20-Year Efficacy of Endoscopic Thoracic Sympathectomy for Primary Hyperhidrosis: A Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Methods
2.3. Surgical Technique
3. Results
3.1. Patient Characteristics
3.2. Localization and Redistribution of Hyperhidrosis
3.3. Level of Anxiety and Satisfaction
3.4. Associated Symptoms with Hyperhidrosis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PREOP | Preoperative |
| POSTOP | Postoperative |
| PH | Primary hyperhidrosis |
| ETS | Endoscopic thoracic sympathectomy |
| QoL | Quality of life |
| IQR | Interquartile range |
| SD | Standard deviation |
| NA | Not applicable |
| VAS | Visual analogue scale |
| CS | Compensatory sweating |
References
- Ramos, R.; Moya, J.; Pérez, J.; Villalonga, R.; Morera, R.; Pujol, R.; Ferrer, G. Hiperhidrosis primaria: Estudio prospectivo de 338 pacientes. Med. Clin. 2003, 121, 201–203. [Google Scholar] [CrossRef] [PubMed]
- Gossot, D.; Kabiri, H.; Calindro, R.; Debrosse, D.; Girard, P.; Grunenwald, D. Early complications of thoracic endoscopic sympathectomy: A prospective study of 940 procedures. Ann. Thorac. Surg. 2001, 71, 1116–1119. [Google Scholar] [CrossRef] [PubMed]
- Dumont, P.; Denoyer, A.; Robin, P. Long-term results of thoracic sympathectomy of hyperhidrosis. Ann. Thorac. Surg. 2004, 78, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Moya, J.; Ramos, R.; Morera, R.; Villalonga, R.; Perna, V.; Macia, I.; Ferrer, G. Thoracic sympathicolysis for primary hyperhidrosis: A review of 918 procedures. Surg. Endosc. 2006, 20, 598–602. [Google Scholar] [CrossRef]
- Prasad, A.; Ali, M.; Kaul, S. Endoscopic thoracic sympathectomy for primary palmar hyperhidrosis. Surg. Endosc. 2010, 24, 1952–1957. [Google Scholar] [CrossRef]
- Ramos, R.; Moya, J.; Turón, V.; Pérez, J.; Villalonga, R.; Morera, R.; Perna, V.; Ferrer, G. Primary hyperhidrosis and anxiety: A prospective preoperative survey of 158 patients. Arch. Bronconeumol. 2005, 41, 88–92. [Google Scholar] [CrossRef]
- Ramos, R.; Moya, J.; Morera, R.; Masuet, C.; Perna, V.; Macia, I.; Escobar, I.; Villalonga, R. An assessment of anxiety in patients with primary hyperhidrosis before and after endoscopic thoracic sympathicolysis. Eur. J. Cardiothorac. Surg. 2006, 30, 228–231. [Google Scholar] [CrossRef]
- Moya, J.; Ramos, R.; Vives, N.; Pérez, J.; Morera, R.; Perna, V.; Villalonga, R.; Ferrer, G. Compensatory sweating after upper thoracic sympathectomy: Prospective study of 123 cases. Arch. Bronconeumol. 2004, 40, 360–363.K. [Google Scholar] [CrossRef]
- Ramos, R.; Moya, J.; Macia, I.; Morera, R.; Escobar, I.; Perna, V.; Rivas, F.; Masuet, C.; Saumench, J.; Villalonga, R. Anatomical redistribution of sweating after T2-T3 thoracoscopic sympathicolysis: A study of 210 patients. Surg. Endosc. 2007, 21, 2030–2033. [Google Scholar] [CrossRef]
