Management of Clamshell Fractures in Total Hip Arthroplasty: A Rarely Recognized Periprosthetic Injury Pattern
Abstract
1. Introduction
2. Epidemiology and Risk Factors
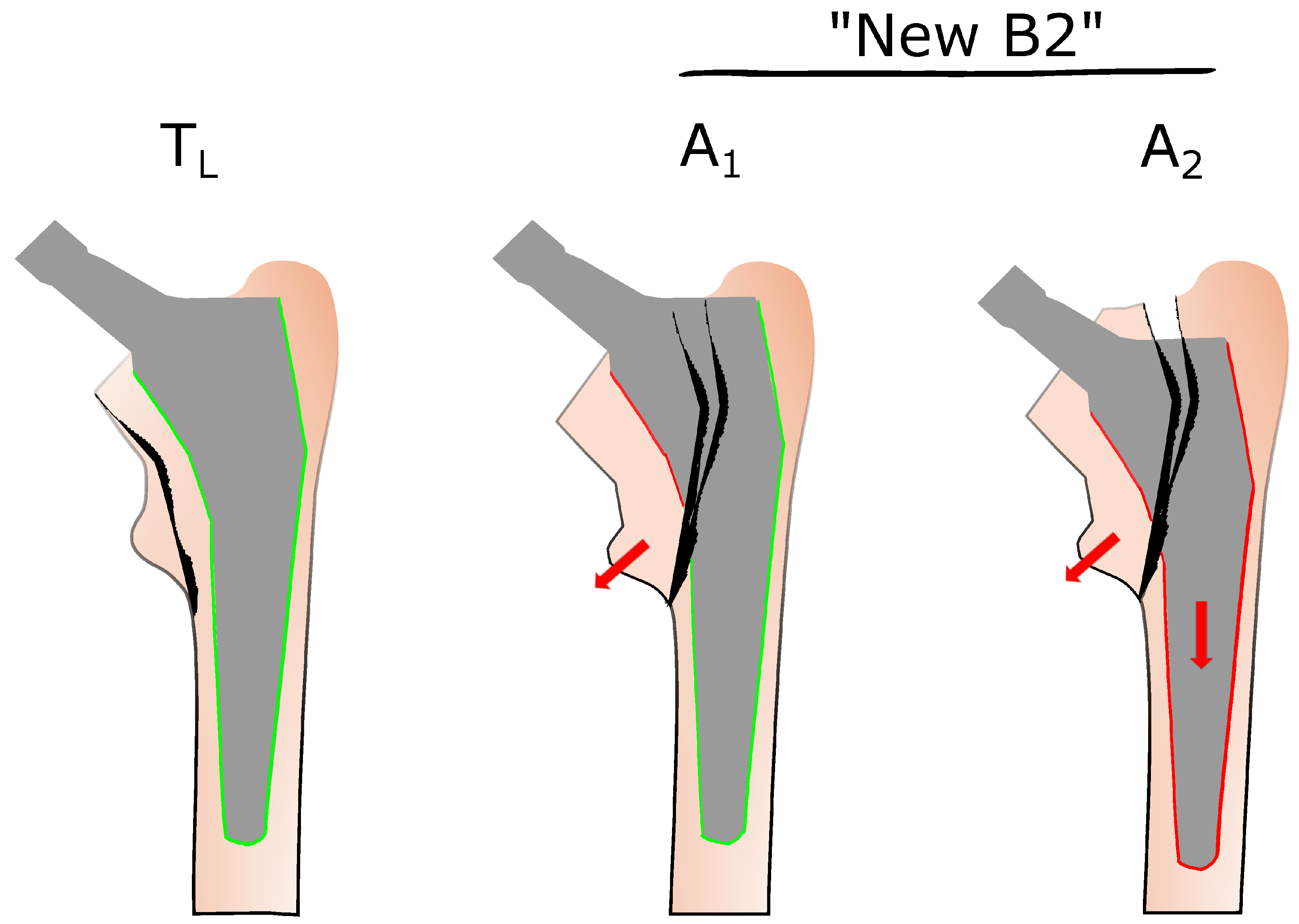
3. Towards Improved Fracture Classification—The Role of Clamshell Fractures in VCS and UCS
4. Biomechanical Considerations
5. Therapeutic Strategies for Clamshell Fractures
6. Limitations
7. Conclusions
- (1)
- According to the increasing number of total hip replacements, PPFFs are gaining growing attention among orthopedic surgeons.
- (2)
- Clamshell fractures may occur intra- and postoperatively and are subject to specific patient and implant related risk factors.
