Heterogeneity in Heart Failure with Preserved Ejection Fraction: A Systematic Review of Phenotypic Classifications and Clinical Implications
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Eligibility Criteria
- Population: adult patients (≥18 years) diagnosed with HFpEF, defined as left ventricular ejection fraction (LVEF) ≥50% or as per study-specific criteria.
- Intervention/focus: studies aiming to define or classify HFpEF phenotypes based on clinical, echocardiographic, hemodynamic, biochemical, or data-driven (e.g., cluster analysis or machine learning) methodologies.
- Study types: observational cohort studies, cross-sectional analyses, post hoc analyses of randomized controlled trials (RCTs), and prospective registries.
- Publication language: English or Spanish.
- Publication date: from January 2010 to April 2025, to capture contemporary understanding of HFpEF phenotyping.
- Reviews, editorials, letters to the editor, case reports, and conference abstracts.
- Studies focused exclusively on HFrEF or HFmrEF (heart failure with mildly reduced ejection fraction).
- Preclinical, animal-based, or in vitro studies.
PICO Framework
- Population (P): adults diagnosed with HFpEF.
- Intervention (I): classification into clinical or mechanistic phenotypes.
- Comparison (C): not applicable; some studies may include internal or external validation of phenotypic models.
- Outcomes (O): description of phenotypic clusters; prognostic stratification; treatment response; methodological characteristics of phenotype derivation.
2.3. Search Strategy
- (“heart failure with preserved ejection fraction” OR “HFpEF” OR “diastolic heart failure”) AND
- (“phenotype” OR “phenotyping” OR “classification” OR “subtype” OR “cluster analysis” OR “latent class” OR “machine learning”) AND
- (“clinical characteristics” OR “prognosis” OR “biomarkers” OR “echocardiography” OR “comorbidities”).
2.4. Study Selection
2.5. Data Extraction and Synthesis
- -
- Study design, setting, and sample size;
- -
- Diagnostic criteria for HFpEF;
- -
- Methodology used for phenotyping (e.g., statistical model, variables considered);
- -
