Impact of an Enhanced Disinfection Protocol on the Incidence of Clostridioides difficile Infections and Antibiotic Consumption in a Hospital Setting: A Retrospective Intervention Study
Abstract
1. Background
- Phase 1 tests (suspension tests) are conducted to determine whether a chemical disinfectant or antiseptic possesses bactericidal, fungicidal, yeasticidal, or sporicidal activity, regardless of its specific area of application. Phase 1 tests cannot be used to support any product-related claims.
- Phase 2/step 1 tests use quantitative suspension methods in which microorganisms are exposed to chemical disinfectants or antiseptics at various concentrations, contact times, and temperatures, often in the presence of interfering substances. These tests confirm product activity under laboratory conditions that resemble the intended use (e.g., on instruments or surfaces, with or without mechanical action, in medical settings). An example of a Phase 2/step 1 standard is EN 17126, which assesses sporicidal activity [10].
- Phase 2/step 2 tests are based on carrier methods conducted under conditions that simulate practical use. For sporicidal activity in the medical area, a carrier-based standard involving mechanical action—EN 17846 [11]—is available. This document applies to four methods of product application for wiping and/or mopping:
- (a)
- soaking any non-specified wipe or mop with the product;
- (b)
- spraying the product onto any non-specified or specified wipe and/or mop;
- (c)
- user impregnation of specified wipes or mops with the product according to the manufacturer’s instructions;
- (d)
- pre-impregnation of specified wipes or mops by the manufacturer as ready-to-use products.
2. Methodology
2.1. Study Aims
- -
- To determine the impact of the disinfection intervention on infection rates and antibiotic stewardship within the clinical settings observed;
- -
- To compare antibiotic consumption trends (in defined daily doses per 1000 patient-days) before, during, and after the implementation of the disinfection intervention;
- -
- To identify any correlations between specific antibiotics linked to an increased risk of CDI and the infection rates, in order to inform future antibiotic stewardship programs.
2.2. Study Design and Setting
2.3. Disinfection Preparations
2.4. Enhanced Disinfection Protocol
Data Collection Method and Tools
2.5. Statistical Analysis
3. Results
3.1. CDI Prevalence During Study Period
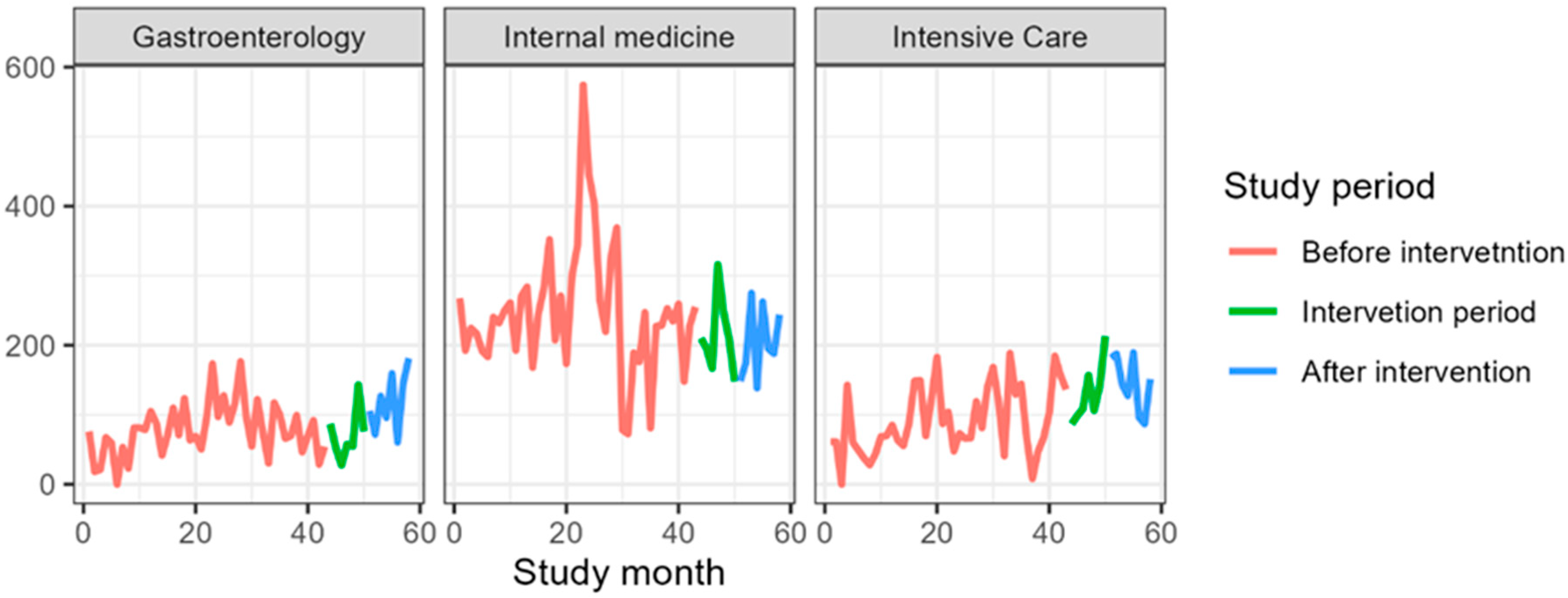
3.2. Gastroenterology
3.3. Internal Medicine Department
3.4. Department of Anesthesiology and Intensive Care
3.5. Overall Antibiotic Consumption
4. Discussion
