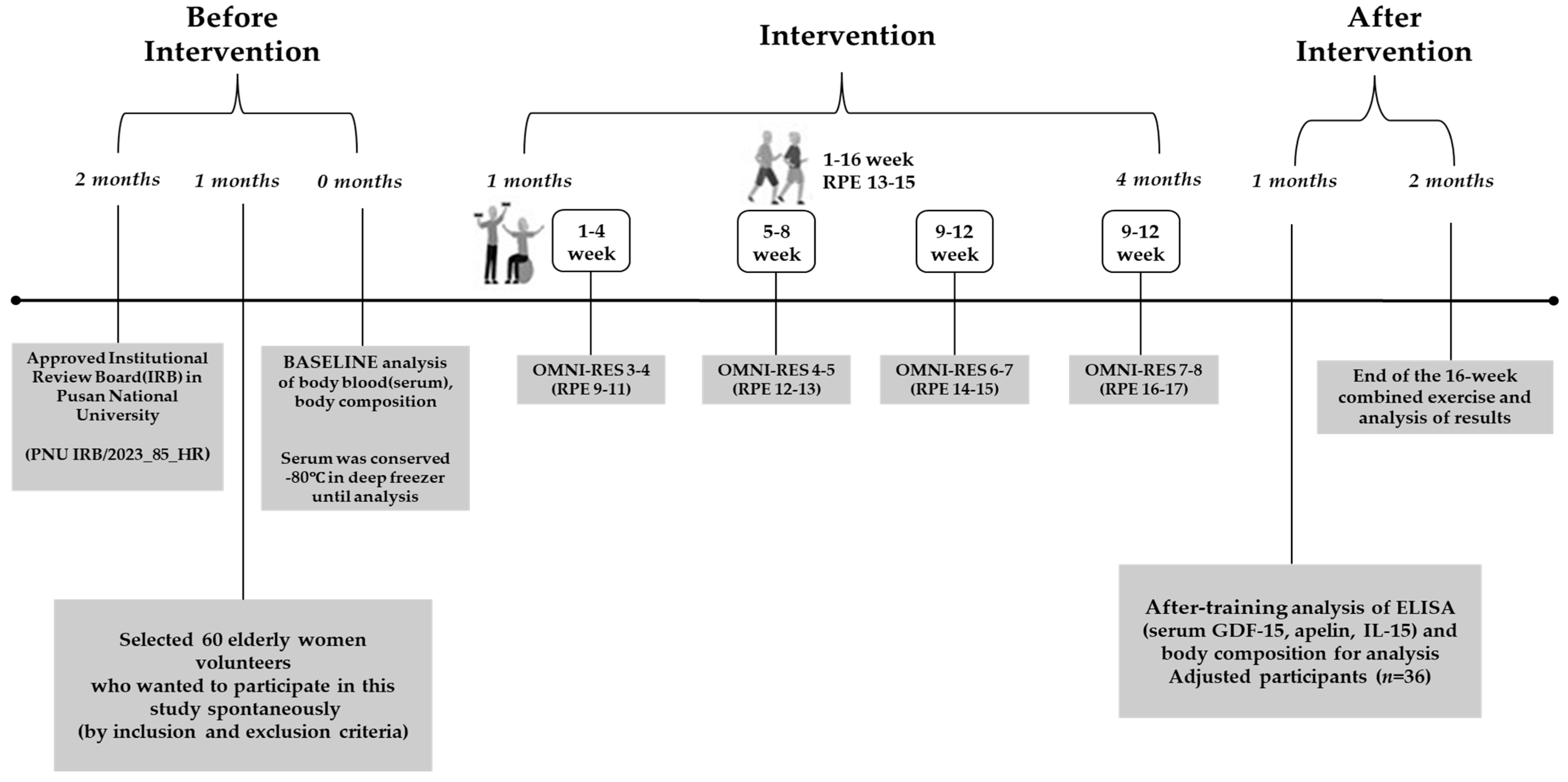
2.2. Exercise Program
2.3. Measurement Items and Analysis Methods
2.3.1. Body Composition
2.3.2. Blood Analysis
2.4. Data Processing
3. Results
3.1. Body Composition
3.2. GDF-15
3.3. Apelin-12
3.4. IL-15
4. Discussion
4.1. Body Composition
4.2. GDF-15
4.3. Apelin-12
4.4. IL-15
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| %BF | Percentage of body fat |
| GDF-15 | Growth differentiation factor-15 |
| IL-15 | Interleukin-15 |
| BMI | Body mass index |
| OMNI-RES | OMNI-Resistance Exercise Scale |
References
- García-Miranda, A.; Garcia-Hernandez, A.; Castañeda-Saucedo, E.; Navarro-Tito, N.; Maycotte, P. Adipokines as regulators of autophagy in obesity-linked cancer. Cells 2022, 11, 3230. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Paik, I.Y.; Ryu, J.H.; Lee, T.H.; Kim, D.E. Effects of aerobic and resistance exercises on circulating apelin-12 and apelin-36 concentrations in obese middle-aged women: A randomized controlled trial. BMC Womens Health 2019, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh Pahlavani, H. Exercise therapy for people with sarcopenic obesity: Myokines and adipokines as effective actors. Front. Endocrinol. 2022, 13, 811751. [Google Scholar] [CrossRef] [PubMed]
- Son, J.S.; Chae, S.A.; Park, B.I.; Min, D.; Song, W. Plasma apelin levels in overweight/obese adults following a single bout of exhaustive exercise: A preliminary cross-sectional study. Endocrinol. Diabetes Nutr. 2019, 66, 278–290. [Google Scholar] [CrossRef]
- Chen, T.C.; Huang, T.H.; Tseng, W.C.; Tseng, K.W.; Hsieh, C.C.; Chen, M.Y.; Chou, T.Y.; Huang, Y.C.; Chen, H.L.; Nosaka, K. Changes in plasma C1q, apelin and adropin concentrations in older adults after descending and ascending stair walking intervention. Sci. Rep. 2021, 11, 17644. [Google Scholar] [CrossRef]
- Johann, K.; Kleinert, M.; Klaus, S. The role of GDF15 as a myomitokine. Cells 2021, 10, 2990. [Google Scholar] [CrossRef]
- Wang, D.; Day, E.A.; Townsend, L.K.; Djordjevic, D.; Jørgensen, S.B.; Steinberg, G. GDF15: Emerging biology and therapeutic applications for obesity and cardiometabolic disease. Nat. Rev. Endocrinol. 2021, 17, 592–607. [Google Scholar] [CrossRef]
- Zhang, H.; Fealy, C.E.; Kirwan, J.P. Exercise training promotes a GDF15-associated reduction in fat mass in older adults with obesity. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E829–E836. [Google Scholar] [CrossRef]