- Number and type of phenotypes identified;
- -
- Baseline characteristics of each phenotype;
- -
- Prognostic implications (e.g., mortality, hospitalization);
- -
- Treatment response stratified by phenotype (if available);
- -
- Due to expected methodological heterogeneity, a narrative synthesis approach was employed, with results summarized in structured tables, with studies grouped by phenotyping method and phenotypic characteristics;
- -
- Risk of bias and quality assessment.
2.6. PRISMA Compliance
3. Results
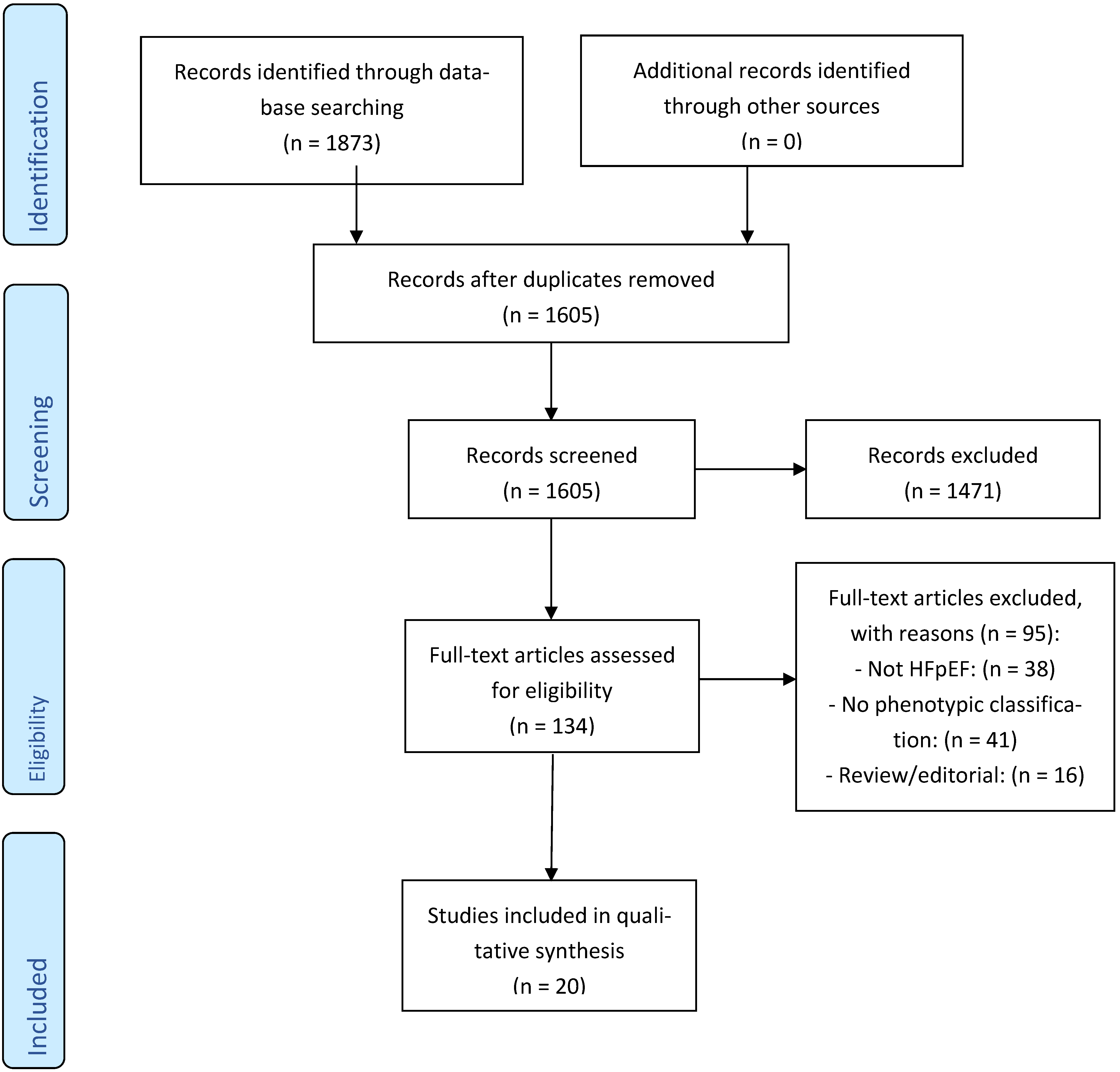
3.1. Study Selection
Characteristics of Included Studies
3.2. Phenotyping Methodologies
3.3. Recurrent Phenotypic Patterns
- The metabolic phenotype typically included patients who were obese, diabetic, hypertensive with high inflammatory markers. This phenotype often displayed preserved systolic and relatively mild diastolic dysfunction, but a higher risk of rehospitalization.
- The atrial fibrillation/cardiometabolic phenotype was characterized by a high prevalence of AF, left atrial enlargement, and elevated NT-proBNP levels. This cluster often had a mixed prognosis, with variable responses to guideline-directed therapy.
- The younger hypertensive phenotype comprised patients with fewer comorbidities, well-preserved diastolic function, and lower natriuretic peptide levels. These individuals tended to have a more favorable prognosis but were under-represented in trials.
- The elderly–frail phenotype encompassed older individuals with sarcopenia, polypharmacy, cognitive impairment, and often reduced functional reserve. This phenotype had the worst quality of life scores and highest all-cause mortality.
- The cardiorenal phenotype was defined by moderate to severe renal impairment, volume overload, and a high prevalence of anemia. Prognosis was generally poor, with frequent hospitalizations and rapid functional decline.
- The right heart–pulmonary phenotype included patients with evidence of pulmonary hypertension, elevated right ventricular systolic pressure, and tricuspid regurgitation. This group often presented with signs of systemic congestion and had poor exercise tolerance.