5. Limitations and Strength of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malone, D.C.; Armstrong, E.P.; Gratie, D.; Pham, S.V.; Amin, A. A systematic review of real-world healthcare resource use and costs of Clostridioides difficile infections. Antimicrob Steward. Healthc. Epidemiol 2023, 3, e17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sammons, J.S.; Localio, R.; Xiao, R.; Coffin, S.E.; Zaoutis, T. Clostridium difficile infection is associated with increased risk of death and prolonged hospitalization in children. Clin. Infect. Dis. 2013, 57, 1–8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sierocka, A.; Kiersnowska, Z.; Lemiech-Mirowska, E.; Marczak, M. Costs Associated with the Treatment of Clostridioides difficile Infections. Int. J. Environ. Res. Public Health 2021, 18, 7647. [Google Scholar] [CrossRef] [PubMed]
- Riddle, D.J.; Dubberke, E.R. Trends in Clostridium difficile Disease: Epidemiology and Intervention. Infect. Med. 2009, 26, 211–220. [Google Scholar] [PubMed] [PubMed Central]
- Cymbal, M.; Chatterjee, A.; Baggott, B.; Auron, M. Management of Clostridioides difficile Infection: Diagnosis, Treatment, and Future Perspectives. Am. J. Med. 2024, 137, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Barker, A.K.; Alagoz, O.; Safdar, N. Interventions to reduce the incidence of hospital-onset Clostridium Difficile infection: An agent-based modeling approach to evaluate clinical effectiveness in adult acute care hospitals. Clin. Infect. Dis. 2018, 66, 1192–1203. [Google Scholar] [CrossRef]
- Carling, P.C.; Parry, M.F.; Olmstead, R. Environmental approaches to controlling Clostridioides difficile infection in healthcare settings. Antimicrob. Resist. Infect. Control 2023, 12, 94. [Google Scholar] [CrossRef]
- Bolten, A.; Schmidt, V.; Steinhauer, K. Use of the European standardization framework established by CEN/TC 216 for effective disinfection strategies in human medicine, veterinary medicine, food hygiene, industry, and domestic and institutional use—A review. GMS Hyg. Infect. Control 2022, 17, 14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tyski, S.; Bocian, E.; Laudy, A.E. Application of normative documents for determination of biocidal activity of disinfectants and antiseptics dedicated to the medical area: A narrative review. J. Hosp. Infect. 2022, 125, 75–91. [Google Scholar] [CrossRef] [PubMed]
- EN 17126; 2018 Chemical Disinfectants and Antiseptics—Quantitative Suspension Test for the Evaluation of Sporicidal Activity of Chemical Disinfectants in the Medical Area—Test Method and Requirements (Phase 2, Step 1). BSI: Sydney, Australia, 2018.
- EN 17846; 2023 Chemical Disinfectants and Antiseptics—Quantitative Test Method for the Evaluation of Sporicidal Activity Against Clostridioides difficile on Non-Porous Surfaces with Mechanical Action Employing Wipes in the Medical Area (4-Field Test)—Test Method and Requirements (Phase 2, Step 2). BSI: Sydney, Australia, 2023.
