Renal Vein Blood Flow Patterns Identify Patients at Risk for Early Kidney Allograft Loss Due to Cardiac Postrenal Vein Congestion
Abstract
1. Introduction
2. Materials and Methods
3. Results
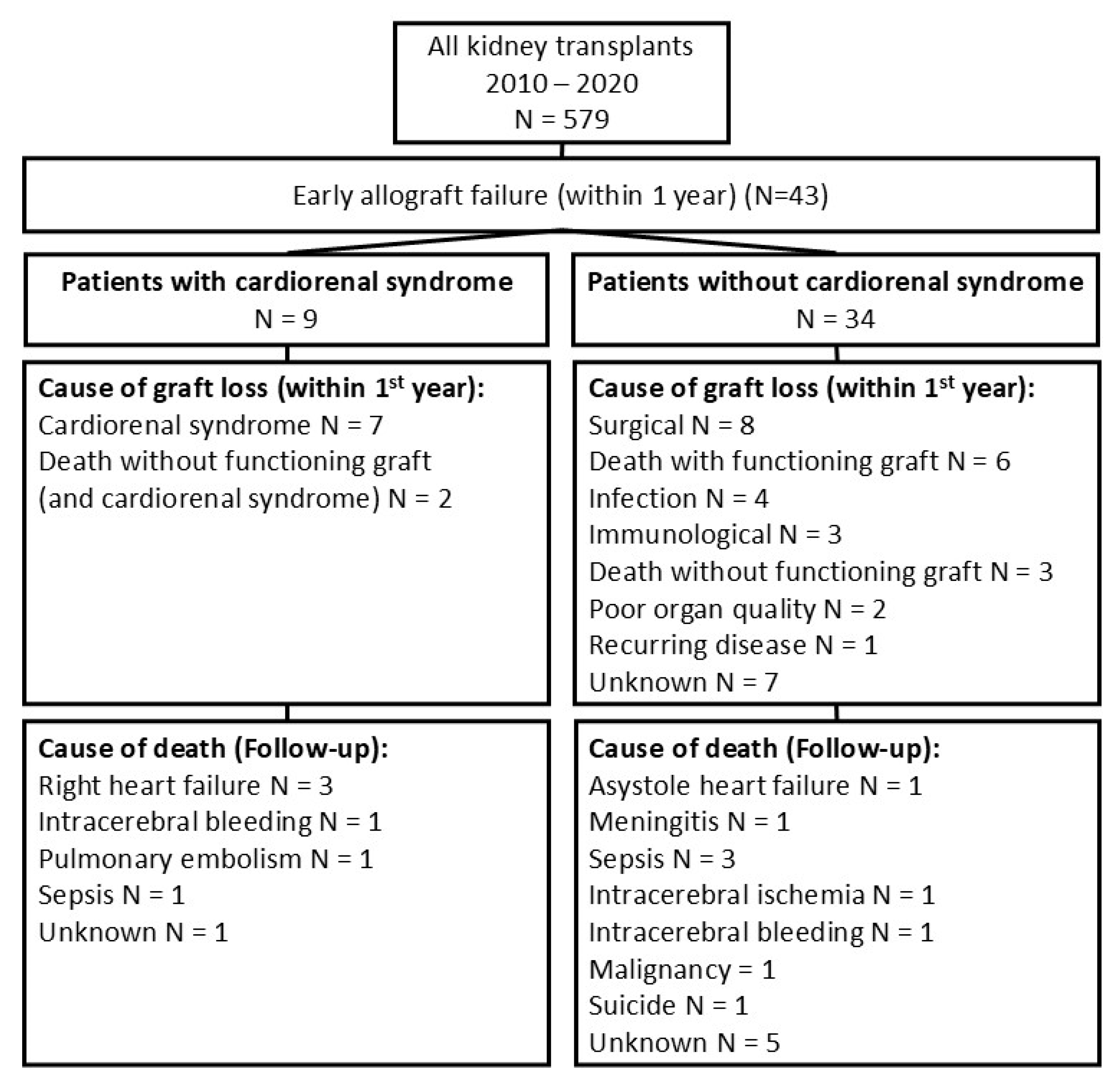
3.1. Patient Characteristics and Clinical Outcomes
3.2. Identifying Patients with Graft Loss Due to Cardiorenal Syndrome
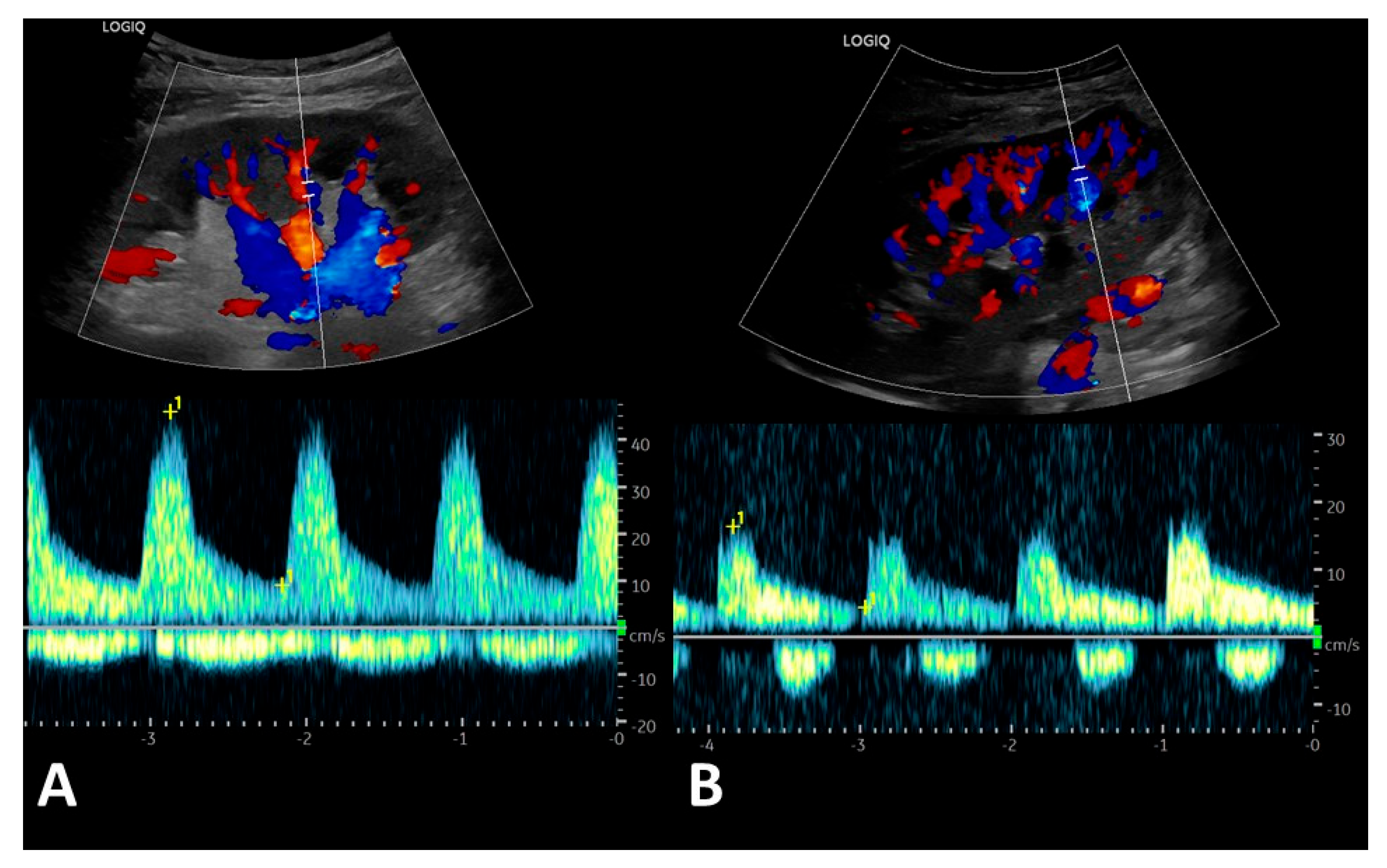
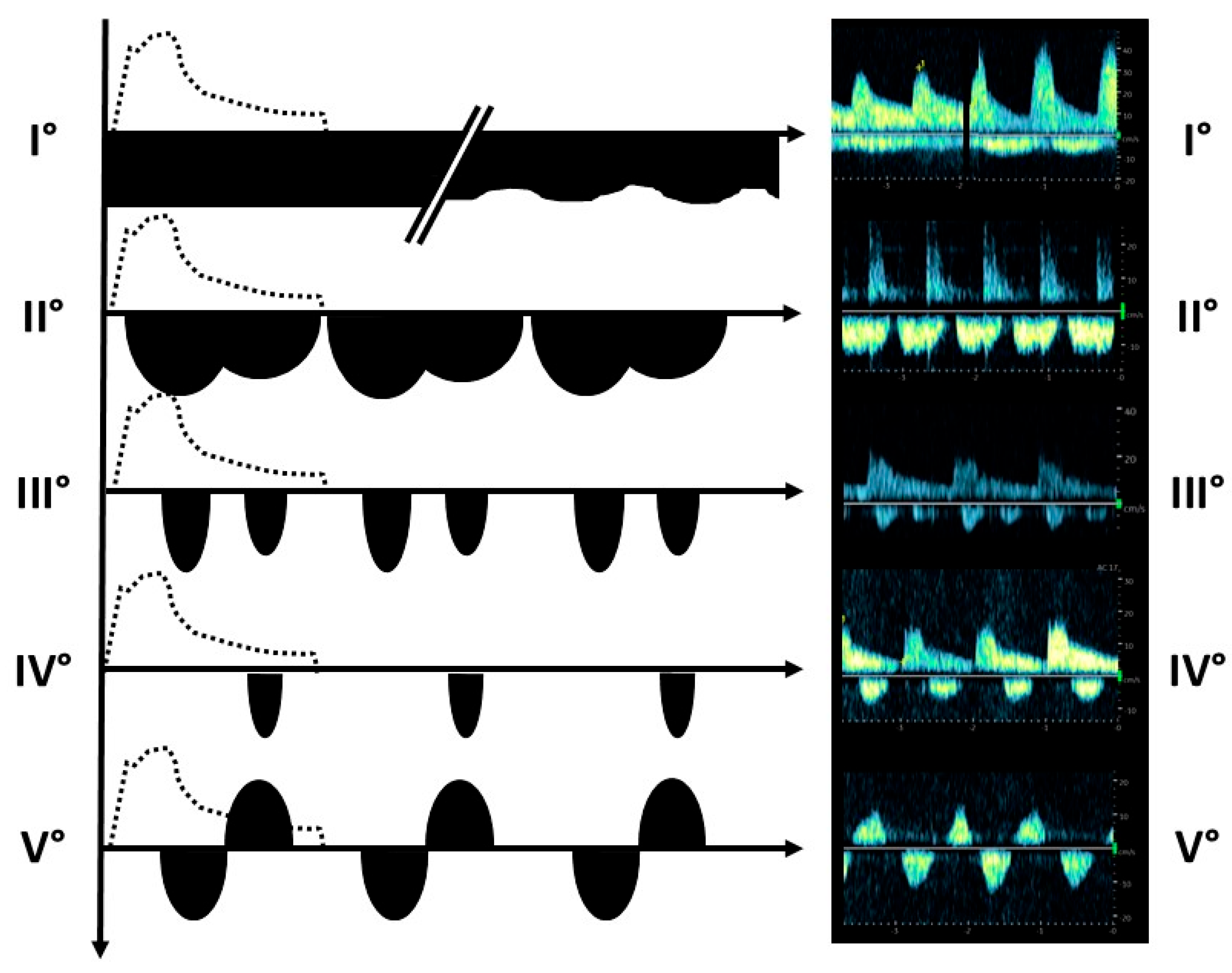
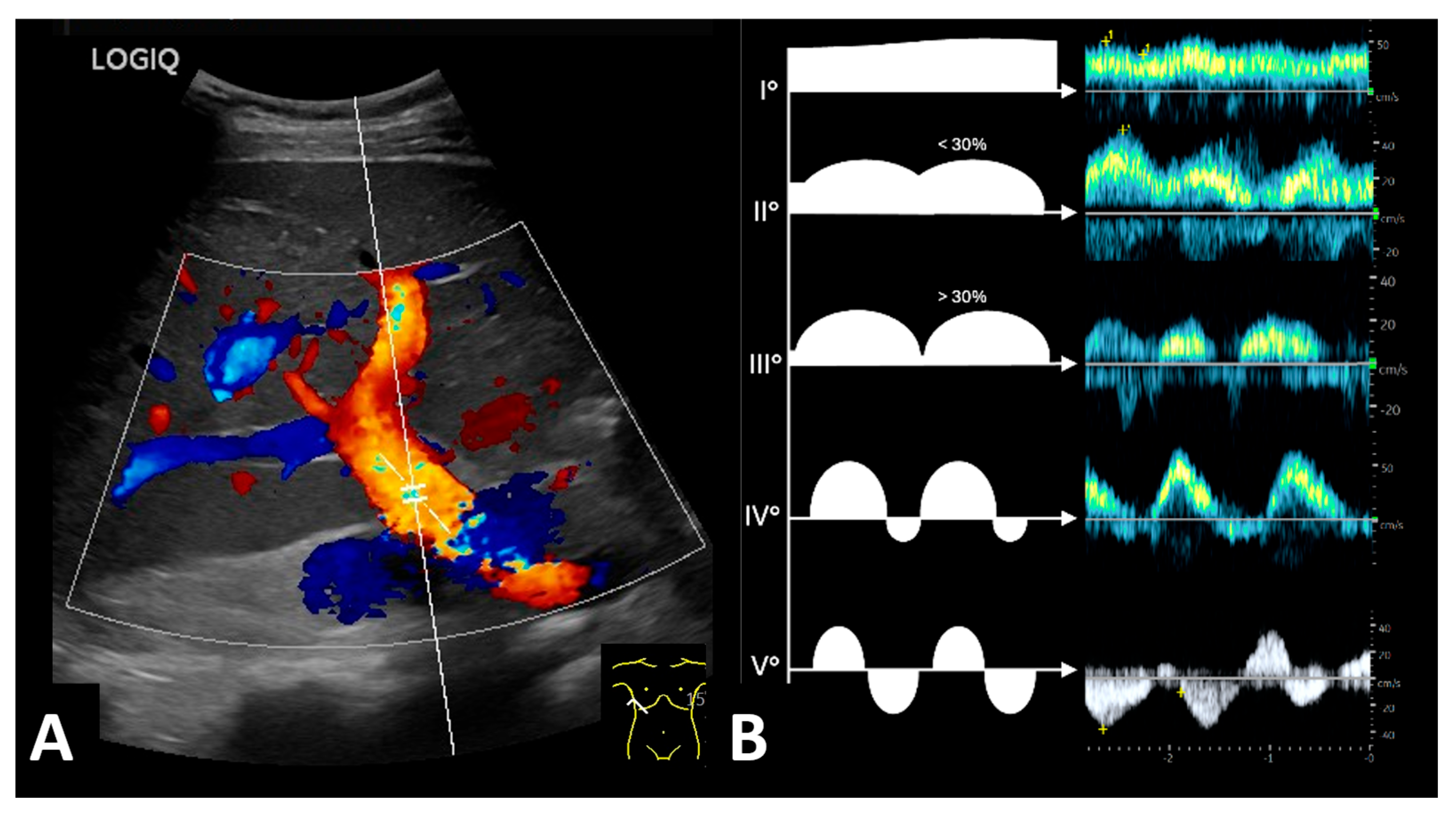
3.3. Post-Transplant Doppler Sonography Parameters of Transplanted Kidneys
3.4. Pre-Transplant Liver Doppler Sonography Identifies Patients at Risk for Cardiorenal Syndrome
3.5. One-Year Survial of Cardiorenal Patients
3.6. Development of Score to Predict Risk of Early Graft Failure Due to Cardiac Postrenal Venous Congestion Before Transplantation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A. | Artery |
| Aa. | Arteries |
| F | Female |
| HLA | Human leucocyte antigen |
| LVEDD | Left ventricular end-diastolic diameter |
| LVEF | Left ventricular ejection fraction |
| M | Male |
| mo. | Months |
| n/a | Not applicable |
| PAH | Pulmonary arterial hypertension |
| PRA | Panel-reactive antibodies |
| RI | Resistive index |
| RVEDD | Right ventricular end-diastolic diameter |
| sPAP | Systolic pulmonary artery pressure |
| TAPSE | Tricuspid annular plane systolic excursion |
| UTI | Urinary tract infection |
| V. | Vein |
| Vv. | Veins |
References
- Suthanthiran, M.; Strom, T.B. Renal transplantation. N. Engl. J. Med. 1994, 331, 365–376. [Google Scholar] [CrossRef]
- Port, F.K.; Wolfe, R.A.; Mauger, E.A.; Berling, D.P.; Jiang, K. Comparison of survival probabilities for dialysis patients vs cadaveric renal transplant recipients. JAMA 1993, 270, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- Schnuelle, P.; Lorenz, D.; Trede, M.; van der Woude, F.J. Impact of renal cadaveric transplantation on survival in end-stage renal failure: Evidence for reduced mortality risk compared with hemodialysis during long-term follow-up. J. Am. Soc. Nephrol. 1998, 9, 2135–2141. [Google Scholar] [CrossRef] [PubMed]