3.4. Prognostic Implications
3.5. Methodological Quality
4. Discussion
4.1. Clinical and Pathophysiological Insights from Recurrent Phenotypes
4.2. Methodological Challenges in Phenotyping
4.3. Implications for Clinical Practice and Therapeutics
4.4. Future Directions and the Promise of Precision Medicine
5. Conclusions and Future Directions
5.1. Future Directions
5.1.1. Development of Standardized, Reproducible Phenotyping Algorithms
5.1.2. Prospective Validation of Phenotypes in Diverse Cohorts
5.1.3. Incorporation of Multi-Omic and Digital Health Data
5.1.4. Design of Phenotype-Stratified Clinical Trials
5.1.5. Implementation of Clinical Tools for Phenotype Identification
5.1.6. Understanding Phenotype Evolution and Transitions
5.1.7. Integration of Patient-Centered Outcomes and Quality of Life
Funding
Conflicts of Interest
References
- Abdin, A.; Böhm, M.; Shahim, B.; Karlström, P. Heart failure with preserved ejection fraction: Epidemiology, pathophysiology, diagnosis and treatment strategies. Int. J. Cardiol. 2024, 412, 132304. [Google Scholar] [CrossRef] [PubMed]
- Dunlay, S.M.; Roger, V.L.; Redfield, M.M. Epidemiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2017, 14, 591–602. [Google Scholar] [CrossRef]
- Borlaug, B.A. Evaluation and management of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2020, 17, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Lyass, A.; Enserro, D.; Larson, M.G.; Ho, J.E.; Kizer, J.R.; Gottdiener, J.S.; Psaty, B.M.; Vasan, R.S. Temporal trends in the incidence of and mortality associated with heart failure with preserved and reduced ejection fraction. JACC Heart Fail. 2018, 6, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Morfino, P.; Aimo, A.; Castiglione, V.; Vergaro, G. Biomarkers of HFpEF: Natriuretic peptides, high-sensitivity troponins and beyond. J. Cardiovasc. Dev. Dis. 2022, 9, 256. [Google Scholar] [CrossRef]
- Luo, L.; Zuo, Y.; Dai, L. Metabolic rewiring and inter-organ crosstalk in diabetic HFpEF. Cardiovasc. Diabetol. 2025, 24, 13. [Google Scholar] [CrossRef]
- Valero-Muñoz, M.; Saw, E.L.; Hekman, R.M.; Blum, B.C.; Hourani, Z.; Granzier, H.; Emili, A.; Sam, F. Proteomic and phosphoproteomic profiling in heart failure with preserved ejection fraction (HFpEF). Front. Cardiovasc. Med. 2022, 9, 966968. [Google Scholar] [CrossRef]
- Heinzel, F.R.; Shah, S.J. The future of heart failure with preserved ejection fraction: Deep phenotyping for targeted therapeutics. Herz 2022, 47, 215–223. [Google Scholar] [CrossRef]
- Segar, M.W.; Patel, K.V.; Ayers, C.; Basit, M.; Tang, W.H.W.; Willett, D.; Berry, J.; Grodin, J.L.; Pandey, A. Phenomapping of patients with heart failure with preserved ejection fraction using machine learning-based unsupervised cluster analysis. Eur. J. Heart Fail. 2020, 22, 1487–1495. [Google Scholar] [CrossRef]
- Hedman, Å.K.; Hage, C.; Sharma, A.; Brosnan, M.J.; Buckbinder, L.; Gan, L.M.; Shah, S.J.; Linde, C.M.; Donal, E.; Daubert, J.-C.; et al. Identification of novel pheno-groups in heart failure with preserved ejection fraction using machine learning. Heart 2020, 106, 342–349. [Google Scholar] [CrossRef]
