Screening and Procedural Guidance for Mitral Transcatheter Edge-to-Edge Repair (M-TEER)
Abstract
1. Introduction
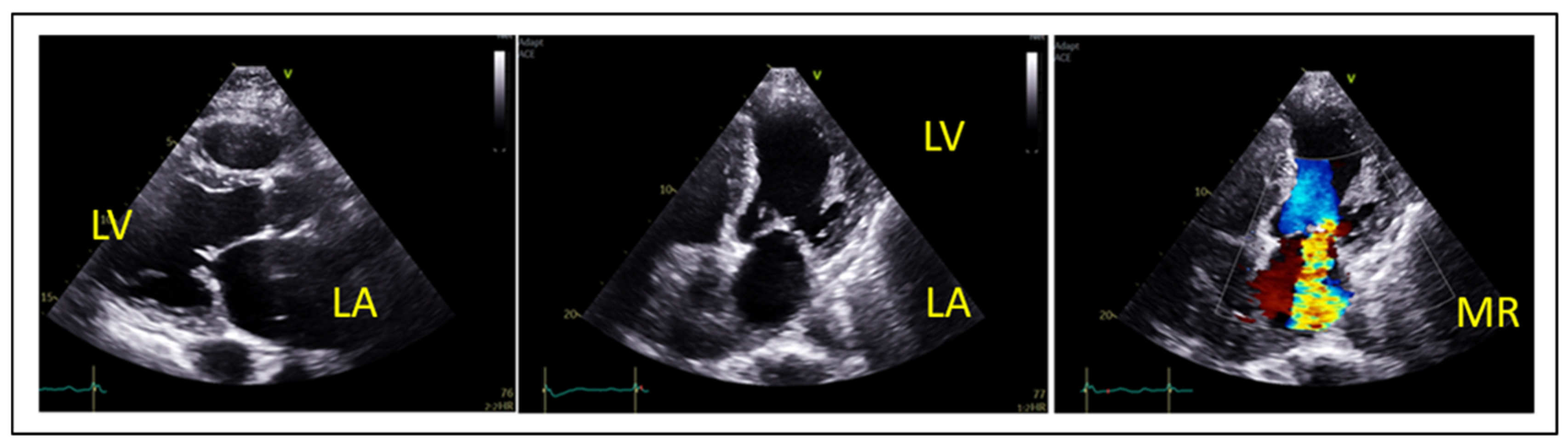
2. Echocardiographic Evaluation of MR
3. Indications for Intervention in Mitral Regurgitation
3.1. Indications for Intervention in Primary MR
3.2. Indications for Intervention in Secondary MR
4. Patient Selection and Screening Criteria
4.1. Selection in Primary Mitral Regurgitation (PMR)
4.2. Selection in Secondary Mitral Regurgitation (SMR)
5. Anatomical Considerations for TEER
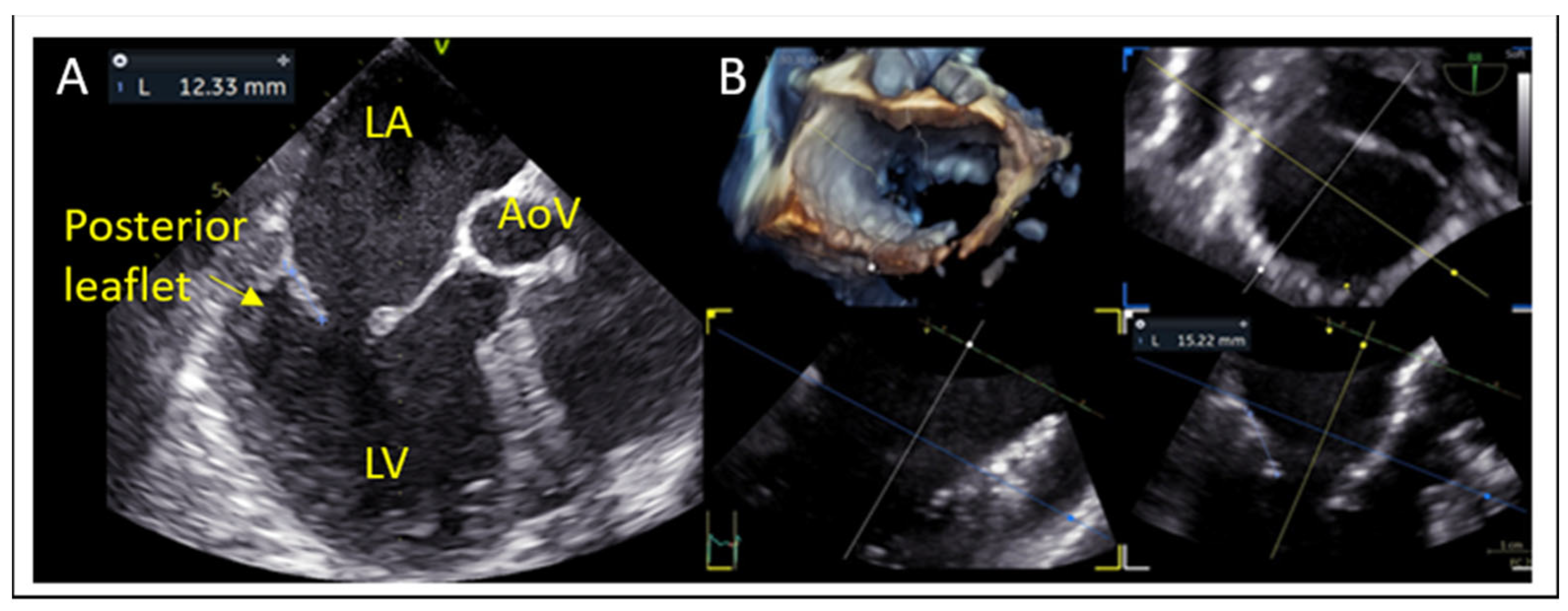
- Posterior leaflet length: A minimum of 7 mm (ideally > 10 mm) is typically required for adequate leaflet grasping.