- Lentine, K.L.; Smith, J.M.; Hart, A.; Miller, J.; Skeans, M.A.; Larkin, L.; Robinson, A.; Gauntt, K.; Israni, A.K.; Hirose, R.; et al. OPTN/SRTR 2020 Annual Data Report: Kidney. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2022, 22 (Suppl. S2), 21–136. [Google Scholar] [CrossRef] [PubMed]
- Rangaswami, J.; Bhalla, V.; Blair, J.E.A.; Chang, T.I.; Costa, S.; Lentine, K.L.; Lerma, E.V.; Mezue, K.; Molitch, M.; Mullens, W.; et al. Cardiorenal Syndrome: Classification, Pathophysiology, Diagnosis, and Treatment Strategies: A Scientific Statement From the American Heart Association. Circulation 2019, 139, e840–e878. [Google Scholar] [CrossRef]
- Basic-Jukic, N.; Juric, I.; Furic-Cunko, V. Cardiorenal Syndrome in Renal Transplant Recipients: Prevalence, Clinical Presentation, Treatment, and Outcome. Cardiorenal Med. 2020, 10, 333–339. [Google Scholar] [CrossRef]
- Samarendra, P.; Ramkumar, M.; Sharma, V.; Kumari, S. Cardiorenal syndrome in renal transplant recipients—It’s the fistula at fault: A case series. Clin. Transplant. 2018, 32, e13417. [Google Scholar] [CrossRef]
- Waiser, J.; Knebel, F.; Rudolph, B.; Wu, K.; Müller, E.; Sanad, W.; Budde, K.; Neumayer, H.-H.; Halleck, F. Renal allograft loss caused by cardiorenal syndrome: Frequency and diagnosis. Transplantation 2015, 99, 1208–1215. [Google Scholar] [CrossRef]
- Husain-Syed, F.; Gröne, H.-J.; Assmus, B.; Bauer, P.; Gall, H.; Seeger, W.; Ghofrani, A.; Ronco, C.; Birk, H.-W. Congestive nephropathy: A neglected entity? Proposal for diagnostic criteria and future perspectives. ESC Heart Fail. 2021, 8, 183–203. [Google Scholar] [CrossRef]