- Meijs, C.; Handoko, M.L.; Savarese, G.; Vernooij, R.W.M.; Vaartjes, I.; Banerjee, A.; Koudstaal, S.; Brugts, J.J.; Asselbergs, F.W.; Uijl, A. Discovering distinct phenotypical clusters in heart failure across the ejection fraction spectrum: A systematic review. Curr. Heart Fail. Rep. 2023, 20, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Casebeer, A.; Horter, L.; Hayden, J.; Beane, R.; Broyles, R. Phenotypic clustering of heart failure with preserved ejection fraction reveals different rates of hospitalization. J. Cardiovasc. Med. 2021, 22, 8–14. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Anand, I.S.; Ge, J.; Lam, C.S.P.; Maggioni, A.P.; Martinez, F.; Packer, M.; Pfeffer, M.A.; Pieske, B.; et al. Angiotensin–neprilysin inhibition in heart failure with preserved ejection fraction. N. Engl. J. Med. 2019, 381, 1609–1620. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Bohm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in heart failure with a preserved ejection fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; McMurray, J.J.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.; Martinez, F.; et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Pfeffer, M.A.; Assmann, S.F.; Boineau, R.; Anand, I.S.; Claggett, B.; Clausell, N.; Desai, A.S.; Diaz, R.; Fleg, J.L.; et al. Spironolactone for heart failure with preserved ejection fraction. N. Engl. J. Med. 2014, 370, 1383–1392. [Google Scholar] [CrossRef]
- Nguyen, N.T.V.; Nguyen, H.A.; Nguyen, H.H.; Truong, B.Q.; Chau, H.N. Phenotype-specific outcome and treatment response in heart failure with preserved ejection fraction with comorbid hypertension and diabetes: A 12-month observational study. J. Pers. Med. 2023, 13, 1218. [Google Scholar] [CrossRef]
- Lin, C.Y.; Sung, H.Y.; Chen, Y.J.; Yeh, H.-I.; Hou, C.J.-Y.; Tsai, C.-T.; Hung, C.-L. Personalized management for heart failure with preserved ejection fraction. J. Pers. Med. 2023, 13, 746. [Google Scholar] [CrossRef]
- Palazzuoli, A.; Severino, P.; D’Amato, A.; Myftari, V.; Tricarico, L.; Correale, M.; Dattilo, G.; Fioretti, F.; Nodari, S. Distinct profiles and new pharmacological targets for heart failure with preserved ejection fraction. Rev. Cardiovasc. Med. 2024, 25, 270. [Google Scholar] [CrossRef]
- Rosano, G.M.C.; Vitale, C. Precision cardiology: Phenotype-targeted therapies for HFmrEF and HFpEF. Int. J. Heart Fail. 2024, 6, 47–59. [Google Scholar] [CrossRef]
- Rabkin, S.W. Evaluating the adverse outcome of subtypes of heart failure with preserved ejection fraction defined by machine learning: A systematic review focused on defining high risk phenogroups. EXCLI J. 2022, 21, 487–518. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Guo, H.; Wang, W.; Wang, X.; Ding, J.; Wang, H. Identifying novel subgroups in heart failure patients with unsupervised machine learning: A scoping review. Front. Cardiovasc. Med. 2022, 9, 895836. [Google Scholar] [CrossRef]
- Shah, S.J.; Katz, D.H.; Selvaraj, S.; Burke, M.A.; Yancy, C.W.; Gheorghiade, M.; Bonow, R.O.; Huang, C.-C.; Deo, R.C. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation 2015, 131, 269–279. [Google Scholar] [CrossRef]
- Pieske, B.; Tschoepe, C.; de Boer, R.A.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.P.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA-PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 40, 3297–3317. [Google Scholar] [CrossRef]