- Srinivasa, V.R.; Hariri, R.; Frank, L.R.; Kingsley, L.; Magee, E.; Pokrywka, M.; Yassin, M.H. Hospital-associated Clostridium difficile infection and reservoirs within the hospital environment. Am. J. Infect. Control 2019, 47, 780–785. [Google Scholar] [CrossRef] [PubMed]
- McDonald, L.C.; Arduino, M. Editorial commentary: Climbing the evidentiary hierarchy for environmental infection control. Clin. Infect. Dis. 2013, 56, 36–39. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rutala, W.A.; Donskey, C.J.; Weber, D.J. Disinfection and sterilization: New technologies. Am. J. Infect. Control 2023, 51, A13–A21. [Google Scholar] [CrossRef] [PubMed]
- Pandalai, S.P.; Dankovic, D.A. A Preliminary Quantitative Risk Assessment for Inhalation Exposure to Glutaraldehyde. J. Appl. Toxicol. 2025, 45, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- White, C.W.; Martin, J.G. Chlorine gas inhalation: Human clinical evidence of toxicity and experience in animal models. Proc. Am. Thorac. Soc. 2010, 7, 257–263. [Google Scholar] [CrossRef]
- Casey, M.L.; Hawley, B.; Edwards, N.; Cox-Ganser, J.M.; Cummings, K.J. Health problems and disinfectant product exposure among staff at a large multispecialty hospital. Am. J. Infect. Control 2017, 45, 1133–1138, Erratum in Am. J. Infect. Control 2018, 46, 599. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vogt, H.; Balej, J.; Bennett, J.E.; Wintzer, P.; Sheikh, S.A.; Gallone, P. Chlorine Oxides and Chlorine Oxygen Acids. In Ullmann’s Encyclopedia of Industrial Chemistry; Ley, C., Ed.; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar] [CrossRef]
- Goh, C.F.; Ming, L.C.; Wong, L.C. Dermatologic reactions to disinfectant use during the COVID-19 pandemic. Clin. Dermatol. 2021, 39, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Rhea, S.; Jones, K.; Endres-Dighe, S.; Munoz, B.; Weber, D.J.; Hilscher, R.; MacFarquhar, J.; Sickbert-Bennett, E.; DiBiase, L.; Marx, A.; et al. CDC MInD-Healthcare Network. Modeling inpatient and outpatient antibiotic stewardship interventions to reduce the burden of Clostridioides difficile infection in a regional healthcare network. PLoS ONE 2020, 15, e0234031. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Allegranzi, B.; Nejad, S.B.; Combescure, C.; Graafmans, W.; Attar, H.; Donaldson, L.; Pittet, D. Burden of endemic health-care-associated infection in developing countries: Systematic review and meta-analysis. Lancet 2011, 377, 228–241. [Google Scholar] [CrossRef]
- Zarb, P.; Coignard, B.; Griskeviciene, J.; Muller, A.; Vankerckhoven, V.; Weist, K.; Goossens, M.M.; Vaerenberg, S.; Hopkins, S.; Catry, B.; et al. The European Centre for Disease Prevention and Control (ECDC) pilot point prevalence survey of healthcare-associated infections and antimicrobial use. Eurosurveillance 2012, 17, 20316. [Google Scholar] [CrossRef]
- 3M™ Clean-Trace™Hygiene Management System, Hygiene Management Guide for Environmental Surfaces. Available online: https://multimedia.3m.com/mws/media/1840682O/3m-cleantrace-hygiene-management-system-for-environmental-surfaces.pdf (accessed on 6 July 2025).
- Tudorica Sirmon, A.; Bădițoiu, L.M.; Anghel, M.; Laitin, S.M.D.; Muntean, D.; Kemper, C.A. Adenosine Triphosphate Bioluminescence Assay versus Microbiological Swab Culture in the Evaluation of Surface Sanitation in a Pediatric Hospital in Romania. Risk Manag. Healthc. Policy 2025, 18, 1669–1681. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mulvey, D.; Redding, P.; Robertson, C.; Woodall, C.; Kingsmore, P.; Bedwell, D.; Dancer, S. Finding a benchmark for monitoring hospital cleanliness. J. Hosp. Infect. 2011, 77, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Hong, M.Y.; Kang, H.M.; Yum, S.K.; Youn, Y.A.; Lee, D.-G.; Kang, J.H. Using adenosine triphosphate bioluminescence level monitoring to identify bacterial reservoirs during two consecutive Enterococcus faecium and Staphylococcus capitis nosocomial infection outbreaks at a neonatal intensive care unit. Antimicrob. Resist. Infect. Control 2023, 12, 68. [Google Scholar] [CrossRef] [PubMed]
- Available online: www.gov.pl/web/urpl/wykaz-produktow-biobojczych2 (accessed on 6 July 2025).