- Flail gap and flail width: Severe primary MR may demonstrate a flail gap > 10 mm and width > 15 mm, which has traditionally limited eligibility but can be managed in experienced centers.
- Coaptation depth and coaptation length: Excessive coaptation depth (>11 mm) or reduced coaptation length (<2 mm) may pose procedural difficulty in secondary MR.
- Mitral valve area (MVA) and pressure gradient (PG): MVA < 4.0 cm2 may raise concern for post-procedural mitral stenosis, especially in patients requiring multiple devices. Cut-off values of 3.0 cm2 for MVA and 4 mmHg for mean PG are used to consider a patient ineligible for this method.
- Mitral annular and leaflet calcification: These may hinder adequate device deployment and leaflet grasping.
- Mitral annulus dimensions: Small dimensions of annulus (annulus area, anterior–posterior and medial-lateral diameters) should also be considered in the screening process.
6. Implications for Clinical Practice
7. Preprocedural Echocardiographic Assessment
7.1. Mitral Valve Anatomy
7.2. Posterior Leaflet Length
7.3. Mitral Valve Area and Gradient
7.4. Flail Gap and Width (PMR)
7.5. Tenting Height and Coaptation Length (SMR)
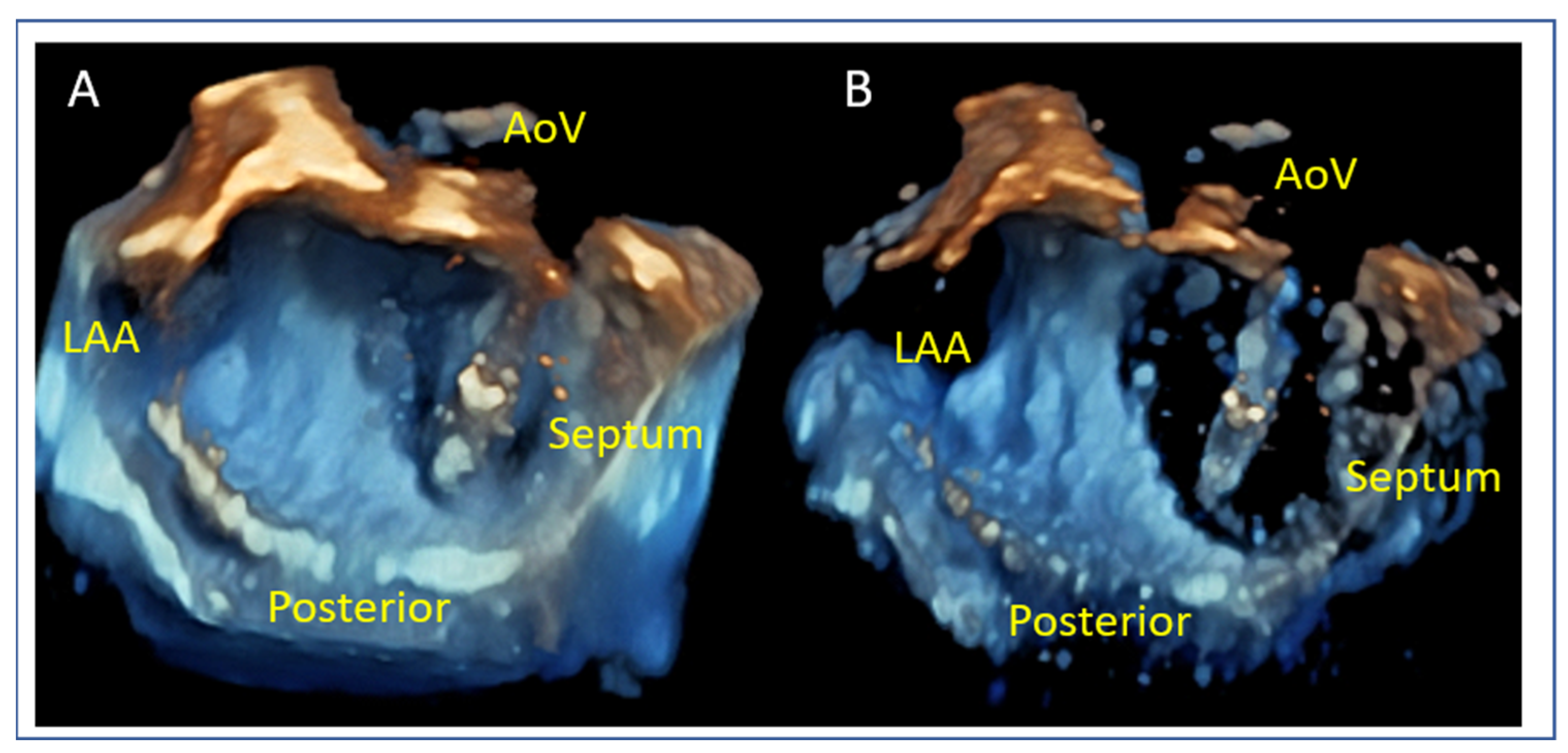
8. Anatomical Challenges
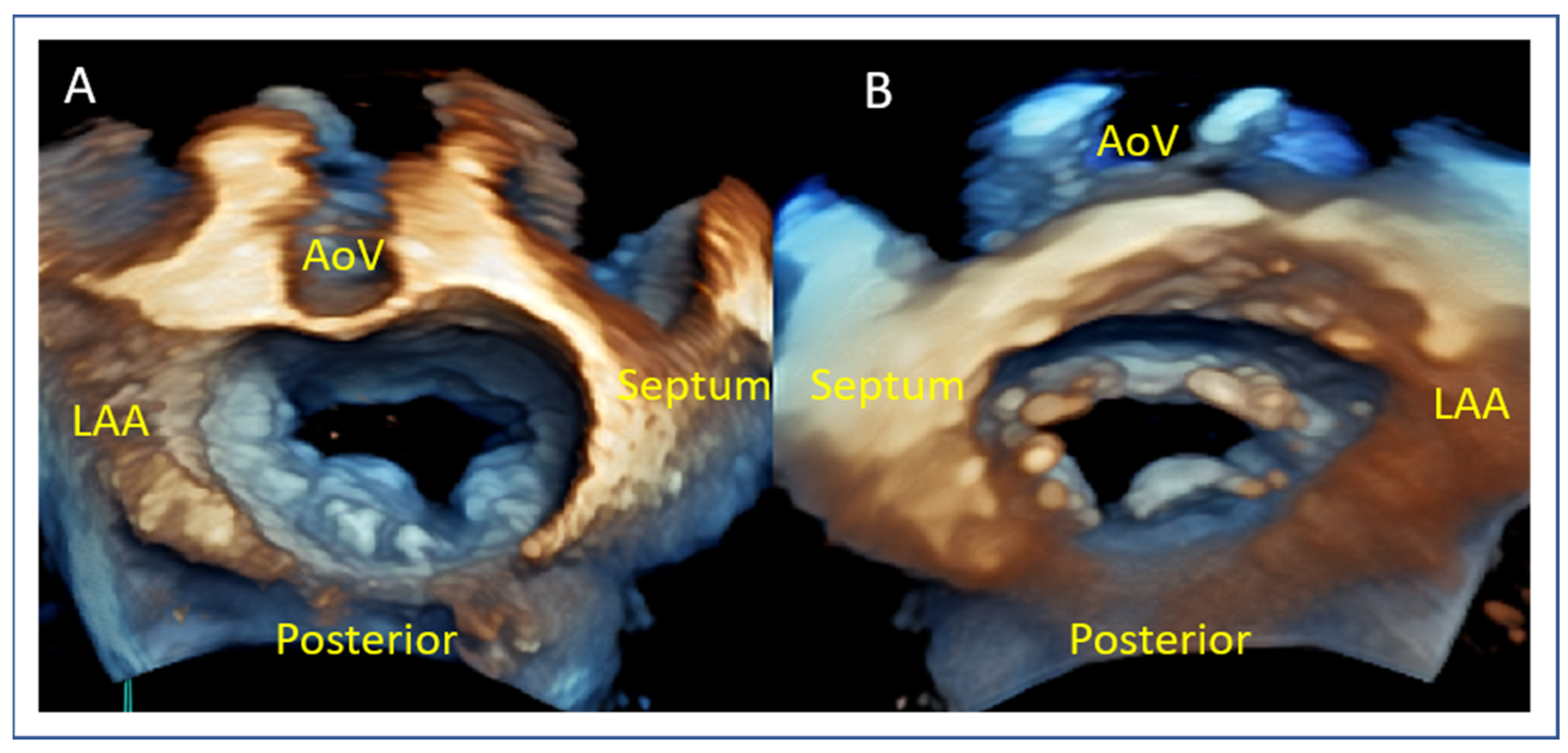
- Posterior Leaflet Cleft-like Indentation
- The posterior leaflet typically has two indentations that differentiate the scallops. A cleft-like indentation is defined as having a depth of at least 50% of the adjacent scallops [43,44] and 3D imaging is the best option to recognize such abnormalities (Figure 9). This feature makes grasping challenging and may lead to residual mitral regurgitation (MR).
- Leaflet and Annular Calcification
- Adequate but Tethered Leaflets
- Posterior leaflet length may be sufficient, but severe tethering reduces coaptation and grasping success (Figure 11).