- Banerjee, A.; Dashtban, A.; Chen, S.; Pasea, L.; Thygesen, J.H.; Fatemifar, G.; Tyl, B.; Dyszynski, T.; Asselbergs, F.W.; Lund, L.H.; et al. Identifying subtypes of heart failure from three electronic health record sources with machine learning: An external, prognostic, and genetic validation study. Lancet Digit Health 2023, 5, e370–e379. [Google Scholar] [CrossRef] [PubMed]
- Nauta, J.F.; Hummel, Y.M.; Tromp, J.; Ouwerkerk, W.; van der Meer, P.; Jin, X.; de Vries, C.J.; van Veldhuisen, D.J.; Voors, A.A. Concomitant pulmonary hypertension in heart failure with preserved ejection fraction: A latent class analysis. Eur. J. Heart Fail. 2020, 22, 1007–1015. [Google Scholar] [CrossRef]
- Flint, K.M.; Shah, S.J.; Lewis, E.F.; Kao, D.P. Variation in clinical and patient-reported outcomes among complex heart failure with preserved ejection fraction phenotypes. ESC Heart Fail 2020, 7, 811–824. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abdelhamid, M.; Al Ghalayini, K.; Al-Humood, K.; Altun, B.; Arafah, M.; Bader, F.; Ibrahim, M.; Sabbour, H.; Shawky Elserafy, A.; Skouri, H.; et al. Regional expert opinion: Management of heart failure with preserved ejection fraction in the Middle East, North Africa and Turkey. ESC Heart Fail 2023, 10, 2773–2787. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tromp, J.; van Veldhuisen, D.J.; Pratt, C.; van der Meer, P.; Edelmann, F.; Narayanan, N.; Scharhag, J.; Nishimura, R.A.; Anker, S.D.; Vasan, R.S.; et al. Multinational clustering of heart failure with preserved ejection fraction phenotypes in Asia. Eur. Heart J. 2022, 43, 2577–2589. [Google Scholar] [CrossRef]
- Aimo, A.; Vergaro, G.; Passino, C.; Corrà, U.; Cannatà, A.; Piepoli, M.F.; Metra, M.; Rengo, F.; Tavazzi, L.; Volterrani, M.; et al. Support vector machine-based clustering in heart failure with preserved ejection fraction. ESC Heart Fail. 2021, 8, 545–555. [Google Scholar] [CrossRef]
- Harada, T.; Kagami, K.; Kato, T.; Obokata, M. Echocardiography in the diagnostic evaluation and phenotyping of heart failure with preserved ejection fraction. J. Cardiol. 2022, 79, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Upadhya, B.; Deo, R.C.; Sudarshan, G.; Horne, B.D.; Hernandez, A.F.; Adabag, A.S.; Zile, M.R.; Baicu, C.F. Combining principal component analysis and cluster analysis to phenotype heart failure with preserved ejection fraction. J. Am. Geriatr. Soc. 2019, 67, 1232–1240. [Google Scholar] [CrossRef]
- Schelbert, E.B.; Drazner, M.H.; Seliger, S.L.; Bahrami, H.; Daniels, L.B.; Gowda, S.; Redfield, M.M.; de Lemos, J.A.; Gopal, D.M. Decision tree classification of heart failure with preserved ejection fraction phenotypes. JACC Cardiovasc. Imaging 2017, 10, 1211–1221. [Google Scholar] [CrossRef]
- Tanaka, H.; Nakamura, M.; Sato, A.; Fujimoto, Y.; Yamamoto, H.; Suzuki, K.; Ueda, Y.; Otsuka, K.; Ito, H. Latent class analysis of a Japanese heart failure with preserved ejection fraction cohort. J. Cardiol. 2020, 76, 345352. [Google Scholar] [CrossRef]
- Chung, H.; Kim, J.; Lee, S.; Park, H.; Choi, D.; Kim, Y.; Lim, Y.; Shin, J.; Hong, G.; Kim, N. Bayesian clustering for phenotyping heart failure with preserved ejection fraction in a South Korean population. Korean Circ. J. 2021, 51, 387–396. [Google Scholar] [CrossRef]