- Browne, K.; Mitchell, B.G. Multimodal environmental cleaning strategies to prevent healthcare-associated infections. Antimicrob. Resist. Infect. Control 2023, 12, 83. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Available online: https://www.cdc.gov/infection-control/media/pdfs/Guidelines-Environmental-Cleaning-Checklist-2010-P.pdf (accessed on 6 July 2025).
- Wagner, A.K.; Soumerai, S.B.; Zhang, F.; Ross-Degnan, D. Segmented regression analysis of interrupted time series studies in medication use research. J. Clin. Pharm. Ther. 2002, 27, 299–309. [Google Scholar] [CrossRef]
- Sestraş, R.E.; Jäntschi, L.; Bolboacă, S.D. Poisson parameters of antimicrobial activity: A quantitative structure-activity approach. Int. J. Mol. Sci. 2012, 13, 5207–5229. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 6 July 2025).
- Sopena, N.; Freixas, N.; Bella, F.; Pérez, J.; Hornero, A.; Limon, E.; Gudiol, F.; Pujol, M. VINCat Clostridioides difficile study group. Impact of a training program on the surveillance of Clostridioides difficile infection. Epidemiol. Infect. 2019, 147, e231. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kociolek, L.K.; Gerding, D.N.; Carrico, R.; Carling, P.; Donskey, C.J.; Dumyati, G.; Kuhar, D.T.; Loo, V.G.; Maragakis, L.L.; Pogorzelska-Maziarz, M.; et al. Strategies to prevent Clostridioides difficile infections in acute-care hospitals: 2022 Update. Infect. Control Hosp. Epidemiol. 2023, 44, 527–549. [Google Scholar] [CrossRef]
- Gemein, S.; Gebel, J.; Christiansen, B.; Martiny, H.; Vossebein, L.; Brill, F.H.H.; Decius, M.; Eggers, M.; Koburger-Janssen, T.; Meckel, M.; et al. Interlaboratory reproducibility of a test method following 4-field test methodology to evaluate the susceptibility of Clostridium difficile spores. J. Hosp. Infect. 2019, 103, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Gemein, S.; Andrich, R.; Christiansen, B.; Decius, M.; Exner, M.; Hunsinger, B.; Imenova, E.; Kampf, G.; Koburger-Janssen, T.; Konrat, K.; et al. Efficacy of five ‘sporicidal’ surface disinfectants against Clostridioides difficile spores in suspension tests and 4-field tests. J. Hosp. Infect. 2022, 122, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Gemein, S. Etablierung Eines Testverfahrens zur Prüfung Sporizider Flächendesinfektionsmittel: Mit Dem Schwerpunkt Clostridium difficile Ribotyp 027. Available online: https://hdl.handle.net/20.500.11811/5062 (accessed on 6 July 2025).
- Werner, S.; Naujox, K.; Rehm, M.-E.; Brückner, E. Method for assessment of the range efficacy of presoaked single-use wipes for surface disinfection. Hyg. Med. 2018, 43, E93–E99. [Google Scholar]
- Dyer, C.; Hutt, L.P.; Burky, R.; Joshi, L.T. Biocide Resistance and Transmission of Clostridium difficile Spores Spiked onto Clinical Surfaces from an American Health Care Facility. Appl. Environ. Microbiol. 2019, 85, e01090-19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Joshi, L.T.; Phillips, D.S.; Williams, C.F.; Alyousef, A.; Baillie, L. Contribution of spores to the ability of Clostridium difficile to adhere to surfaces. Appl. Environ. Microbiol. 2012, 78, 7671–7679. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Przondo, J. Związki powierzchniowo czynne i ich zastosowanie w produktach chemii gospodarczej. Wydawnictwo Politechniki Radomskiej, Radom. 2010. Available online: https://www.scribd.com/document/483307228/Zwi%C4%85zki-powierzchniowo-czynne-i-ich-zastosowanie-w-chemii-gospodarczej-Jan-Przondo (accessed on 6 July 2025).