9. Intraprocedural Guidance
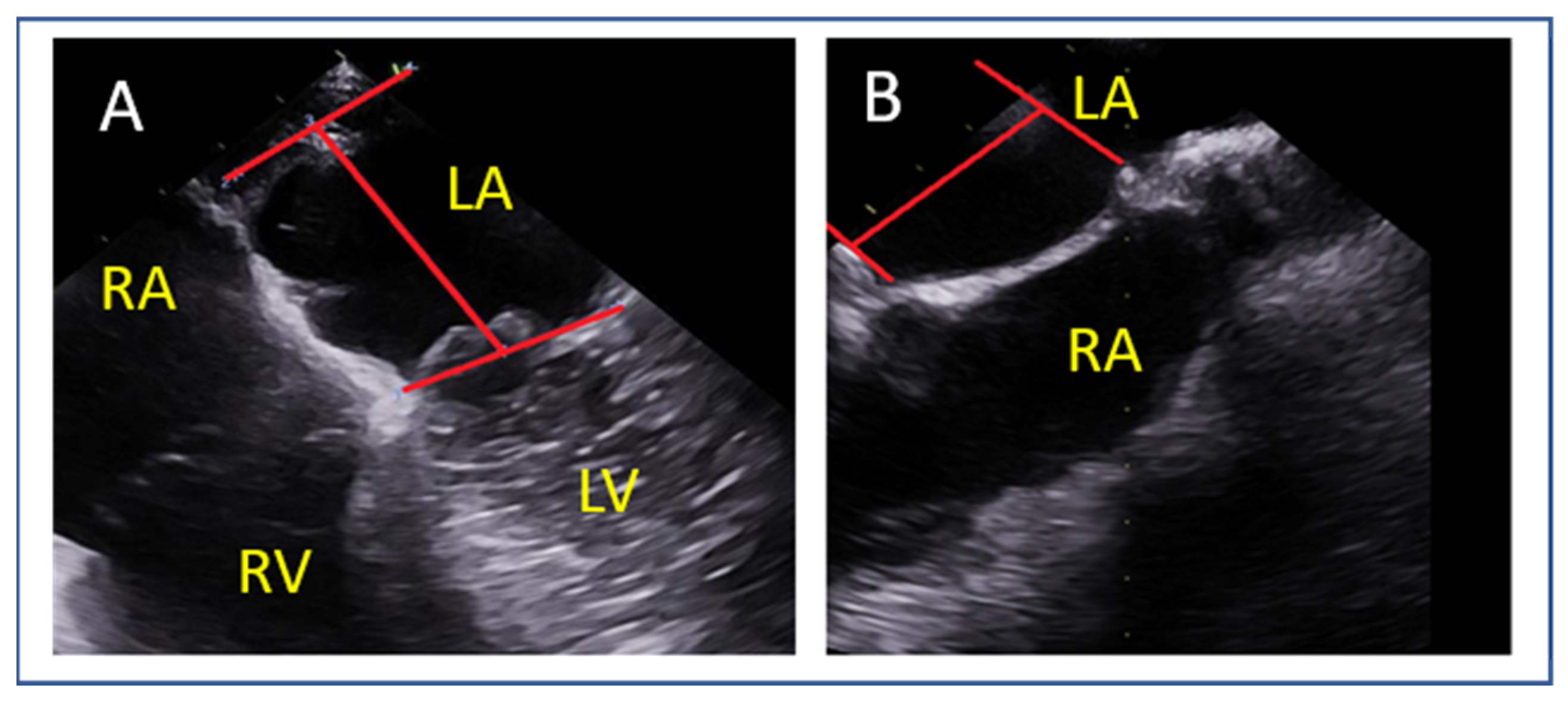
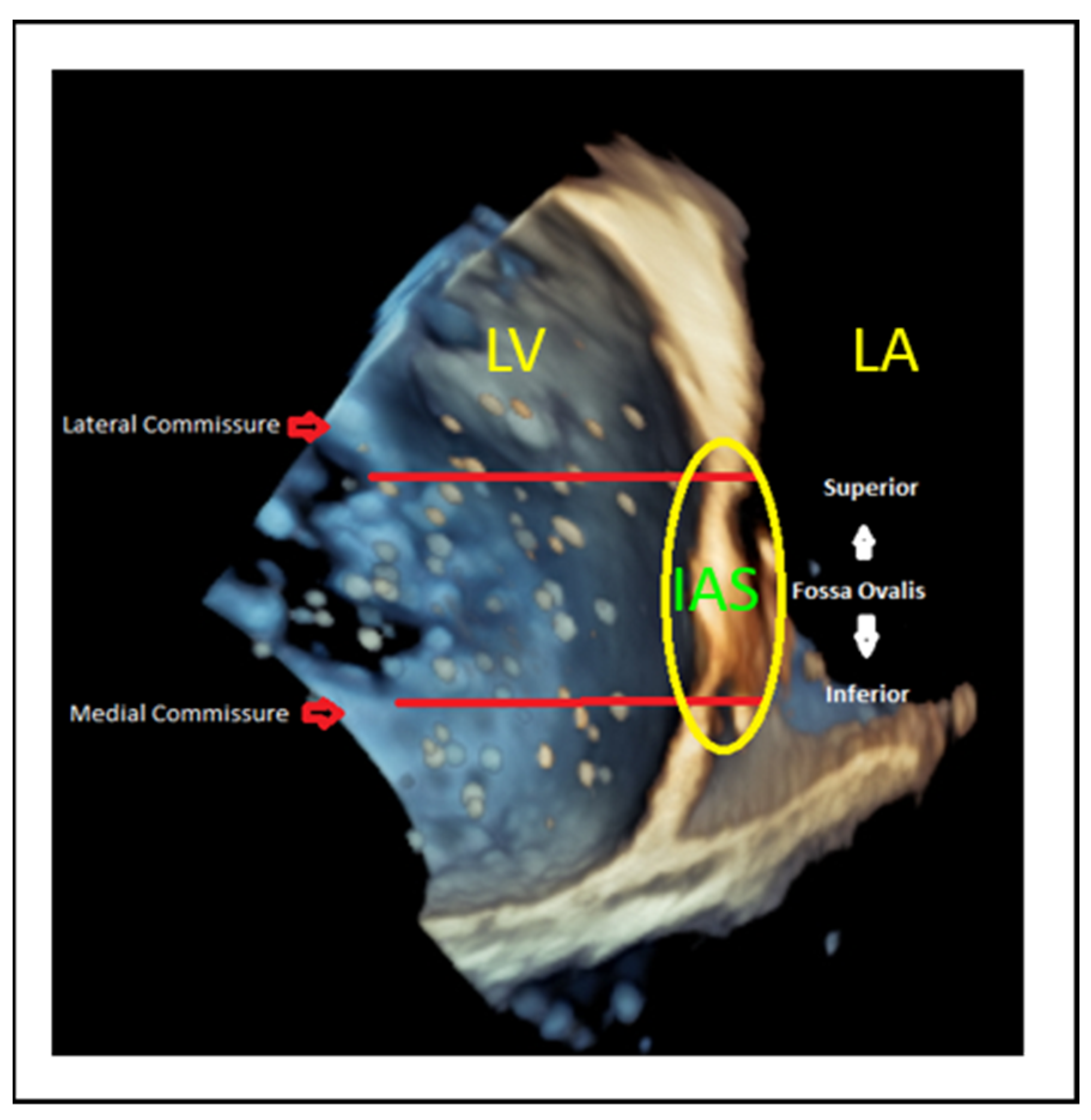
9.1. Transseptal Puncture
- Lateral commissure: A superior and lower puncture height, approximately 3.5 cm, is preferred to facilitate access [46].