- de La Espriella-Juan, R.; Núñez, E.; Miñana, G.; Sanchis, J.; Bayés-Genís, A.; González, J.; Chorro, J.; Núñez, J. Intrarenal venous flow in cardiorenal syndrome: A shining light into the darkness. ESC Heart Fail. 2018, 5, 1173–1175. [Google Scholar] [CrossRef]
- Puzzovivo, A.; Monitillo, F.; Guida, P.; Leone, M.; Rizzo, C.; Grande, D.; Ciccone, M.M.; Iacoviello, M. Renal Venous Pattern: A New Parameter for Predicting Prognosis in Heart Failure Outpatients. J. Cardiovasc. Dev. Dis. 2018, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Beaubien-Souligny, W.; Benkreira, A.; Robillard, P.; Bouabdallaoui, N.; Chassé, M.; Desjardins, G.; Lamarche, Y.; White, M.; Bouchard, J.; Denault, A. Alterations in Portal Vein Flow and Intrarenal Venous Flow Are Associated With Acute Kidney Injury After Cardiac Surgery: A Prospective Observational Cohort Study. J. Am. Heart Assoc. 2018, 7, e009961. [Google Scholar] [CrossRef]
- Spiegel, R.; Teeter, W.; Sullivan, S.; Tupchong, K.; Mohammed, N.; Sutherland, M.; Leibner, E.; Rola, P.; Galvagno, S.M.; Murthi, S.B. The use of venous Doppler to predict adverse kidney events in a general ICU cohort. Crit. Care 2020, 24, 615. [Google Scholar] [CrossRef] [PubMed]
- Trpkov, C.; Grant, A.D.; Fine, N.M. Intrarenal Doppler Ultrasound Renal Venous Stasis Index Correlates With Acute Cardiorenal Syndrome in Patients With Acute Decompensated Heart Failure. CJC Open 2021, 3, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Husain-Syed, F.; Birk, H.-W.; Ronco, C.; Schörmann, T.; Tello, K.; Richter, M.J.; Wilhelm, J.; Sommer, N.; Steyerberg, E.; Bauer, P.; et al. Doppler-Derived Renal Venous Stasis Index in the Prognosis of Right Heart Failure. J. Am. Heart Assoc. 2019, 8, e013584. [Google Scholar] [CrossRef]