- Guo, A.; Li, K.; Xiao, Q. Sarcopenic obesity: Myokines as potential diagnostic biomarkers and therapeutic targets? Exp. Gerontol. 2020, 139, 111022. [Google Scholar] [CrossRef]
- Ahima, R.S.; Park, H.K. Connecting myokines and metabolism. Endocrinol. Metab. 2015, 30, 235–245. [Google Scholar] [CrossRef]
- Lutz, C.T.; Quinn, L.S. Sarcopenia, obesity, and natural killer cell immune senescence in aging: Altered cytokine levels as a common mechanism. Aging 2012, 4, 535. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, K.; Yamaguchi, A. Sarcopenic obesity and endocrinal adaptation with age. Int. J. Endocrinol. 2013, 2013, 204164. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, G.; Blake, C.; Cunningham, C.; Lennon, O.; Perrotta, C. What exercise prescription is optimal to improve body composition and cardiorespiratory fitness in adults living with obesity? A network meta-analysis. Obes. Rev. 2021, 22, e13137. [Google Scholar] [CrossRef] [PubMed]
- Grajek, A.; Sprung, V.S. Global self-esteem, physical activity, and body composition changes following a 12-week dietary and physical activity intervention in older women. Int. J. Environ. Res. Public Health 2022, 19, 13220. [Google Scholar] [CrossRef]
- Park, W.I.; Jung, W.S.; Hong, K.S.; Kim, Y.Y.; Kim, S.W.; Park, H.Y. Effects of moderate combined resistance-and aerobic-exercise for 12 weeks on body composition, cardiometabolic risk factors, blood pressure, arterial stiffness, and physical functions, among obese older men: A pilot study. Int. J. Environ. Res. Public Health 2020, 17, 7233. [Google Scholar] [CrossRef]
- Kim, B.Y.; Kang, S.M.; Kang, J.H.; Kang, S.Y.; Kim, K.K.; Kim, K.B.; Kim, B.; Kim, S.J.; Kim, Y.H.; Kim, J.H.; et al. Korean Society for the Study of Obesity Guidelines for the Management of Obesity in Korea. J. Obes. Metab. Syndr. 2021, 30, 81. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hyun, S.J.; Ha, S.M.; Kim, J.S.; Kim, D.Y. Effects of combined exercise program on inflammatory factors related to cardiovascular disease in elderly women. J. Korean Assoc. Phys. Educ. Sport. Girls Women. 2019, 33, 153–167. [Google Scholar] [CrossRef]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2018. [Google Scholar]
- Colado, J.C.; Furtado, G.E.; Teixeira, A.M.; Flandez, J.; Naclerio, F. Concurrent and construct validation of a new scale for rating perceived exertion during elastic resistance training in the elderly. J. Sports Sci. Med. 2020, 19, 175–186. [Google Scholar]