- Xu, J.; Liang, W.; Cai, J.; Xiong, J.; Huang, C.; Xu, Z.; Guan, J. Long term outcomes and risk factors of compensatory hyperhidrosis after thoracoscopic sympathectomy in primary palmar hyperhidrosis patients: A retrospective single-center study. J. Cardiothorac. Surg. 2024, 19, 590. [Google Scholar] [CrossRef]
- Raveglia, F.; Orlandi, R.; Guttadauro, A.; Cioffi, U.; Cardillo, G.; Cioffi, G.; Scarci, M. How to prevent, reduce, and treat severe post sympathetic chain compensatory hyperhidrosis: 2021 state of the art. Front. Surg. 2022, 8, 814916. [Google Scholar] [CrossRef]
- Horslen, L.C.; Wilshire, C.L.; Louie, B.E.; Vallières, E. Long-term impact of endoscopic thoracic sympathectomy for primary palmar hyperhidrosis. Ann. Thorac. Surg. 2018, 106, 1008–1012. [Google Scholar] [CrossRef]
- Martínez-Hernández, N.J.; Estors-Guerrero, M.; Galbis-Caravajal, J.M.; Hervás-Marín, D.; Roig-Bataller, A. Endoscopic thoracic sympathectomy for primary hyperhidrosis: An over a decade-long follow-up on efficacy, impact, and patient satisfaction. J. Thorac. Dis. 2024, 16, 8292–8299. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Askari, A.; Kordzadeh, A.; Lee, G.H.; Harvey, M. Endoscopic thoracic sympathectomy for primary hyperhidrosis: A 16-year follow-up in a single UK centre. Surgeon 2013, 11, 130–133. [Google Scholar] [CrossRef]
- Lima, S.O.; Neto, J.M.; Fontes, L.M.; Figueiredo, M.B.G.d.A.; Santos, J.M.; Santana, V.R. Evaluation of quality of life (QOL) of young patients with primary hyperhidrosis (PH) before and after endoscopic thoracic sympathectomy (ETS). J. Am. Acad. Dermatol. 2023, 88, e197–e201. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Kumaya, Y.; Hirayama, Y.; Oda, H.; Cho, H.; Huang, C.L. Single-center experience of thoracoscopic sympathectomy for palmar hyperhidrosis with long-term postoperative questionnaire survey. Gen. Thorac. Cardiovasc. Surg. 2024, 72, 732–737. [Google Scholar] [CrossRef]
- Turhan, K.; Kavurmaci, Ö.; Akçam, T.I.; Ergönül, A.G.; Özdil, A.; Çakan, A.; Çağirici, U. Long-term outcomes and course of compensatory sweating after endoscopic sympathicotomy. Thorac. Cardiovasc. Surg. 2022, 70, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Li, D.C.; Hulbert, A.; Waldbaum, B.; Ober, C.; Hooker, C.M.; Huang, P.; Molena, D.; Yang, S.C.; Ito, T.; Perry-Parrish, C.; et al. Endoscopic thoracic sympathectomy for primary focal hyperhidrosis: Impact on psycho-social symptomatology and psychotropic medication use. Eur. J. Cardiothorac. Surg. 2018, 54, 904–911. [Google Scholar] [CrossRef]
- Shabat, S.; Furman, D.; Kupietzky, A.; Srour, B.B.; Mordechai-Heyn, T.; Grinbaum, R.; Mazeh, H.; Mizrahi, I. Long-term outcomes of endoscopic thoracoscopic sympathectomy for primary focal palmar hyperhidrosis: High patient satisfaction rates despite significant compensatory hyperhidrosis. Surg. Laparosc. Endosc. Percutan Tech. 2022, 32, 730–735. [Google Scholar] [CrossRef]