- Lim, W.Y.; Tan, C.H.; Lee, K.H.; Wong, Y.H.; Ng, C.L.; Cheung, F.; Ho, S.Y.; Chan, Y.H.; Ching, C.K.; Tan, T.B. Neural network-based clustering for identification of phenotypes in heart failure with preserved ejection fraction: A Malaysian cohort. Int. J. Cardiol. 2022, 361, 45–52. [Google Scholar] [CrossRef]
- Jasinska-Piadlo, A.; Campbell, P. Management of patients with heart failure and preserved ejection fraction. Heart 2023, 109, 874–883. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nouraei, H.; Rabkin, S.W. A new approach to the clinical subclassification of heart failure with preserved ejection fraction. Int. J. Cardiol. 2021, 331, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Hegde, S.M.; Iyengar, A.P.; Shah, S.J.; Nair, G.M.; Menon, S.; Ramesh, S.; Verma, S.; Bhatia, D. Development of a clinical severity index for risk stratification in heart failure with preserved ejection fraction. Indian. Heart J. 2019, 71, 192–198. [Google Scholar] [CrossRef]
- Gori, M.; Santini, M.; Mele, D.; Rengo, F.; Passantino, A.; Iacoviello, M.; Garofalo, O.; Marra, A.M.; Vigorito, C.; Michelin, M.; et al. Comorbidity-driven phenotyping in heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2014, 16, 1006–1014. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Nishimura, R.A.; Sorajja, P.; Lam, C.S.; Redfield, M.M.; Kass, D.A.; O’Connor, C.M.; Stevenson, L.W.; Felker, G.M. Invasive hemodynamic profiling in heart failure with preserved ejection fraction. Circ. Heart Fail. 2015, 8, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Obokata, M.; Reddy, Y.N.V.; Pislaru, S.V.; Melenovsky, V.; Borlaug, B.A. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 2017, 136, 6–19. [Google Scholar] [CrossRef] [PubMed]
- van Woerden, G.; van Veldhuisen, D.J.; Westenbrink, B.D.; de Boer, R.A.; Rienstra, M.; Gorter, T.M. Connecting epicardial adipose tissue and heart failure with preserved ejection fraction: Mechanisms, management and modern perspectives. Eur. J. Heart Fail. 2022, 24, 2238–2250. [Google Scholar] [CrossRef] [PubMed]
- Kosiborod, M.N.; Abildstrøm, S.Z.; Borlaug, B.A.; Butler, J.; Rasmussen, S.; Davies, M.; Hovingh, G.K.; Kitzman, D.W.; Lindegaard, M.L.; Møller, D.V. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N. Engl. J. Med. 2023, 389, 1069–1084. [Google Scholar] [CrossRef]
- Pugliese, N.R.; Pellicori, P.; Filidei, F.; De Biase, N.; Maffia, P.; Guzik, T.J.; Masi, S.; Taddei, S.; Cleland, J.G.F. Inflammatory pathways in heart failure with preserved left ventricular ejection fraction: Implications for future interventions. Cardiovasc. Res. 2023, 118, 3536–3555. [Google Scholar] [CrossRef]
- Kao, D.P.; Lewsey, J.D.; Anand, I.S.; Massie, B.M.; Zile, M.R.; Carson, P.E.; McKelvie, R.S.; Komajda, M.; McMurray, J.J.; Yusuf, S. Characterization of subgroups of heart failure patients with preserved ejection fraction with possible implications for prognosis and treatment response. Eur. J. Heart Fail. 2015, 17, 925–935. [Google Scholar] [CrossRef]
- Bekfani, T.; Pellicori, P.; Morris, D.A.; Ebner, N.; Valentova, M.; Steinbeck, L.; Sandek, A.; Herrmann-Lingen, C.; Wachter, R.; Düngen, H.D.; et al. Sarcopenia in patients with heart failure with preserved ejection fraction: Impact on muscle strength, exercise capacity and quality of life. Int. J. Cardiol. 2016, 222, 416. [Google Scholar] [CrossRef]