- Ohara, H.; Yoshihisa, A.; Horikoshi, Y.; Ishibashi, S.; Matsuda, M.; Yamadera, Y.; Sugawara, Y.; Ichijo, Y.; Hotsuki, Y.; Watanabe, K.; et al. Renal Venous Stasis Index Reflects Renal Congestion and Predicts Adverse Outcomes in Patients With Heart Failure. Front. Cardiovasc. Med. 2022, 9, 772466. [Google Scholar] [CrossRef]
- Yoshihisa, A.; Watanabe, K.; Sato, Y.; Ishibashi, S.; Matsuda, M.; Yamadera, Y.; Ichijo, Y.; Yokokawa, T.; Misaka, T.; Oikawa, M.; et al. Intrarenal Doppler ultrasonography reflects hemodynamics and predicts prognosis in patients with heart failure. Sci. Rep. 2020, 10, 22257. [Google Scholar] [CrossRef]
- Bansal, S.; Prasad, A.; Linas, S. Right Heart Failure—Unrecognized Cause of Cardiorenal Syndrome. J. Am. Soc. Nephrol. 2018, 29, 1795–1798. [Google Scholar] [CrossRef]
- Mehta, K.S.; Shirkande, A.K.; Bhurke, S.P.; Pajai, A.E.; Swami, R.S.; Jadhav, S.N. Pulmonary Hypertension in Various Stages of Chronic Kidney Disease in Indian Patients. Indian J. Nephrol. 2019, 29, 95–101. [Google Scholar] [CrossRef]
- Reddy, Y.N.V.; Obokata, M.; Dean, P.G.; Melenovsky, V.; Nath, K.A.; Borlaug, B.A. Long-term cardiovascular changes following creation of arteriovenous fistula in patients with end stage renal disease. Eur. Heart J. 2017, 38, 1913–1923. [Google Scholar] [CrossRef]
- Hetz, P.; Pirklbauer, M.; Müller, S.; Posch, L.; Gummerer, M.; Tiefenthaler, M. Prophylactic Ligature of AV Fistula Prevents High Output Heart Failure after Kidney Transplantation. Am. J. Nephrol. 2020, 51, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Zlotnick, D.M.; Axelrod, D.A.; Chobanian, M.C.; Friedman, S.; Brown, J.; Catherwood, E.; Costa, S.P. Non-invasive detection of pulmonary hypertension prior to renal transplantation is a predictor of increased risk for early graft dysfunction. Nephrol. Dial. Transpl. 2010, 25, 3090–3096. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Garcia, R.; Torosoff, M.; Fein, S.A.; Ashar, A.; Chandolias, N.; Conti, D.; Lyubarova, R. Influence of mildly and moderately elevated pulmonary artery systolic pressure on post-renal transplantation survival and graft function. Echocardiography 2019, 36, 22–27. [Google Scholar] [CrossRef]