- Cai, S.-W.; Shen, N.; Li, D.-X.; Wei, B.; An, J.; Zhang, J.-H. Compensatory sweating after restricting or lowering the level of sympathectomy: A systematic review and meta-analysis. Clinics 2015, 70, 214–219. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Y.; Zou, W.; Mao, N.; Tang, J.; Jiang, L.; Zou, G.; Yang, L.; Yu, B.; Wei, G. Impact of endoscopic thoracic R4 sympathicotomy combined with R3 ramicotomy for primary palmar hyperhidrosis. Front. Surg. 2023, 10, 1144299. [Google Scholar] [CrossRef] [PubMed]
- Loizzi, D.; Mongiello, D.; Bevilacqua, M.T.; Raveglia, F.; Fiorelli, A.; Congedo, M.T.; Ardò, N.P.; Sollitto, F. Surgical management of compensatory sweating: A systematic review. Front. Surg. 2023, 10, 1160827. [Google Scholar] [CrossRef] [PubMed]


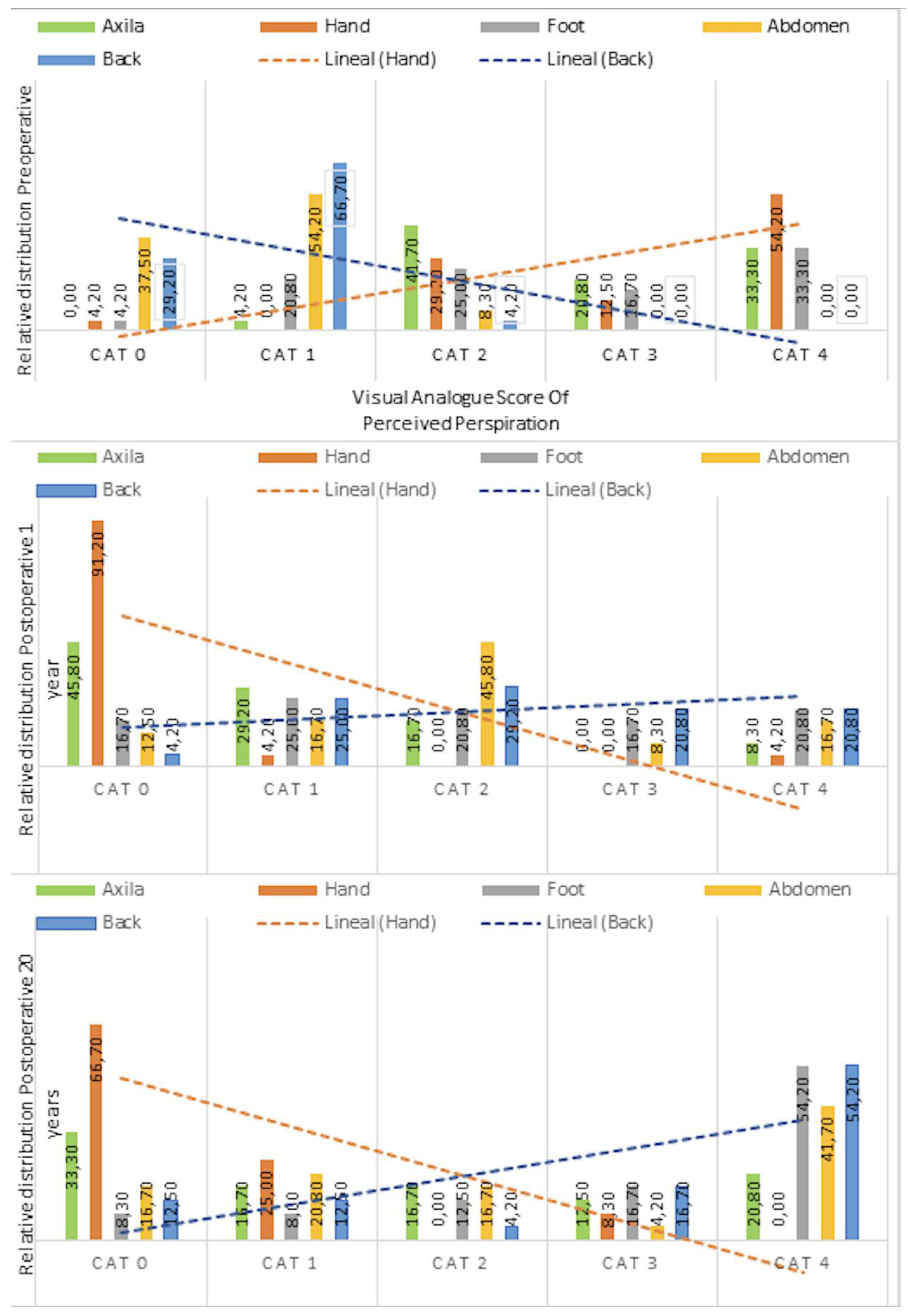
| Anatomical Region | Preoperative (n = 24) | 1 Year Postoperative (n = 24) | 20 Years Postoperative (n = 24) | p Value |
|---|---|---|---|---|
| Median (IQR) | ||||
| Axilla | 3.0 (2.0, 4.0) | 1.0 (0.0, 1.2) | 1.5 (0.0, 3.0) | <0.001 |
| Hand | 4.0 (2.0, 4.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 1.0) | <0.001 |
| Foot | 2.5 (1.8, 4.0) | 2.0 (1.0, 3.0) | 4.0 (2.0, 4.0) | 0.053 |
| Abdomen | 1.0 (0.0, 1.0) | 2.0(1.0, 2.2) | 2.0 (1.0, 4.0) | <0.001 |
| Back | 1.0 (0.0, 1.0) | 2.0 (1.0, 3.0) | 4.0 (1.8, 4.0) | <0.001 |
| Preoperative (n = 24) | 1 Year Postop (n = 24) | 20 Years Postop (n = 24) | p Value | |
|---|---|---|---|---|
| Mean ± SD | ||||
| Anxiety level | 2 ±1.02 | 0.37 ± 0.57 | 0.16 ± 0.81 | <0.001 |
| Degree of satisfaction | NA | 3.33 ± 0.70 | 3.04 ± 1.30 | 0.306 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ureña, A.; Grando, L.; Rodriguez-Gussinyer, L.; Macía, I.; Rivas, F.; Quiroga, N.I.; Moreno, C.; Michavilla, X.; Ramos, R. 20-Year Efficacy of Endoscopic Thoracic Sympathectomy for Primary Hyperhidrosis: A Cohort Study. J. Clin. Med. 2025, 14, 4831. https://doi.org/10.3390/jcm14144831
Ureña A, Grando L, Rodriguez-Gussinyer L, Macía I, Rivas F, Quiroga NI, Moreno C, Michavilla X, Ramos R. 20-Year Efficacy of Endoscopic Thoracic Sympathectomy for Primary Hyperhidrosis: A Cohort Study. Journal of Clinical Medicine. 2025; 14(14):4831. https://doi.org/10.3390/jcm14144831
Chicago/Turabian StyleUreña, Anna, Leandro Grando, Lluisa Rodriguez-Gussinyer, Ivan Macía, Francisco Rivas, Nestor Iván Quiroga, Camilo Moreno, Xavier Michavilla, and Ricard Ramos. 2025. "20-Year Efficacy of Endoscopic Thoracic Sympathectomy for Primary Hyperhidrosis: A Cohort Study" Journal of Clinical Medicine 14, no. 14: 4831. https://doi.org/10.3390/jcm14144831
APA StyleUreña, A., Grando, L., Rodriguez-Gussinyer, L., Macía, I., Rivas, F., Quiroga, N. I., Moreno, C., Michavilla, X., & Ramos, R. (2025). 20-Year Efficacy of Endoscopic Thoracic Sympathectomy for Primary Hyperhidrosis: A Cohort Study. Journal of Clinical Medicine, 14(14), 4831. https://doi.org/10.3390/jcm14144831