- Matson, T.E.; Renz, A.D.; Takemoto, M.L.; McClure, L.A.; Rosenberg, D.E. Physical activity participation among older adults: Trends and disparities. J. Aging Health 2018, 30, 1260–1275. [Google Scholar]
- Scherer, P.E. The multifaceted roles of adipose tissue—Therapeutic targets for diabetes and beyond: The 2015 Banting Lecture. Diabetes 2018, 67, 1452–1462. [Google Scholar] [CrossRef]
- Cai, L.; Li, C.; Wang, Y.; Mo, Y.; Yin, J.; Ma, X. Increased serum GDF15 related to improvement in metabolism by lifestyle intervention among young overweight and obese adults. Diabetes Metab. Syndr. Obes. 2021, 14, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Townsend, L.K.; DesOrmeaux, G.J.; Frangos, S.M.; Batchuluun, B.; Dumont, L.; Kuhre, R.E.; Ahmadi, E.; Hu, S.; Rebalka, I.A.; et al. GDF15 promotes weight loss by enhancing energy expenditure in muscle. Nature 2023, 619, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Eddy, A.C.; Trask, A.J. GDF-15 and the regulation of energy balance: Emerging roles in obesity and metabolism. J. Endocrinol. 2021, 248, R41–R55. [Google Scholar]
- Keipert, S.; Ost, M. GDF-15 as a stress-induced myokine with metabolic regulatory function. Nat. Rev. Endocrinol. 2021, 17, 592–605. [Google Scholar]
- Kleinert, M.; Clemmensen, C.; Sjøberg, K.A.; Carl, C.S.; Jeppesen, J.F.; Wojtaszewski, J.F.; Kiens, B.; Richter, E.A. Exercise increases circulating GDF15 in humans. Mol. Metab. 2018, 9, 187–191. [Google Scholar] [CrossRef]
- Tchou, I.; Margeli, A.; Tsironi, M.; Skenderi, K.; Barnet, M.; Kanaka-Gantenbein, C.; Papassotiriou, I.; Beris, P. Growth-differentiation factor-15, endoglin and NT-proBNP induction in athletes participating in an ultramarathon foot race. Biomarkers 2009, 14, 418–422. [Google Scholar] [CrossRef]
- Vinel, C.; Lukjanenko, L.; Batut, A.; Deleruyelle, S.; Pradere, J.P.; Le Gonidec, S.; Dortignac, A.; Geoffre, N.; Pereira, O.; Karaz, S.; et al. The exerkine apelin reverses age-associated sarcopenia. Nat. Med. 2018, 24, 1360–1371. [Google Scholar] [CrossRef]
- Sheibani, S.; Hanachi, P.; Refahiat, M.A. Effect of aerobic exercise on serum concentration of apelin, TNF-α and insulin in obese women. Iran. J. Basic. Med. Sci. 2012, 15, 1196–1201. [Google Scholar]