- Issa, N.; Krowka, M.J.; Griffin, M.D.; Hickson, L.J.; Stegall, M.D.; Cosio, F.G. Pulmonary hypertension is associated with reduced patient survival after kidney transplantation. Transplantation 2008, 86, 1384–1388. [Google Scholar] [CrossRef] [PubMed]
- Sadat, B.; Tirunagari, D.; Karthikeyan, V.; Patel, A.; van Harn, M.; Saleem, M.M.; Ananthasubramaniam, K. Clinical impact of pre-kidney transplant pulmonary hypertension on post-transplant outcomes. Int. J. Cardiovasc. Imaging 2021, 37, 1979–1986. [Google Scholar] [CrossRef]
- Obi, C.; Frost, A.E.; Graviss, E.A.; Nguyen, D.T.; Gaber, A.O.; Suki, W.N. The Association of Pretransplant Pulmonary Hypertension With Patient and Graft Survival After Kidney Transplantation: A Retrospective Cohort Study. Transplant. Proc. 2020, 52, 3023–3032. [Google Scholar] [CrossRef]

| All Patients | Patients with Other Reasons for Graft Loss (N = 34) | Patients with Assumed Cardiorenal Syndrome (N = 9) | p-Value | |
|---|---|---|---|---|
| Recipient age | 57 ± 12 | 56 ± 12 | 61 ± 10 | 0.276 |
| Time on dialysis (mo.) | 37 ± 31 | 39 ± 31 | 27 ± 28 | 0.332 |
| Donor age | 55 ± 17 | 54 ± 17 | 59 ± 16 | 0.419 |
| Multi-organ recipient | 7 | 5 | 2 | |
| Heart transplantation | 3 | 1 | 2 | |
| HLA mismatches (cumulative for A, B, DR) | 3.6 ± 1.6 | 3.5 ± 1.8 | 4.1 ± 0.7 | 0.347 |
| PRA (%) | 6 ± 16 | 6 ± 16 | 7 ± 14 | 0.812 |
| Living donation | 3 | 3 | 0 | |
| Cold ischemia time (hours) | 11.7 ± 4.7 | 11.6 ± 4.9 | 12.2 ± 3.3 | 0.775 |
| Warm ischemia time (min.) | 44 ± 18 | 43 ± 19 | 48 ± 16 | 0.581 |
| Histological evidence of rejection (including borderline rejection) | 12/35 (34%) (NA 7) | 10/27 (37%) (NA 6) | 2/8 (25%) (NA 1) | |