- Dychdala, G.R. Chlorine and Chlorine Compounds in Disinfection, Sterilisation and Preservation, 3rd ed.; Block, S.S., Ed.; Lea and Febiger: Philadelphia, PA, USA, 1983; pp. 157–182. [Google Scholar]
- Buttgen, S.; Gebel, J.; Rheinbaben, F.V.; Hornei, B.; Engelhart, S.; Exner, M. Efficacy of surface and instrument disinfectants with sporicidal claims against spores of Clostridium difficile ribotype 027. Hyg. Med. 2008, 33, 194–200. [Google Scholar]
- Commission for Hospital Hygiene and Infection Prevention (KRINKO). Hygiene requirements for cleaning and disinfection of surfaces: Recommendation of the Commission for Hospital Hygiene and Infection Prevention (KRINKO) at the Robert Koch Institute. GMS Hyg. Infect. Control 2024, 19, Doc13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brown, L.; Marshall, A.; Conway, L.; Otter, J.; Norville, P.; Clarke, J. Assessing the stability and sporicidal efficacy of oxidizing disinfectants. J. Hosp. Infect. 2024, 149, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Nkemngong, C.A.; Chaggar, G.K.; Li, X.; Teska, P.J.; Oliver, H.F. Disinfectant wipes transfer Clostridioides difficile spores from contaminated surfaces to uncontaminated surfaces during the disinfection process. Antimicrob. Resist. Infect. Control 2020, 9, 176. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lawley, T.D.; Clare, S.; Deakin, L.J.; Goulding, D.; Yen, J.L.; Raisen, C.; Brandt, C.; Lovell, J.; Cooke, F.; Clark, T.G.; et al. Use of purified Clostridium difficile spores to facilitate evaluation of health care disinfection regimens. Appl. Environ. Microbiol. 2010, 76, 6895–6900. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Freier, L.; Zacharias, N.; Gemein, S.; Gebel, J.; Engelhart, S.; Exner, M.; Mutters, N.T. Environmental Contamination and Persistence of Clostridioides difficile in Hospital Wastewater Systems. Appl. Environ. Microbiol. 2023, 89, e0001423. [Google Scholar] [CrossRef]
- EN 16615; 2015 Chemical Disinfectants and Antiseptics—Quantitative Suspension Test for the Evaluation of Sporicidal Activity of Chemical Disinfectants in the Medical Area—Test Method and Requirements (Phase 2, Step 1). BSI: Sydney, Australia, 2015.
- Available online: https://standards.cencenelec.eu/dyn/www/f?p=205:22:0::::FSP_ORG_ID,FSP_LANG_ID:6197,25&cs=17E2B30DF09AC0FA861986AA85042A41B (accessed on 6 July 2025).
- Hawes, A.M.; Desai, A.; Patel, P.K. Did Clostridioides difficile testing and infection rates change during the COVID-19 pandemic? Anaerobe 2021, 70, 102384. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Turner, N.A.; Krishnan, J.; Nelson, A.; Polage, C.R.; Sinkowitz-Cochran, R.L.; Fike, L.; Kuhar, D.T.; Kutty, P.K.; Snyder, R.L.; Anderson, D.J. CDC’s Hospital-Onset Clostridioides difficile Prevention Framework in a Regional Hospital Network. JAMA Netw. Open. 2024, 7, e243846. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Long-Term Health and Economic Effects of Antimicrobial Resistance in the United States. Stewardship and Infection Prevention. In Combating Antimicrobial Resistance and Protecting the Miracle of Modern Medicine; Palmer, G.H., Buckley, G.J., Eds.; National Academies Press: Washington, DC, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK577289/ (accessed on 6 July 2025).