- Ji, E.; Park, S.J.; Jang, I.Y.; Baek, J.Y.; Jo, Y.; Jung, H.W.; Lee, E.; Ryu, D.; Kim, B.J. Circulating apelin levels and muscle health in older adults. J. Nutr. Health Aging 2025, 29, 12–19. [Google Scholar] [CrossRef]
- Fujie, S.; Sato, K.; Miyamoto-Mikami, E.; Hasegawa, N.; Fujita, S.; Sanada, K.; Hamaoka, T.; Iemitsu, M. Reduction of arterial stiffness by exercise training is associated with increasing plasma apelin level in middle-aged and older adults. PLoS ONE 2014, 9, e111002. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, H.J.; Park, J.S. Effects of 12-week combined exercise on IL-15 expression and metabolic indicators in elderly women. J. Exerc. Rehabil. 2020, 16, 317–324. [Google Scholar]
- Hingorjo, M.R.; Qureshi, M.A.; Mehdi, A. Serum interleukin-15 and its relationship with visceral fat and markers of obesity in adults. J. Pak. Med. Assoc. 2018, 68, 764–768. [Google Scholar]
- Tolouei Azar, J.; Shabkhiz, F.; Khalafi, M. The effects of eight weeks of resistance training on serum levels of IL-15, IL-6, TNF-α, and insulin resistance in older type 2 diabetic men. J. Sport Biosci. 2021, 12, 391–406. [Google Scholar]
- Kim, K.; Kim, H.; Yoon, M. Effects of Resistance Exercise and Fermented Soybean Consumption on Glucose Tolerance and Expressions of Immune Senescence-Related Myokines in Middle-Aged Obese Rats. J. Obes. Metab. Syndr. 2018, 27, 186–195. [Google Scholar] [CrossRef]
| Variables Group | Age (yrs) | Height (cm) | Weight (kg) | BMI (kg/m2) | %BF (%) |
|---|---|---|---|---|---|
| OG (n = 15) | 76.67 ± 4.86 | 151.07 ± 4.06 | 62.57 ± 6.41 | 27.37 ± 2.26 | 38.69 ± 4.97 |
| NG (n = 14) | 77.57 ± 6.12 | 149.61 ± 5.54 | 47.89 ± 7.10 | 21.36 ± 2.49 | 25.46 ± 4.35 |
| Variable | Graph. | Pre | Post | Diff (%) | Paired-t | F | |
|---|---|---|---|---|---|---|---|
| Weight (kg) | OG (n = 15) | 62.57 ± 6.41 | 61.43 ± 6.40 | −1.83 | 5.056 *** | Group | 33.046 *** |
| NG (n = 14) | 47.89 ± 7.10 | 47.06 ± 7.40 | −1.79 | 2.244 * | Time | 21.254 *** | |
| t-value | −5.850 *** | −5.609 *** | 0.039 | G × T | 0.504 | ||
| BMI (kg/m2) | OG (n = 15) | 27.37 ± 2.26 | 26.87 ± 2.29 | −1.83 | 5.149 *** | Group | 44.947 *** |
| NG (n = 14) | 21.36 ± 2.50 | 20.97 ± 2.58 | −1.86 | 2.368 * | Time | 22.301 *** | |
| t-value | −6.806 *** | −6.532 *** | −0.030 | G × T | 0.321 | ||
| %BF (%) | OG (n = 15) | 38.69 ± 4.97 | 36.85 ± 6.14 | −4.89 | 2.211 * | Group | 49.166 *** |