| Histological evidence of singular acute tubular necrosis | 11/35 (31%) (NA 7) | 8/27 (29%) (NA 6) | 3/8 (37%) (NA 1) | |
| One-year survival rate | 28/43 (65.1%) | 24/34 (70.6%) | 4/9 (44.4%) | 0.129 |
| Overall survival rate | 22/43 (51.2%) | 20/34 (58.8%) | 2/9 (22.2%) | 0.027 |
| Patient survival (years) | 5.4 ± 4.9 | 6.3 ± 5.0 | 2.0 ± 3.2 | 0.005 |
| Death age | 59 ± 11 | 59 ± 11 | 60 ± 11 | 0.985 |
| All Patients (N = 43) | Patients with Other Reasons for Graft Loss (N = 34) | Patients with Assumed Cardiorenal Syndrome (N = 9) | p-Value (Pearson’s Chi2, Student’s t-Test) | |
|---|---|---|---|---|
| Cardiac disease | ||||
| Any heart disease (myocardial, valvular, and/or coronary heart disease, %) | 80.0 (20/34) | 46.2 (12/26) | 100 (8/8) | p = 0.07, φ = 0.46 |
| Any valvular heart disease (%) | 36.4 (12/33) | 20.0 (5/25) | 87.5 (7/8) | p ≤ 0.001, φ = 0.60 |
| Coronary artery disease (%) | 28.6 (12/42) | 23.5 (8/34) | 50.0 (4/8) | p = 0.136, φ = 0.23 |
| Atrial fibrillation (%) | 4.8 (2/42) | 2.9 (1/34) | 12.5 (1/8) | p = 0.253, φ = 0.18 |
| Right heart | ||||
| Any right heart disease (%) | 50.0 (15/30) | 36.4 (8/22) | 87.5 (7/8) | p = 0.01, φ = 0.45 |
| Right atrial area dilatation (>20 cm2) | 48.0 (12/25) | 36.8 (7/19) | 83.3 (5/6) | p ≤ 0.05, φ = 0.40 |
| RVEDD enlarged (>36 mm) | 28.6 (8/28) | 9.5 (2/21) | 85.7 (6/7) | p ≤ 0.001, φ = 0.73 |
| TAPSE reduced (<16 mm) | 14.3 (4/28) | 0.0 (0/22) | 66.7 (4/6) | p ≤ 0.001, φ = 0.78 |
| TAPSE (mm) | 17 ± 3 | 20.5 ± 0.5 | 16 ± 3 | n/a |
Tricuspid regurgitation
| 75.9 (22/29) 10.3 (3/29) 13.8 (4/29) | 90.5 (19/21) 4.8 (1/21) 4.8 (1/21) | 37.5 (3/8) 25.0 (2/8) 37.5 (3/8) | p ≤ 0.001, φ = 0.56 |
| sPAP elevated (>33 mmHg; %) | 37.5 (9/24) | 18.6 (3/16) | 75.0 (6/8) | p = 0.005, φ = 0.56 |
| Mean pulmonary artery pressure (mmHg) | 33 ± 14 (n/a 19) | 28 ± 9 (n/a 18) | 44 ± 18 (n/a 1) | 0.015 |
| Left heart | ||||