- (3)
- Therapeutic options for managing CFs primarily depend on the design and stability of the femoral stem. They may include open reduction and internal fixation, stem revision, or a combination of both.
- (4)
- Clamshell fractures with a loose stem should be treated according to the established principles for conventional Vancouver B2 fractures.
- (5)
- Future research should focus on distinct stem designs and the respective evaluation of associated fracture patterns.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CF | Clamshell fracture |
| ORIF | Open reduction and internal fixation |
| PPFF | Periprosthetic femoral fracture |
| THA | Total hip arthroplasty |
| UCS | Unified Classification System for periprosthetic femoral fractures |
| VCS | Vancouver classification system |
References
- Lindahl, H. Epidemiology of periprosthetic femur fracture around a total hip arthroplasty. Injury 2007, 38, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Grimberg, A.; Kirschner, S.; Lützner, J.; Melsheimer, O.; Morlock, M.; Steinbrück, A. Endoprothesenregister Deutschland (EPRD): Jahresbericht 2024; Endoprothesenregister Deutschland (EPRD): Berlin, Germany, 2025. [Google Scholar]
- Troelsen, A.; Malchau, E.; Sillesen, N.; Malchau, H. A Review of Current Fixation Use and Registry Outcomes in Total Hip Arthroplasty: The Uncemented Paradox. Clin. Orthop. Relat. Res. 2013, 471, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Bunyoz, K.I.; Malchau, E.; Malchau, H.; Troelsen, A. Has the Use of Fixation Techniques in THA Changed in This Decade? The Uncemented Paradox Revisited. Clin. Orthop. Relat. Res. 2020, 478, 697–704. [Google Scholar] [CrossRef]
- Franklin, J.; Malchau, H. Risk factors for periprosthetic femoral fracture. Injury 2007, 38, 655–660. [Google Scholar] [CrossRef]
- Staunton, P.; Alhojailan, K.; Desgagne, C.; Epure, L.; Zukor, D.; Huk, O.; Antoniou, J. Acute Periprosthetic Hip Fractures with Short, Uncemented Femoral Stems. J. Arthroplast. 2024, 39, S248–S253. [Google Scholar] [CrossRef]
- Kastner, P.; Zderic, I.; Gueorguiev, B.; Pastor, T.; Luger, M.; Gotterbarm, T.; Schopper, C. The Effect of Cerclage Banding Distally to a Clamshell Fracture Pattern in Total Hip Arthroplasty—A Biomechanical Study. Bioengineering 2023, 10, 1397. [Google Scholar] [CrossRef]
- Duncan, C.P.; Masri, B.A. Fractures of the femur after hip replacement. Instr. Course Lect. 1995, 44, 293–304. [Google Scholar]
- Van Houwelingen, A.P.; Duncan, C.P. The Pseudo ALT Periprosthetic Fracture: It’s Really a B2. Orthopedics 2011, 34, e479–e481. [Google Scholar] [CrossRef]
- Abdel, M.P.; Watts, C.D.; Houdek, M.T.; Lewallen, D.G.; Berry, D.J. Epidemiology of periprosthetic fracture of the femur in 32 644 primary total hip arthroplasties: A 40-year experience. Bone Jt. J. 2016, 98-B, 461–467. [Google Scholar] [CrossRef]
- Cottino, U.; Dettoni, F.; Caputo, G.; Bonasia, D.E.; Rossi, P.; Rossi, R. Incidence and pattern of periprosthetic hip fractures around the stem in different stem geometry. Int. Orthop. (SICOT) 2020, 44, 53–59. [Google Scholar] [CrossRef]
- Capello, W.N.; D’Antonio, J.A.; Naughton, M. Periprosthetic Fractures Around a Cementless Hydroxyapatite-coated Implant: A New Fracture Pattern Is Described. Clin. Orthop. Relat. Res. 2014, 472, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Mallory, T.H.; Kraus, T.J.; Vaughn, B.K. Intraoperative Femoral Fractures Associated with Cementless Total Hip Arthroplasty. Orthopedics 1989, 12, 231–239. [Google Scholar] [CrossRef]