| NG (n = 14) | 25.46 ± 4.35 | 24.69 ± 4.37 | −2.95 | 1.994 | Time | 7.701 ** | |
| t-value | −7.605 *** | −6.102 *** | 0.704 | G × T | 1.314 | ||
| Variable | Group | Pre | Post | diff (%) | Paired-t | F | |
|---|---|---|---|---|---|---|---|
| GDF-15 (pg/mL) | OG (n = 15) | 1271.30 ± 375.93 | 1403.76 ± 435.79 | 132.46 | −1.485 | Group | 4.564 * |
| NG (n = 14) | 1457.16 ± 794.08 | 1259.46 ± 405.17 | −197.7 | 1.503 | Time | 0.173 | |
| t-value | 0.815 | −0.922 | −2.103 * | G × T | 4.423 * | ||
| Variable | Group | Pre | Post | diff (%) | Paired-t | F | |
|---|---|---|---|---|---|---|---|
| Apelin-12 (pg/mL) | OG (n = 15) | 251.09 ± 42.23 | 251.71 ± 45.30 | 0.62 | −0.078 | Group | 0.548 |
| NG (n = 14) | 241.18 ± 34.87 | 234.29 ± 48.83 | −6.69 | 1.220 | Time | 0.401 | |
| t-value | −0.686 | −0.996 | −0.758 | G × T | 0.575 | ||
| Variable | Group | Pre | Post | diff (%) | Paired-t | F | |
|---|---|---|---|---|---|---|---|
| IL-15 (pg/mL) | OG (n = 15) | 1.85 ± 0.91 | 1.39 ± 0.57 | −0.45 | 1.700 | Group | 1.37 |
| NG (n = 14) | 2.19 ± 0.91 | 1.68 ± 0.57 | −0.51 | 1.973 | Time | 6.70 * | |
| t-value | 1.013 | 1.352 | −0.15 | G × T | 0.02 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Bressel, E.; Kim, M.; Kim, T.; Koh, S.; Kim, D. The Effects of a Combined Exercise Intervention on Body Composition, GDF-15, Apelin-12, and IL-15 Among Older Korean Women According to Obesity Status. J. Clin. Med. 2025, 14, 4981. https://doi.org/10.3390/jcm14144981
Kim J, Bressel E, Kim M, Kim T, Koh S, Kim D. The Effects of a Combined Exercise Intervention on Body Composition, GDF-15, Apelin-12, and IL-15 Among Older Korean Women According to Obesity Status. Journal of Clinical Medicine. 2025; 14(14):4981. https://doi.org/10.3390/jcm14144981
Chicago/Turabian StyleKim, Jeongsook, Eadric Bressel, Minkyo Kim, Taekyu Kim, Suhan Koh, and Doyeon Kim. 2025. "The Effects of a Combined Exercise Intervention on Body Composition, GDF-15, Apelin-12, and IL-15 Among Older Korean Women According to Obesity Status" Journal of Clinical Medicine 14, no. 14: 4981. https://doi.org/10.3390/jcm14144981
APA StyleKim, J., Bressel, E., Kim, M., Kim, T., Koh, S., & Kim, D. (2025). The Effects of a Combined Exercise Intervention on Body Composition, GDF-15, Apelin-12, and IL-15 Among Older Korean Women According to Obesity Status. Journal of Clinical Medicine, 14(14), 4981. https://doi.org/10.3390/jcm14144981