| Any left heart disease (%) | 80.0 (20/34) | 46.2 (12/26) | 100 (8/8) | p = 0.01, φ = 0.44 |
| Left atrial area (>20 cm2; %) | 52.9 (18/34) | 38.5 (10/26) | 100 (8/8) | p = 0.002, φ = 0.52 |
| Left ventricular end-diastolic diameter (LVEDD) enlargement (F > 52 mm; M > 58 mm; %) | 28.6 (6/21) | 21.4 (3/14) | 42.8 (3/7) | p = 0.31, φ = 0.22 |
LVEF (%)
| 80.0 (28/35) 8.6 (3/35) 5.7 (2/35) 5.7 (2/35) | 85.2 (23/27) 11.1 (3/27) 3.7 (1/27) 0.0 (0/27) | 62.8 (5/8) 0.0 (0/8) 12.5 (1/8) 25.0 (2/8) | p = 0.03, φ = 0.50 |
| Evidence of diastolic dysfunction (%) | 29.0 (9/31) | 19.2 (5/26) | 80.0 (4/5) | p = 0.006, φ = 0.49 |
| Liver duplex sonography | ||||
| Evidence of venous congestion in liver duplex sonography (%) | 22.9 (8/35) | 7.4 (2/27) | 75.0 (6/8) | p ≤ 0.001, φ = 0.68 |
| All Patients (N = 43) | Patients with Other Reasons for Graft Loss (N = 34) | Patients with Assumed Cardiorenal syndrome (N = 9) | p-Value | |
|---|---|---|---|---|
| Renal length (cm) | 10.4 ± 1.1 | 10.6 ± 1.1 | 10.1 ± 1.1 | 0.281 |
| Resistive Index (RI) | 0.73 ± 0.09 | 0.72 ± 0.09 | 0.75 ± 0.1 | 0.397 |
| Renal vein profile | ||||
| 33 1 0 3 6 | 33 1 0 0 0 | 0 0 0 3 6 |
| Unexplained Renal Insufficiency | Severe Heart Disease | Severe Volume Overload | Acute Tubular Necrosis | Combination of All | Renal Venous Congestion Grades IV and V | |
|---|---|---|---|---|---|---|
| Patient 1 | Yes | Yes | Yes | Yes | Yes | Yes (V) |
| Patient 2 | Yes | Yes | Yes | No | No | Yes (V) |
| Patient 3 | Yes | Yes | Yes | N/a | Yes | Yes (V) |
| Patient 4 | Yes | Yes | Yes | No | No | Yes (IV) |
| Patient 5 | Yes | Yes | Yes | Yes | Yes | Yes (V) |
| Patient 6 | Yes | Yes | Yes | No | No | Yes (V) |
| Patient 7 | Yes | Yes | Yes | No | No | Yes (V) |
| Patient 8 | No (rejection) | Yes | Yes | No | No | Yes (IV) |
| Patient 9 | Yes | Yes | Yes | Yes | Yes | Yes (IV) |
| Score 2 | Score 1 | Criterion | Points |