- Kubde, D.; Badge, A.K.; Ugemuge, S.; Shahu, S. Importance of Hospital Infection Control. Cureus 2023, 15, e50931. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alslamah, T.; Abalkhail, A. The National Strategies for and Challenges in Infection Prevention and Control of the Healthcare System in the Kingdom of Saudi Arabia (Review Study). Vaccines 2022, 10, 1302. [Google Scholar] [CrossRef] [PubMed]



| Characteristic | 01.2019–07.2022, N = 43 1 | 08.2022–02.2023, N = 7 1 | 03.2023–10.2023, N = 8 1 | p-Value 2 |
|---|---|---|---|---|
| Number of infections | months | months | months | 0.79 |
| 0 | 27 (63%) | 5 (71%) | 5 (63%) | |
| 1 | 12 (28%) | 1 (14%) | 3 (38%) | |
| 2 | 4 (9.3%) | 1 (14%) | 0 (0%) |
| Characteristic | 01.2019–07.2022, N = 43 1 | 08.2022–02.2023, N = 7 1 | 03.2023–10.2023, N = 8 1 | p-Value 2 |
|---|---|---|---|---|
| Number of infections | months | months | months | 0.10 |
| 0 | 17 (40%) | 3 (43%) | 8 (100%) | |
| 1 | 13 (30%) | 4 (57%) | 0 (0%) | |
| 2 | 8 (19%) | 0 (0%) | 0 (0%) | |
| 3 | 1 (2.3%) | 0 (0%) | 0 (0%) | |
| 4 | 4 (9.3%) | 0 (0%) | 0 (0%) |
| Characteristic | 01.2019–07.2022, N = 43 1 | 08.2022–02.2023, N = 7 1 | 03.2023–10.2023, N = 8 1 | p-Value 2 |
|---|---|---|---|---|
| Number of infections | months | months | months | 0.77 |
| 0 | 24 (56%) | 3 (43%) | 3 (38%) | |
| 1 | 10 (23%) | 2 (29%) | 3 (38%) | |
| 2 | 5 (12%) | 2 (29%) | 2 (25%) | |
| 3 | 3 (7.0%) | 0 (0%) | 0 (0%) | |
| 4 | 1 (2.3%) | 0 (0%) | 0 (0%) |
| Characteristics | Before Intervention N = 43 | Intervention Period N = 7 | After Intervention N = 8 |
|---|---|---|---|
| Fluoroquinolones | |||
| Mean (SD) | 186.5 (57.0) | 148.6 (42.5) | 220.4 (46.7) |
| Median | 177.1 | 143.4 | 233.4 |
| IQR | [143.2, 210.5] | [130.7, 172.4] | [202.3, 254.0] |
| Min–Max | 92.3–331.0 | 81.8–208.7 | 122.9–265.9 |
| Cephalosporins | |||
| Mean (SD) | 78.0 (37.5) | 71.2 (36.8) | 118.5 (42.5) |
| Median | 73.8 | 57.6 | 116.4 |
| IQR | [54.7, 98.7] | [53.5, 81.5] | [89.9, 149.0] |
| Min–Max | 0.0–176.4 | 27.8–143.0 | 60.6–181.5 |
| Amoxicillin | |||
| Mean (SD) | 7.7 (9.3) | 6.6 (5.4) | 3.3 (7.8) |
| Median | 4.4 | 7.1 | 0.0 |
| IQR | [0.0, 13.1] | [2.1, 11.2] | [0.0, 0.9] |
| Min–Max | 0.0–33.8 | 0.0–12.6 | 0.0–22.4 |
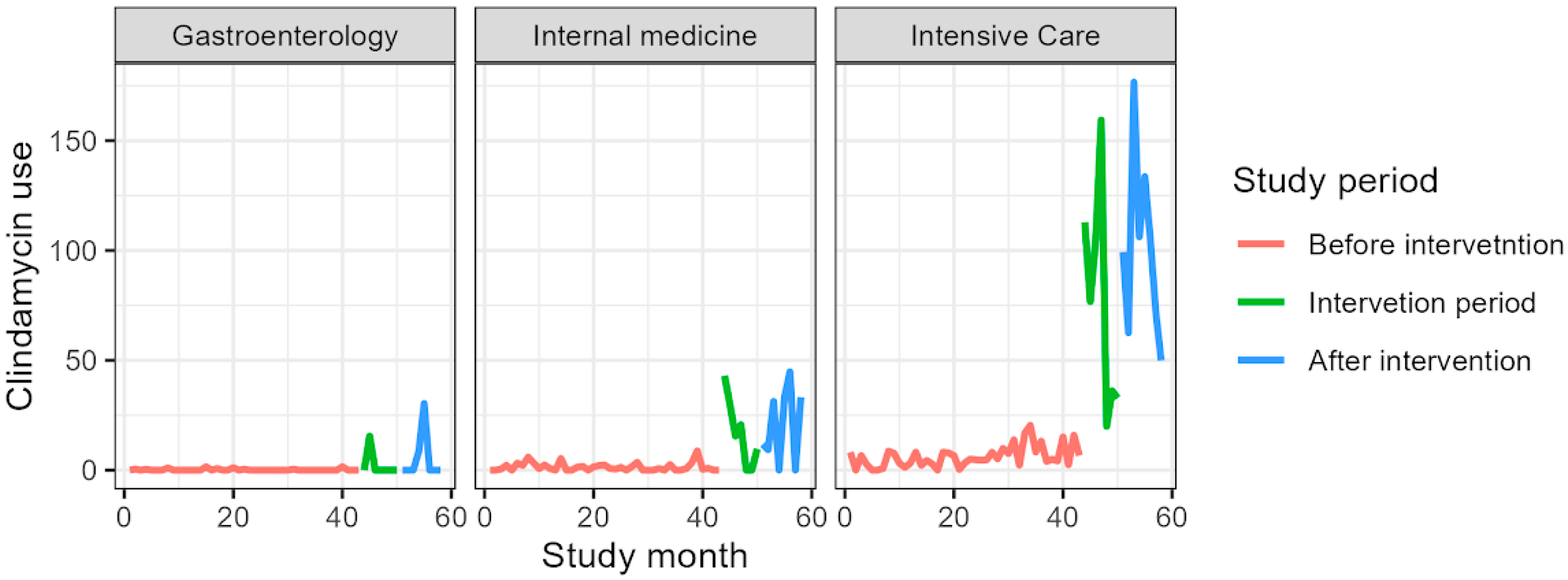
| Clindamycin | |||
| Mean (SD) | 0.2 (0.4) | 2.2 (5.8) | 4.8 (10.7) |
| Median | 0.0 | 0.0 | 0.0 |
| IQR | [0.0, 0.0] | [0.0, 0.0] | [0.0, 2.1] |
| Min–Max | 0.0–1.6 | 0.0–15.5 | 0.0–30.3 |
| Carbapenems | |||
| Mean (SD) | 4.6 (2.5) | 35.7 (28.5) | 57.9 (46.3) |