- Duncan, C.P.; Haddad, F.S. The Unified Classification System (UCS): Improving our understanding of periprosthetic fractures. Bone Jt. J. 2014, 96, 713–716. [Google Scholar] [CrossRef]
- Huang, J.-F.; Jiang, X.-J.; Shen, J.-J.; Zhong, Y.; Tong, P.-J.; Fan, X.-H. Modification of the Unified Classification System for periprosthetic femoral fractures after hip arthroplasty. J. Orthop. Sci. 2018, 23, 982–986. [Google Scholar] [CrossRef]
- Egrise, F.; Gastaud, O.; Cointat, C.; Raffaelli, A.; Tabutin, J. Identification and treatment of potentially destabilizing Vancouver B-lesser trochanter periprosthetic fracture (“New B2”): A 33-case series. Orthop. Traumatol. Surg. Res. 2022, 108, 103357. [Google Scholar] [CrossRef]
- Karam, J.; Campbell, P.; Desai, S.; Hunter, M. Periprosthetic proximal femoral fractures in cemented and uncemented stems according to Vancouver classification: Observation of a new fracture pattern. J. Orthop. Surg. Res. 2020, 15, 100. [Google Scholar] [CrossRef]
- Watanabe, N.; Ogawa, T.; Takada, R.; Amano, Y.; Jinno, T.; Koga, H.; Yoshii, T.; Okawa, A.; Miyatake, K. Association of osteoporosis and high serum homocysteine levels with intraoperative periprosthetic fracture during total hip arthroplasty: A propensity-score matching analysis. Arch. Orthop. Trauma Surg. 2023, 143, 7219–7227. [Google Scholar] [CrossRef]
- Poss, R.; Ewald, F.C.; Thomas, W.H.; Sledge, C.B. Complications of total hip-replacement arthorplasty in patients with rheumatoid arthritis. J. Bone Jt. Surg. Am. 1976, 58, 1130–1133. [Google Scholar] [CrossRef]
- Moldovan, F.; Moldovan, L. A Modeling Study for Hip Fracture Rates in Romania. J. Clin. Med. 2025, 14, 3162. [Google Scholar] [CrossRef]
- Luger, M.; Feldler, S.; Pisecky, L.; Klasan, A.; Gotterbarm, T.; Schopper, C. Periprosthetic Femoral Fractures in Cementless Short Versus Straight Stem Total Hip Arthroplasty: A Propensity Score Matched Analysis. J. Arthroplast. 2023, 38, 751–756. [Google Scholar] [CrossRef]
- Mondanelli, N.; Troiano, E.; Facchini, A.; Ghezzi, R.; Di Meglio, M.; Nuvoli, N.; Peri, G.; Aiuto, P.; Colasanti, G.B.; Giannotti, S. Treatment Algorithm of Periprosthetic Femoral Fracturens. Geriatr. Orthop. Surg. Rehabil. 2022, 13, 21514593221097608. [Google Scholar] [CrossRef] [PubMed]
- Masri, B.A.; Meek, R.M.D.; Duncan, C.P. Periprosthetic Fractures Evaluation and Treatment. Clin. Orthop. Relat. Res. 2004, 420, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Brady, O.H.; Garbuz, D.S.; Masri, B.A.; Duncan, C.P. Classification of the hip. Orthop. Clin. N. Am. 1999, 30, 215–220. [Google Scholar] [CrossRef]
- Pepke, W.; Nadorf, J.; Ewerbeck, V.; Streit, M.R.; Kinkel, S.; Gotterbarm, T.; Maier, M.W.; Kretzer, J.P. Primary stability of the Fitmore stem: Biomechanical comparison. Int. Orthop. 2014, 38, 483–488. [Google Scholar] [CrossRef]
- Kastner, P.; Zderic, I.; Gueorguiev, B.; Richards, G.; Schauer, B.; Hipmair, G.; Gotterbarm, T.; Schopper, C. Cementless femoral stem revision in total hip arthroplasty: The periprosthetic clamshell fracture. A biomechanical investigation. J. Orthop. Res. 2023, 41, 641–648. [Google Scholar] [CrossRef]
- Carli, A.V.; Negus, J.J.; Haddad, F.S. Periprosthetic femoral fractures and trying to avoid them: What is the contribution of femoral component design to the increased risk of periprosthetic femoral fracture? Bone Jt. J. 2017, 99, 50–59. [Google Scholar] [CrossRef]
- Fottner, A.; Woiczinski, M.; Kistler, M.; Schröder, C.; Schmidutz, T.F.; Jansson, V.; Schmidutz, F. Influence of undersized cementless hip stems on primary stability and strain distribution. Arch. Orthop. Trauma Surg. 2017, 137, 1435–1441. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Kim, J.T.; Kim, K.-C.; Ha, Y.-C.; Koo, K.-H. Conservative Treatment for Minimally Displaced Type B Periprosthetic Femoral Fractures. J. Arthroplast. 2017, 32, 3529–3532. [Google Scholar] [CrossRef]
