Establishment of Norms for Facial Discriminative Sensitivity in Healthy Women Aged 45–60 Years: A Reference Framework
Abstract
1. Introduction
1.1. Background/Rationale
1.2. Objectives
2. Method
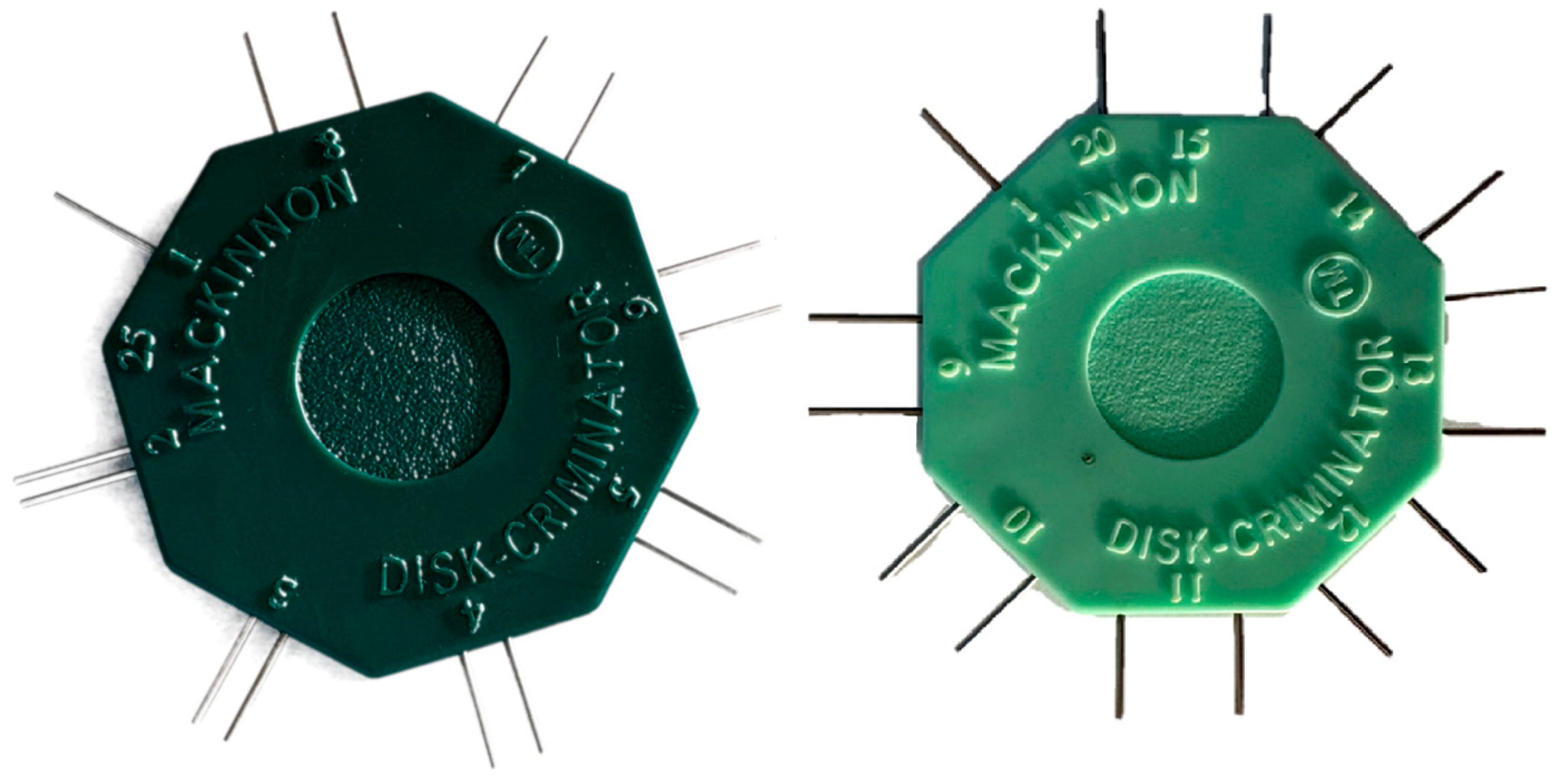
2.1. Study Design
2.2. Setting
2.3. Participants
2.4. Variables
2.5. Data Sources and Measurement
2.6. Bias
2.7. Study Size
2.8. Quantitative Variables
2.9. Statistical Methods
2.10. Ethical Considerations
3. Results
3.1. Sample Characteristics
3.2. Main Results
3.3. Inter-Individual Variability
3.4. Comparison with the Literature Data
4. Discussion
4.1. Interpretation of Results
4.2. Reliability of Measurements and Data Robustness
4.3. Influence of Age
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yıldız, T.; Selimen, D. The Impact of Facial Aesthetic and Reconstructive Surgeries on Patients’ Quality of Life. Indian J. Surg. 2015, 77, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Siemionow, M.; Gharb, B.B.; Rampazzo, A. Pathways of Sensory Recovery after Face Transplantation. Plast. Reconstr. Surg. 2011, 127, 1875–1889. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.Q.; Hadlock, T.A. Improving Outcomes Tracking in Facial Plastic and Reconstructive Surgery. Facial Plast. Surg. Aesthetic Med. 2020, 22, 319–320. [Google Scholar] [CrossRef]
- Meyer, R.A.; Rath, E.M. Sensory Rehabilitation after Trigeminal Nerve Injury or Nerve Repair. Oral Maxillofac. Surg. Clin. N. Am. 2001, 13, 365–376. [Google Scholar] [CrossRef]
- McGlone, F.; Reilly, D. The Cutaneous Sensory System. Neurosci. Biobehav. Rev. 2010, 34, 148–159. [Google Scholar] [CrossRef]
- Primavera, G.; Berardesca, E. Sensitive Skin: Mechanisms and Diagnosis. Int. J. Cosmet. Sci. 2005, 27, 1–10. [Google Scholar] [CrossRef]
- Dellon, A.L. Management of Peripheral Nerve Problems in the Upper and Lower Extremity Using Quantitative Sensory Testing. Hand Clin. 1999, 15, 697–715. [Google Scholar] [CrossRef]
- Dellon, A.L.; Andonian, E.; DeJesus, R.A. Measuring Sensibility of the Trigeminal Nerve. Plast. Reconstr. Surg. 2007, 120, 1546–1550. [Google Scholar] [CrossRef]
- Lee Dellon, A.; Kallman, C.H. Evaluation of Functional Sensation in the Hand. J. Hand Surg. 1983, 8, 865–870. [Google Scholar] [CrossRef]