| Median | 4.2 | 26.7 | 51.0 |
| IQR | [3.1, 5.4] | [17.3, 40.9] | [32.4, 64.4] |
| Min–Max | 0.8–12.9 | 13.5–93.6 | 11.9–160.7 |
| Characteristics | Before Intervention N = 43 | Intervention Period N = 7 | After Intervention N = 8 |
|---|---|---|---|
| Fluoroquinolones | |||
| Mean (SD) | 171.4 (166.9) | 136.4 (56.2) | 123.2 (19.3) |
| Median | 140.4 | 120.8 | 124.0 |
| IQR | [117.1, 176.5] | [95.7, 178.4] | [116.3, 132.9] |
| Min–Max | 24.0–1196.1 | 69.2–216.9 | 86.0–148.9 |
| Cephalosporins | |||
| Mean (SD) | 247.3 (92.0) | 213.5 (55.6) | 203.3 (51.5) |
| Median | 240.3 | 209.8 | 191.6 |
| IQR | [192.6, 270.9] | [181.1, 229.2] | [168.4, 248.7] |
| Min–Max | 72.6–574.2 | 147.9–316.0 | 138.6–274.9 |
| Amoxicillin | |||
| Mean (SD) | 8.4 (12.8) | 9.0 (14.7) | 4.9 (8.1) |
| Median | 5.8 | 6.0 | 1.0 |
| IQR | [0.0, 11.9] | [1.2, 6.6] | [0.0, 5.4] |
| Min–Max | 0.0–62.3 | 0.0–41.6 | 0.0–22.7 |
| Clindamycin | |||
| Mean (SD) | 1.4 (1.9) | 16.9 (15.7) | 20.5 (17.2) |
| Median | 0.7 | 15.5 | 21.5 |
| IQR | [0.0, 2.2] | [4.8, 25.2] | [6.9, 33.2] |
| Min–Max | 0.0–8.8 | 0.0–43.0 | 0.0–44.8 |
| Carbapenems | |||
| Mean (SD) | 6.0 (2.3) | 59.6 (23.5) | 75.6 (24.3) |
| Median | 5.6 | 67.2 | 80.1 |
| IQR | [4.4, 6.8] | [41.1, 75.6] | [54.6, 83.3] |
| Min–Max | 2.3–12.6 | 27.0–89.2 | 45.1–121.1 |
| Characteristics | Before Intervention N = 43 | Intervention Period N = 7 | After Intervention N = 8 |
|---|---|---|---|
| Fluoroquinolones | |||
| Mean (SD) | 63.0 (41.4) | 81.1 (61.9) | 55.9 (23.6) |
| Median | 55.7 | 51.9 | 58.6 |
| IQR | [31.2, 96.4] | [42.4, 92.9] | [40.3, 73.2] |
| Min–Max | 0.0–172.0 | 36.5–208.8 | 15.9–86.7 |
| Cephalosporins | |||
| Mean (SD) | 91.4 (48.6) | 130.3 (44.0) | 145.6 (40.0) |
| Median | 73.1 | 108.7 | 147.5 |
| IQR | [60.8, 132.9] | [102.5, 149.2] | [119.7, 183.2] |
| Min–Max | 0.0–188.4 | 86.8–213.1 | 87.0–189.3 |
| Amoxicillin | |||
| Mean (SD) | 31.6 (30.0) | 18.2 (26.2) | 6.9 (13.4) |
| Median | 27.4 | 0.0 | 0.0 |
| IQR | [5.5, 44.3] | [0.0, 29.4] | [0.0, 5.3] |
| Min–Max | 0.0–126.4 | 0.0–68.3 | 0.0–36.1 |
| Clindamycin | |||
| Mean (SD) | 6.1 (4.9) | 77.5 (51.2) | 100.8 (40.9) |
| Median | 5.0 | 76.8 | 102.4 |
| IQR | [2.7, 8.2] | [34.4, 109.0] | [70.0, 113.1] |
| Min–Max | 0.0–20.4 | 19.9–159.4 | 49.9–176.6 |
| Carbapenems | |||
| Mean (SD) | 13.4 (6.8) | 159.4 (85.3) | 214.6 (57.9) |
| Median | 12.9 | 171.8 | 208.0 |
| IQR | [8.1, 16.1] | [116.8, 188.6] | [165.0, 257.8] |
| Min–Max | 2.5–35.0 | 31.9–301.4 | 144.5–301.7 |
| Beta | 95% CI 1 | p-Value | |