- Medial commissure: A more inferior puncture, closer to the inferior vena cava (IVC), with a higher height of 4.5–5 cm is recommended for better alignment [47].
- Ventricular functional MR: The puncture height should be set 1 cm lower than the usual height to match the coaptation depth [46].
9.2. Navigating the Device
9.3. Device Alignment and Implantation
9.4. Leaflet Grasping
9.5. Device Deployment and Release
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFMR | Atrial functional MR |
| BTT | Bridge-to-transplant |
| CRT | Cardiac resynchronization therapy |
| DMR | Degenerative mitral regurgitation |
| EROA | Effective regurgitant orifice area |
| GDMT | Guideline-directed medical therapy |
| HF | Heart failure |
| IVC | Inferior vena cava |
| LA | Left atrium |
| LAVI | Left atrial volume index |
| LV | Left ventricle |
| LVAD | Left ventricular assist device |
| LVEDV | Left ventricular end-diastolic volume |
| LVEF | Left ventricular ejection fraction |
| LVESD | Left ventricular end-systolic diameter |
| MPR | Multiplanar reconstruction |
| MR | Mitral regurgitation |
| M-TEER | Mitral transcatheter edge-to-edge repair |
| MV | Mitral valve |
| MVA | Mitral valve area |
| NYHA | New York Heart Association Class |
| PCI | Percutaneous coronary intervention |
| PMR | Primary mitral regurgitation |
| RF | Regurgitant fraction |
| RVol | Regurgitant volume |
| SMR | Secondary mitral regurgitation |
| SPAP | Systolic pulmonary artery pressure |
| TAVI | Transcatheter aortic valve implantation |
| TEE/TOE | Transesophageal echocardiography |
| TR | Tricuspid regurgitation |
| VC | Vena contracta |
References
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Iung, B.; Baron, G.; Butchart, E.G.; Delahaye, F.; Gohlke-Bärwolf, C.; Levang, O.W.; Tornos, P.; Vanoverschelde, J.-L.; Vermeer, F.; Boersma, E.; et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur. Heart J. 2003, 24, 1231–1243. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi William, A.; Levine Robert, A.; Flachskampf, F.; Grayburn, P.; Gillam, L.; Leipsic, J.; Thomas James, D.; Kwong Raymond, Y.; Vandervoort, P.; Chandrashekhar, Y. Atrial Functional Mitral Regurgitation. JACC Cardiovasc. Imaging 2022, 15, 1870–1882. [Google Scholar] [CrossRef]
- Iliakis, P.; Dimitriadis, K.; Pyrpyris, N.; Beneki, E.; Theofilis, P.; Tsioufis, P.; Kamperidis, V.; Aznaouridis, K.; Aggeli, K.; Tsioufis, K. Atrial Functional Mitral Regurgitation: From Diagnosis to Current Interventional Therapies. J. Clin. Med. 2024, 13, 5035. [Google Scholar] [CrossRef] [PubMed]
- Prakash, R.; Horsfall, M.; Markwick, A.; Pumar, M.; Lee, L.; Sinhal, A.; Joseph, M.X.; Chew, D.P. Prognostic impact of moderate or severe mitral regurgitation (MR) irrespective of concomitant comorbidities: A retrospective matched cohort study. BMJ Open 2014, 4, e004984. [Google Scholar] [CrossRef] [PubMed]
- Enriquez-Sarano, M.; Akins, C.W.; Vahanian, A. Mitral regurgitation. Lancet 2009, 373, 1382–1394. [Google Scholar] [CrossRef]
- Sannino, A.; Smith, R.L., 2nd; Schiattarella, G.G.; Trimarco, B.; Esposito, G.; Grayburn, P.A. Survival and Cardiovascular Outcomes of Patients with Secondary Mitral Regurgitation: A Systematic Review and Meta-analysis. JAMA Cardiol. 2017, 2, 1130–1139. [Google Scholar] [CrossRef]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef]
- Papadopoulos, K.; Ikonomidis, I.; Vannan, M.A. The added value of three-dimensional transthoracic echocardiography in mitral annular disjunction: A case report. Front. Cardiovasc. Med. 2024, 11, 1366444. [Google Scholar] [CrossRef]
- Grayburn, P.A. How to measure severity of mitral regurgitation: Valvular heart disease. Postgrad. Med. J. 2008, 94, 376–383. [Google Scholar] [CrossRef]
- Lancellotti, P.; Pibarot, P.; Chambers, J.; La Canna, G.; Pepi, M.; Dulgheru, R.; Dweck, M.; Delgado, V.; Garbi, M.; Vannan, M.A.; et al. Multi-modality imaging assessment of native valvular regurgitation: An EACVI and ESC council of valvular heart disease position paper. Eur. Heart J. Cardiovasc. Imaging 2022, 23, e171–e232. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 43, 561–632. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 77, e25–e197. [Google Scholar] [CrossRef] [PubMed]