- Kesarwani, A.; Antonyshyn, O.; Mackinnon, S.E.; Gruss, J.S.; Novak, C.; Kelly, L. Facial Sensibility Testing in the Normal and Posttraumatic Population. Ann. Plast. Surg. 1989, 22, 416–425. [Google Scholar] [CrossRef]
- Vriens, J.P.M.; van der Glas, H.W. Extension of Normal Values on Sensory Function for Facial Areas Using Clinical Tests on Touch and Two-Point Discrimination. Int. J. Oral Maxillofac. Surg. 2009, 38, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.; Samman, N. Facial Skin Sensibility in a Young Healthy Chinese Population. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 107, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.L.; Shamaskin, R.G.; Harkins, S.W. Assessment of Recovery from Injury to Inferior Alveolar and Mental Nerves. Oral Surg. Oral Med. Oral Pathol. 1987, 64, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Fogaça, W.C.; Sturtz, G.P.; Surjan, R.C.T.; Ferreira, M.C. Evaluation of Cutaneous Sensibility on Infraorbital Nerve Area. J. Craniofac. Surg. 2005, 16, 953–956. [Google Scholar] [CrossRef]
- Novak, C.B.; Ross, B.; Mackinnon, S.E.; Nedzelski, J.M. Facial Sensibility in Patients with Unilateral Facial Nerve Paresis. Otolaryngol. Neck Surg. 1993, 109, 506–513. [Google Scholar] [CrossRef]
- Montagna, W.; Carlisle, K. Structural Changes in Aging Human Skin. J. Invest. Dermatol. 1979, 73, 47–53. [Google Scholar] [CrossRef]
- Cerimele, D.; Celleno, L.; Serri, F. Physiological Changes in Ageing Skin. Br. J. Dermatol. 1990, 122, 13–20. [Google Scholar] [CrossRef]
- Kijowska, J.; Grzegorczyk, J.; Gliwa, K.; Jędras, A.; Sitarz, M. Epidemiology, Diagnostics, and Therapy of Oral Cancer—Update Review. Cancers 2024, 16, 3156. [Google Scholar] [CrossRef]
- Van Sickels, J.E.; Zysset, M.; Nishioka, G.J.; Thrash, W.J. A Comparative Study of Normal Sensibility of the Inferior Alveolar Nerve and the Infraorbital Nerve. Oral Surg. Oral Med. Oral Pathol. 1989, 67, 255–257. [Google Scholar] [CrossRef]
- Albornoz, C.A.; Nichols, S.E.; Wang, J.V.; Saedi, N.; Munavalli, G.S. Optimizing Skin Tightening in Aesthetics in Men. Clin. Dermatol. 2022, 40, 244–248. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Corniani, G.; Saal, H.P. Tactile Innervation Densities across the Whole Body. J. Neurophysiol. 2020, 124, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.L.; McNulty, P.A. Age-Related Changes in Cutaneous Sensation in the Healthy Human Hand. Age 2013, 35, 1077–1089. [Google Scholar] [CrossRef] [PubMed]