| RA area > 20 cm2 | 1 | ||
| RVEDD > 36 mm | 1 | ||
| PAP > 33 mmHg | 1 | ||
| TAPSE < 16 mm | 1 | ||
| Tricuspid regurgitation II° | 1 | ||
| Tricuspid regurgitation III° | 2 | ||
| + | Liver duplex sonography with evidence of venous congestion | 1 |
| Score | AUC | CI | Sensitivity | Specificity | Cut-Off |
|---|---|---|---|---|---|
| Score 1 | 0.914 | 0.741, 1.000 | 87.5% | 87.5% | >1 |
| Score 2 | 0.914 | 0.741, 1.000 | 87.5% | 91.7% | >2 |
| Liver duplex sonography with evidence of venous congestion | 0.831 | 0.618, 1.000 | 71.4% | 94.7% | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Putz, F.J.; Kranert, P.C.; Banas, M.C.; Schierling, W.; Jung, E.M.; Bergler, T.; Banas, B. Renal Vein Blood Flow Patterns Identify Patients at Risk for Early Kidney Allograft Loss Due to Cardiac Postrenal Vein Congestion. J. Clin. Med. 2025, 14, 4897. https://doi.org/10.3390/jcm14144897
Putz FJ, Kranert PC, Banas MC, Schierling W, Jung EM, Bergler T, Banas B. Renal Vein Blood Flow Patterns Identify Patients at Risk for Early Kidney Allograft Loss Due to Cardiac Postrenal Vein Congestion. Journal of Clinical Medicine. 2025; 14(14):4897. https://doi.org/10.3390/jcm14144897
Chicago/Turabian StylePutz, Franz Josef, Paul Christian Kranert, Miriam C. Banas, Wilma Schierling, Ernst Michael Jung, Tobias Bergler, and Bernhard Banas. 2025. "Renal Vein Blood Flow Patterns Identify Patients at Risk for Early Kidney Allograft Loss Due to Cardiac Postrenal Vein Congestion" Journal of Clinical Medicine 14, no. 14: 4897. https://doi.org/10.3390/jcm14144897
APA StylePutz, F. J., Kranert, P. C., Banas, M. C., Schierling, W., Jung, E. M., Bergler, T., & Banas, B. (2025). Renal Vein Blood Flow Patterns Identify Patients at Risk for Early Kidney Allograft Loss Due to Cardiac Postrenal Vein Congestion. Journal of Clinical Medicine, 14(14), 4897. https://doi.org/10.3390/jcm14144897