- Enriquez-Sarano, M.; Avierinos, J.F.; Messika-Zeitoun, D.; Detaint, D.; Capps, M.; Nkomo, V.; Scott, C.; Schaff, H.V.; Tajik, A.J. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N. Engl. J. Med. 2005, 352, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Bartko, P.E.; Arfsten, H.; Heitzinger, G.; Pavo, N.; Toma, A.; Strunk, G.; Hengstenberg, C.; Hülsmann, M.; Goliasch, G. A Unifying Concept for the Quantitative Assessment of Secondary Mitral Regurgitation. J. Am. Coll. Cardiol. 2019, 73, 2506–2517. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, P.B.; Schwammenthal, E.; Levine, R.A.; Vandervoort, P.M. Exercise Dynamics in Secondary Mitral Regurgitation: Pathophysiology and Therapeutic Implications. Circulation 2017, 135, 297–314. [Google Scholar] [CrossRef]
- Onishi, H.; Izumo, M.; Naganuma, T.; Nakamura, S.; Akashi, Y.J. Dynamic Secondary Mitral Regurgitation: Current Evidence and Challenges for the Future. Front. Cardiovasc. Med. 2022, 9, 883450. [Google Scholar] [CrossRef]
- Muneretto, C.; D’Alonzo, M.; Baudo, M.; Como, L.; Segala, A.; Zanin, F.; Rosati, F.; Benussi, S.; Di Bacco, L. Outcomes of patients with mitral annular disjunction undergoing mitral valve repair†. Eur. J. Cardiothorac. Surg. 2025, 67, ezaf029. [Google Scholar] [CrossRef]
- Goldstein, D.; Moskowitz, A.J.; Gelijns, A.C.; Ailawadi, G.; Parides, M.K.; Perrault, L.P.; Hung, J.W.; Voisine, P.; Dagenais, F.; Gillinov, A.M.; et al. Two-Year Outcomes of Surgical Treatment of Severe Ischemic Mitral Regurgitation. N. Engl. J. Med. 2016, 374, 344–353. [Google Scholar] [CrossRef]
- Baldus, S.; Doenst, T.; Pfister, R.; Gummert, J.; Kessler, M.; Boekstegers, P.; Lubos, E.; Schröder, J.; Thiele, H.; Walther, T.; et al. Transcatheter Repair versus Mitral-Valve Surgery for Secondary Mitral Regurgitation. N. Engl. J. Med. 2024, 391, 1787–1798. [Google Scholar] [CrossRef]
- Tanaka, T.; Sugiura, A.; Öztürk, C.; Vogelhuber, J.; Tabata, N.; Wilde, N.; Zimmer, S.; Nickenig, G.; Weber, M. Transcatheter Edge-to-Edge Repair for Atrial Secondary Mitral Regurgitation. JACC Cardiovasc. Interv. 2022, 15, 1731–1740. [Google Scholar] [CrossRef]
- von Stein, P.; Stolz, L.; Haurand Jean, M.; Gröger, M.; Rudolph, F.; Mustafa, D.; Jobst, J.; Mues Christoph, A.; Mahabadi Amir, A.; Hoerbrand Isabel, A.; et al. Transcatheter Edge-to-Edge Repair for Atrial and Ventricular Secondary Mitral Regurgitation. JACC Cardiovasc. Interv. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Lindenfeld, J.; Abraham, W.T.; Kar, S.; Lim, D.S.; Mishell, J.M.; Whisenant, B.; Grayburn, P.A.; Rinaldi, M.; Kapadia, S.R.; et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N. Engl. J. Med. 2018, 379, 2307–2318. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Friede, T.; von Bardeleben, R.S.; Butler, J.; Shahzeb Khan, M.; Diek, M.; Heinrich, J.; Geyer, M.; Placzek, M.; Ferrari, R.; et al. Hospitalization of Symptomatic Patients with Heart Failure and Moderate to Severe Functional Mitral Regurgitation Treated with MitraClip: Insights from RESHAPE-HF2. J. Am. Coll. Cardiol. 2024, 84, 2347–2363. [Google Scholar] [CrossRef]
- Obadia, J.-F.; Messika-Zeitoun, D.; Leurent, G.; Iung, B.; Bonnet, G.; Piriou, N.; Lefèvre, T.; Piot, C.; Rouleau, F.; Carrié, D.; et al. Percutaneous Repair or Medical Treatment for Secondary Mitral Regurgitation. N. Engl. J. Med. 2018, 379, 2297–2306. [Google Scholar] [CrossRef] [PubMed]
- Marut, B.; Lee Frost, C.; Istratoaie, S.; Leurent, G.; L’official, G.; Auffret, V.; Oger, E.; Donal, E. Reverse remodeling according to the aetiology after mitral TEER. Eur. Heart J. Cardiovasc. Imaging 2025, 26 (Suppl. 1), jeae333.146. [Google Scholar] [CrossRef]
- Feldman, T.; Foster, E.; Glower, D.D.; Kar, S.; Rinaldi, M.J.; Fail, P.S.; Smalling, R.W.; Siegel, R.; Rose, G.A.; Engeron, E.; et al. Percutaneous repair or surgery for mitral regurgitation. N. Engl. J. Med. 2011, 364, 1395–1406. [Google Scholar] [CrossRef]
- Grayburn, P.A.; Sannino, A.; Packer, M. Proportionate and Disproportionate Functional Mitral Regurgitation: A New Conceptual Framework That Reconciles the Results of the MITRA-FR and COAPT Trials. JACC Cardiovasc. Imaging 2019, 12, 353–362. [Google Scholar] [CrossRef]
- Namazi, F.; van der Bijl, P.; Fortuni, F.; Mertens, B.J.A.; Kamperidis, V.; van Wijngaarden, S.E.; Stone, G.W.; Narula, J.; Ajmone Marsan, N.; Vahanian, A.; et al. Regurgitant Volume/Left Ventricular End-Diastolic Volume Ratio: Prognostic Value in Patients with Secondary Mitral Regurgitation. JACC Cardiovasc. Imaging 2021, 14, 730–739. [Google Scholar] [CrossRef]