- Blum, A.; Gondim-Teixeira, P.; Gabiache, E.; Roche, O.; Sirveaux, F.; Olivier, P.; Coudane, H.; Raymond, A.; Louis, M.; Grandhaye, M.; et al. Developments in imaging methods used in hip arthroplasty: A diagnostic algorithm. Diagn. Interv. Imaging 2016, 97, 735–747. [Google Scholar] [CrossRef]
- Laurer, H.L.; Wutzler, S.; Possner, S.; Geiger, E.V.; El Saman, A.; Marzi, I.; Frank, J. Outcome after operative treatment of Vancouver type B1 and C periprosthetic femoral fractures: Open reduction and internal fixation versus revision arthroplasty. Arch. Orthop. Trauma Surg. 2011, 131, 983–989. [Google Scholar] [CrossRef]
- Corten, K.; Vanrykel, F.; Bellemans, J.; Frederix, P.R.; Simon, J.-P.; Broos, P.L.O. An algorithm for the surgical treatment of periprosthetic fractures of the femur around a well-fixed femoral component. J. Bone Jt. Surg. Br. Vol. 2009, 91, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.-Q.; Li, X.-S.; Fan, M.-Q.; Yao, Z.-Y.; Song, Z.-F.; Tong, P.-J.; Huang, J.-F. Surgical treatment of specific Unified Classification System B fractures: Potentially destabilising lesser trochanter periprosthetic fractures. Sci. Rep. 2023, 13, 14330. [Google Scholar] [CrossRef]
- Mulay, S.; Hassan, T.; Birtwistle, S.; Power, R. Management of Types B2 and B3 Femoral Periprosthetic Fractures by a Tapered, Fluted, and Distally Fixed Stem. J. Arthroplast. 2005, 20, 751–756. [Google Scholar] [CrossRef]
- Padilla-Rojas, L.G.; Garín-Zertuche, D.E.; López-Almejo, L.; Garabano, G.; Pesciallo, C.Á.; Leal, J.A.; Pinzón, A.; Giordano, V.; Esteves-Pires, R. Periprosthetic fracture management of the proximal femur. OTA Int. 2023, 6, e246. [Google Scholar] [CrossRef]
- Abdel, M.P.; Cottino, U.; Mabry, T.M. Management of periprosthetic femoral fractures following total hip arthroplasty: A review. Int. Orthop. (SICOT) 2015, 39, 2005–2010. [Google Scholar] [CrossRef]
- Patsiogiannis, N.; Kanakaris, N.K.; Giannoudis, P.V. Periprosthetic hip fractures: An update into their management and clinical outcomes. EFORT Open Rev. 2021, 6, 75–92. [Google Scholar] [CrossRef]
- Khan, T.; Grindlay, D.; Ollivere, B.J.; Scammell, B.E.; Manktelow, A.R.J.; Pearson, R.G. A systematic review of Vancouver B2 and B3 periprosthetic femoral fractures. Bone Jt. J. 2017, 99, 17–25. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haussner, F.; Fuchs, M.; Oltmanns, M.; Reichel, H.; Freitag, T. Management of Clamshell Fractures in Total Hip Arthroplasty: A Rarely Recognized Periprosthetic Injury Pattern. J. Clin. Med. 2025, 14, 4896. https://doi.org/10.3390/jcm14144896
Haussner F, Fuchs M, Oltmanns M, Reichel H, Freitag T. Management of Clamshell Fractures in Total Hip Arthroplasty: A Rarely Recognized Periprosthetic Injury Pattern. Journal of Clinical Medicine. 2025; 14(14):4896. https://doi.org/10.3390/jcm14144896
Chicago/Turabian StyleHaussner, Felix, Michael Fuchs, Moritz Oltmanns, Heiko Reichel, and Tobias Freitag. 2025. "Management of Clamshell Fractures in Total Hip Arthroplasty: A Rarely Recognized Periprosthetic Injury Pattern" Journal of Clinical Medicine 14, no. 14: 4896. https://doi.org/10.3390/jcm14144896
APA StyleHaussner, F., Fuchs, M., Oltmanns, M., Reichel, H., & Freitag, T. (2025). Management of Clamshell Fractures in Total Hip Arthroplasty: A Rarely Recognized Periprosthetic Injury Pattern. Journal of Clinical Medicine, 14(14), 4896. https://doi.org/10.3390/jcm14144896