- Catley, M.J.; Tabor, A.; Wand, B.M.; Moseley, G.L. Assessing Tactile Acuity in Rheumatology and Musculoskeletal Medicine—How Reliable Are Two-Point Discrimination Tests at the Neck, Hand, Back and Foot? Rheumatol. Oxf. Engl. 2013, 52, 1454–1461. [Google Scholar] [CrossRef]




| Area | 1R | 2R | 3R | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| n | 20 | 19 | 20 | 19 | 20 | 19 | 20 | 20 |
| Mean TPD (SD) | 14.3 (±3.7) | 10.4 (±2.5) | 13.5 (±4.3) | 8.4 (±1.8) | 5.3 (±0.8) | 8.0 (±1.9) | 2.9 (±0.7) | 3.2 (±1.0) |
| 95% CI Mean | [12.6–16.1] | [9.2–11.6] | [11.5–15.5] | [7.6–9.3] | [4.9–5.7] | [7.1–8.9] | [2.6–3.2] | [2.7–3.7] |
| Coefficient of variation | 0.3 | 0.2 | 0.3 | 0.2 | 0.2 | 0.2 | 0.2 | 0.3 |
| p-value of Shapiro–Wilk | 0.30 | 0.20 | 0.30 | 0.20 | 0.80 | 0.40 | 0.60 | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarhan, F.-R.; Davergne, T.; Couturaud, C.; Testelin, S.; Dakpé, S. Establishment of Norms for Facial Discriminative Sensitivity in Healthy Women Aged 45–60 Years: A Reference Framework. J. Clin. Med. 2025, 14, 4884. https://doi.org/10.3390/jcm14144884
Sarhan F-R, Davergne T, Couturaud C, Testelin S, Dakpé S. Establishment of Norms for Facial Discriminative Sensitivity in Healthy Women Aged 45–60 Years: A Reference Framework. Journal of Clinical Medicine. 2025; 14(14):4884. https://doi.org/10.3390/jcm14144884
Chicago/Turabian StyleSarhan, François-Régis, Thomas Davergne, Christine Couturaud, Sylvie Testelin, and Stéphanie Dakpé. 2025. "Establishment of Norms for Facial Discriminative Sensitivity in Healthy Women Aged 45–60 Years: A Reference Framework" Journal of Clinical Medicine 14, no. 14: 4884. https://doi.org/10.3390/jcm14144884
APA StyleSarhan, F.-R., Davergne, T., Couturaud, C., Testelin, S., & Dakpé, S. (2025). Establishment of Norms for Facial Discriminative Sensitivity in Healthy Women Aged 45–60 Years: A Reference Framework. Journal of Clinical Medicine, 14(14), 4884. https://doi.org/10.3390/jcm14144884