- Berrill, M.; Beeton, I.; Fluck, D.; John, I.; Lazariashvili, O.; Stewart, J.; Ashcroft, E.; Belsey, J.; Sharma, P.; Baltabaeva, A. Disproportionate Mitral Regurgitation Determines Survival in Acute Heart Failure. Front. Cardiovasc. Med. 2021, 8, 742224. [Google Scholar] [CrossRef]
- Anker, S.D.; Friede, T.; von Bardeleben, R.S.; Butler, J.; Khan, M.S.; Diek, M.; Heinrich, J.; Geyer, M.; Placzek, M.; Ferrari, R.; et al. Transcatheter Valve Repair in Heart Failure with Moderate to Severe Mitral Regurgitation. N. Engl. J. Med. 2024, 391, 1799–1809. [Google Scholar] [CrossRef] [PubMed]
- Asgar, A.W.; Tang, G.H.L.; Rogers, J.H.; Rottbauer, W.; Morse, M.A.; Denti, P.; Mahoney, P.; Rinaldi, M.J.; Asch, F.M.; Zamorano, J.L.; et al. Evaluating Mitral TEER in the Management of Moderate Secondary Mitral Regurgitation Among Heart Failure Patients. JACC Heart Fail. 2025, 13, 213–225. [Google Scholar] [CrossRef]
- Giannini, C.; Petronio, A.S.; De Carlo, M.; Guarracino, F.; Conte, L.; Fiorelli, F.; Pieroni, A.; Di Bello, V. Integrated reverse left and right ventricular remodelling after MitraClip implantation in functional mitral regurgitation: An echocardiographic study. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Scandura, S.; Ussia, G.P.; Capranzano, P.; Caggegi, A.; Sarkar, K.; Cammalleri, V.; Mangiafico, S.; Chiarandà, M.; Immè, S.; Di Pasqua, F.; et al. Left Cardiac Chambers Reverse Remodeling after Percutaneous Mitral Valve Repair with the MitraClip System. J. Am. Soc. Echocardiogr. 2012, 25, 1099–1105. [Google Scholar] [CrossRef]
- Papadopoulos, K.; Ikonomidis, I.; Chrissoheris, M.; Chalapas, A.; Kourkoveli, P.; Parissis, J.; Spargias, K. MitraClip and left ventricular reverse remodelling: A strain imaging study. ESC Heart Fail. 2020, 7, 1409–1418. [Google Scholar] [CrossRef]
- Citro, R.; Baldi, C.; Lancellotti, P.; Silverio, A.; Provenza, G.; Bellino, M.; Di Muro, M.R.; Mastrogiovanni, G.; De Rosa, R.; Galasso, G.; et al. Global longitudinal strain predicts outcome after MitraClip implantation for secondary mitral regurgitation. J. Cardiovasc. Med. 2017, 18, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Godino, C.; Munafò, A.; Scotti, A.; Estévez-Loureiro, R.; Portolés Hernández, A.; Arzamendi, D.; Fernández Peregrina, E.; Taramasso, M.; Fam, N.P.; Ho, E.C.; et al. MitraClip in secondary mitral regurgitation as a bridge to heart transplantation: 1-year outcomes from the International MitraBridge Registry. J. Heart Lung Transpl. 2020, 39, 1353–1362. [Google Scholar] [CrossRef]
- Jung, R.G.; Simard, T.; Kovach, C.; Flint, K.; Don, C.; Di Santo, P.; Adamo, M.; Branca, L.; Valentini, F.; Benito-González, T.; et al. Transcatheter Mitral Valve Repair in Cardiogenic Shock and Mitral Regurgitation: A Patient-Level, Multicenter Analysis. JACC Cardiovasc. Interv. 2021, 14, 1–11. [Google Scholar] [CrossRef]
- Hausleiter, J.; Stocker, T.J.; Adamo, M.; Karam, N.; Swaans, M.J.; Praz, F. Mitral valve transcatheter edge-to-edge repair. EuroIntervention 2023, 18, 957–976. [Google Scholar] [CrossRef]
- Gavazzoni, M.; Taramasso, M.; Zuber, M.; Russo, G.; Pozzoli, A.; Miura, M.; Maisano, F. Conceiving MitraClip as a tool: Percutaneous edge-to-edge repair in complex mitral valve anatomies. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 1059–1067. [Google Scholar] [CrossRef]
- Boekstegers, P.; Hausleiter, J.; Baldus, S.; von Bardeleben, R.S.; Beucher, H.; Butter, C.; Franzen, O.; Hoffmann, R.; Ince, H.; Kuck, K.H.; et al. Percutaneous interventional mitral regurgitation treatment using the Mitra-Clip system. Clin. Res. Cardiol. 2014, 103, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Adamo, M.; Chiari, E.; Curello, S.; Maiandi, C.; Chizzola, G.; Fiorina, C.; Frontini, M.; Cuminetti, G.; Pezzotti, E.; Rovetta, R.; et al. Mitraclip therapy in patients with functional mitral regurgitation and missing leaflet coaptation: Is it still an exclusion criterion? Eur. J. Heart Fail. 2016, 18, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Ring, L.; Rana, B.S.; Ho, S.Y.; Wells, F.C. The prevalence and impact of deep clefts in the mitral leaflets in mitral valve prolapse. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 595–602. [Google Scholar] [CrossRef]