|---|---|---|---|
| Fluoroquinolones | |||
| Before intervention | — | — | |
| Intervention period | −18.3 | −61.9, 25.3 | 0.4 |
| After intervention | −7.2 | −48.4, 34.0 | 0.7 |
| Clindamycin | |||
| Before intervention | — | — | |
| Intervention period | 29.7 | 19.9, 39.5 | <0.001 |
| After intervention | 39.5 | 30.2, 48.7 | <0.001 |
| Cephalosporins | |||
| Before intervention | — | — | |
| Intervention period | −0.5 | −29.2, 28.1 | >0.9 |
| After intervention | 16.9 | −10.2, 44.0 | 0.2 |
| Amoxicillin | |||
| Before intervention | — | — | |
| Intervention period | −4.6 | −13.3, 4.0 | 0.3 |
| After intervention | −10.9 | −19.1, −2.7 | 0.009 |
| Carbapenems | |||
| Before intervention | — | — | |
| Intervention period | 76.9 | 60.4, 93.4 | <0.001 |
| After intervention | 108.0 | 92.4, 123.7 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarka, P.; Hreczuch, W.; Chruściel, A.; Piotrowski, M.; Olczak-Pieńkowska, A.; Warda, K.; Rabczenko, D.; Kanecki, K.; Nitsch-Osuch, A. Impact of an Enhanced Disinfection Protocol on the Incidence of Clostridioides difficile Infections and Antibiotic Consumption in a Hospital Setting: A Retrospective Intervention Study. J. Clin. Med. 2025, 14, 4904. https://doi.org/10.3390/jcm14144904
Tarka P, Hreczuch W, Chruściel A, Piotrowski M, Olczak-Pieńkowska A, Warda K, Rabczenko D, Kanecki K, Nitsch-Osuch A. Impact of an Enhanced Disinfection Protocol on the Incidence of Clostridioides difficile Infections and Antibiotic Consumption in a Hospital Setting: A Retrospective Intervention Study. Journal of Clinical Medicine. 2025; 14(14):4904. https://doi.org/10.3390/jcm14144904
Chicago/Turabian StyleTarka, Patryk, Wiesław Hreczuch, Arkadiusz Chruściel, Michał Piotrowski, Anna Olczak-Pieńkowska, Karol Warda, Daniel Rabczenko, Krzysztof Kanecki, and Aneta Nitsch-Osuch. 2025. "Impact of an Enhanced Disinfection Protocol on the Incidence of Clostridioides difficile Infections and Antibiotic Consumption in a Hospital Setting: A Retrospective Intervention Study" Journal of Clinical Medicine 14, no. 14: 4904. https://doi.org/10.3390/jcm14144904
APA StyleTarka, P., Hreczuch, W., Chruściel, A., Piotrowski, M., Olczak-Pieńkowska, A., Warda, K., Rabczenko, D., Kanecki, K., & Nitsch-Osuch, A. (2025). Impact of an Enhanced Disinfection Protocol on the Incidence of Clostridioides difficile Infections and Antibiotic Consumption in a Hospital Setting: A Retrospective Intervention Study. Journal of Clinical Medicine, 14(14), 4904. https://doi.org/10.3390/jcm14144904