- La Canna, G.; Arendar, I.; Maisano, F.; Monaco, F.; Collu, E.; Benussi, S.; De Bonis, M.; Castiglioni, A.; Alfieri, O. Real-time three-dimensional transesophageal echocardiography for assessment of mitral valve functional anatomy in patients with prolapse-related regurgitation. Am. J. Cardiol. 2011, 107, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Praz, F.; Braun, D.; Unterhuber, M.; Spirito, A.; Orban, M.; Brugger, N.; Brinkmann, I.; Spring, K.; Moschovitis, A.; Nabauer, M.; et al. Edge-to-Edge Mitral Valve Repair with Extended Clip Arms: Early Experience from a Multicenter Observational Study. JACC Cardiovasc. Interv. 2019, 12, 1356–1365. [Google Scholar] [CrossRef]
- Radinovic, A.; Mazzone, P.; Landoni, G.; Agricola, E.; Regazzoli, D.; Della-Bella, P. Different transseptal puncture for different procedures: Optimization of left atrial catheterization guided by transesophageal echocardiography. Ann. Card. Anaesth. 2016, 19, 589–593. [Google Scholar] [CrossRef]
- Harb, S.C.; Cohen, J.A.; Krishnaswamy, A.; Kapadia, S.R.; Miyasaka, R.L. Targeting the Future: Three-Dimensional Imaging for Precise Guidance of the Transseptal Puncture. Struct. Heart 2025, 9, 100340. [Google Scholar] [CrossRef]
- Stone, G.W.; Adams, D.H.; Abraham, W.T.; Kappetein, A.P.; Généreux, P.; Vranckx, P.; Mehran, R.; Kuck, K.H.; Leon, M.B.; Piazza, N.; et al. Clinical Trial Design Principles and Endpoint Definitions for Transcatheter Mitral Valve Repair and Replacement: Part 2: Endpoint Definitions: A Consensus Document from the Mitral Valve Academic Research Consortium. J. Am. Coll. Cardiol. 2015, 66, 308–321. [Google Scholar] [CrossRef]
- El Shaer, A.; Chavez Ponce, A.A.; Ali, M.T.; Oguz, D.; Pislaru, S.V.; Nkomo, V.T.; Padang, R.; Eleid, M.F.; Guerrero, M.; Reeder, G.S.; et al. Pulmonary Vein Flow Morphology After Transcatheter Mitral Valve Edge-to-Edge Repair as Predictor of Survival. J. Am. Soc. Echocardiogr. 2024, 37, 530–537. [Google Scholar] [CrossRef]
- Sato, H.; Cavalcante, J.L.; Enriquez-Sarano, M.; Bae, R.; Fukui, M.; Bapat, V.N.; Sorajja, P. Significance of Spontaneous Echocardiographic Contrast in Transcatheter Edge-to-Edge Repair for Mitral Regurgitation. J. Am. Soc. Echocardiogr. 2023, 36, 87–95. [Google Scholar] [CrossRef]
- Maisano, F.; Franzen, O.; Baldus, S.; Schäfer, U.; Hausleiter, J.; Butter, C.; Ussia, G.P.; Sievert, H.; Richardt, G.; Widder, J.D.; et al. Percutaneous mitral valve interventions in the real world: Early and 1-year results from the ACCESS-EU, a prospective, multicenter, nonrandomized post-approval study of the MitraClip therapy in Europe. J. Am. Coll. Cardiol. 2013, 62, 1052–1061. [Google Scholar] [CrossRef] [PubMed]















| COAPT-Eligible Characteristics | COAPT-Ineligible Characteristics |
|---|---|
|
|
| Optimal | Challenging | Unsuitable |
|---|---|---|
| A2/P2 pathology | Commissural (A1/P1, A3/P3) | MVA < 3.0 cm2 |
| MVA > 4 cm2 | MVA > 3.0 cm2 | Posterior leaflet length <7 mm and cleft |
| Posterior leaflet lenght > 10 mm | Posterior leaflet length 7–10 mm or cleft | Calcification in grasping zone |
| No calcification | No calcification in grasping zone, annulus calcification | Rheumatic mitral valve disease |
| DMR criteria: flail gap < 10 mm, flail width < 15 mm | DMR criteria: flail width > 15 mm | Multiple segments, Barlow |
| FMR criteria: tenting height < 10 mm | FMR criteria: Tenting height > 10 mm | |
| Normal leaflets and mobility | Carpentier IIIB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zygouri, A.; Rasmeehirun, P.; L’Official, G.; Papadopoulos, K.; Ikonomidis, I.; Donal, E. Screening and Procedural Guidance for Mitral Transcatheter Edge-to-Edge Repair (M-TEER). J. Clin. Med. 2025, 14, 4902. https://doi.org/10.3390/jcm14144902
Zygouri A, Rasmeehirun P, L’Official G, Papadopoulos K, Ikonomidis I, Donal E. Screening and Procedural Guidance for Mitral Transcatheter Edge-to-Edge Repair (M-TEER). Journal of Clinical Medicine. 2025; 14(14):4902. https://doi.org/10.3390/jcm14144902
Chicago/Turabian StyleZygouri, Andromahi, Prayuth Rasmeehirun, Guillaume L’Official, Konstantinos Papadopoulos, Ignatios Ikonomidis, and Erwan Donal. 2025. "Screening and Procedural Guidance for Mitral Transcatheter Edge-to-Edge Repair (M-TEER)" Journal of Clinical Medicine 14, no. 14: 4902. https://doi.org/10.3390/jcm14144902
APA StyleZygouri, A., Rasmeehirun, P., L’Official, G., Papadopoulos, K., Ikonomidis, I., & Donal, E. (2025). Screening and Procedural Guidance for Mitral Transcatheter Edge-to-Edge Repair (M-TEER). Journal of Clinical Medicine, 14(14), 4902. https://doi.org/10.3390/jcm14144902