- Mone, P.; Lombardi, A.; Gambardella, J.; Pansini, A.; Macina, G.; Morgante, M.; Frullone, S.; Santulli, G. Empagliflozin Improves Cognitive Impairment in Frail Older Adults with Type 2 Diabetes and Heart Failure with Preserved Ejection Fraction. Diabetes Care 2022, 45, 1247–1251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reddy, Y.N.V.; Carter, R.E.; Obokata, M.; Redfield, M.M.; Borlaug, B.A. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation 2018, 138, 861–870. [Google Scholar] [CrossRef]
- Sanchis, L.; Andrea, R.; Falces, C.; Poyatos, S.; Vidal, B.; Sitges, M. Left atrial dysfunction relates to symptom onset in patients with heart failure and preserved ejection fraction. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 62–67. [Google Scholar] [CrossRef]
- Fudim, M.; Carlisle, M.A.; Devaraj, S.; Ajam, T.; Ambrosy, A.P.; Pokorney, S.D.; Al-Khatib, S.M.; Kamalesh, M. One-year mortality after implantable cardioverter-defibrillator placement within the Veterans Affairs Health System. Eur. J. Heart Fail. 2020, 22, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, D.; Lam, C.S.P.; Van Veldhuisen, D.J.; Van Gelder, I.C.; Voors, A.A.; Rienstra, M. Heart failure with preserved ejection fraction and atrial fibrillation: Vicious twins. J. Am. Coll. Cardiol. 2016, 68, 2217–2228. [Google Scholar] [CrossRef]
- Metra, M.; Torp-Pedersen, C.; Swedberg, K.; Cleland, J.G.; Di Lenarda, A.; Komajda, M.; Remme, W.J.; Lutiger, B.; Scherhag, A.; Lukas, M.A.; et al. Influence of heart rate, blood pressure, and beta-blocker dose on outcome and the differences in outcome between carvedilol and metoprolol tartrate in patients with chronic heart failure: Results from the COMET trial. Eur. Heart J. 2005, 26, 2259–2268. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; McCullough, P.; Anker, S.D.; Anand, I.; Aspromonte, N.; Bagshaw, S.M.; Bellomo, R.; Berl, T.; Bobek, I.; Cruz, D.N.; et al. Cardio-renal syndromes: Report from the consensus conference of the acute dialysis quality initiative. Eur. Heart J. 2010, 31, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Jefferies, J.L.; Bartone, C.; Menon, S.; Egnaczyk, G.F.; O’Brien, T.M.; Chung, E.S. Ultrafiltration in heart failure with preserved ejection fraction: Comparison with systolic heart failure patients. Circ. Heart Fail. 2013, 6, 733–739. [Google Scholar] [CrossRef]
- Rangaswami, J.; Bhalla, V.; Blair, J.E.A.; Chang, T.I.; Costa, S.; Lentine, K.L.; Lerma, E.V.; Mezue, K.; Molitch, M.; Mullens, W.; et al. Cardiorenal syndrome: Classification, pathophysiology, diagnosis, and treatment strategies: A scientific statement from the American Heart Association. Circulation 2019, 139, e840–e878. [Google Scholar] [CrossRef]
- Damman, K.; Testani, J.M. The kidney in heart failure: An update. Eur. Heart J. 2015, 36, 1437–1444. [Google Scholar] [CrossRef]
- Guazzi, M.; Naeije, R. Right heart phenotype in heart failure with preserved ejection fraction. Circ. Heart Fail. 2021, 14, e007840. [Google Scholar] [CrossRef]
- Gorter, T.M.; Hoendermis, E.S.; van Veldhuisen, D.J.; Voors, A.A.; Lam, C.S.P.; Geelhoed, B.; Willems, T.P.; van Melle, J.P. Right ventricular dysfunction in heart failure with preserved ejection fraction: A systematic review and meta-analysis. Eur. J. Heart Fail. 2016, 18, 1472–1487. [Google Scholar] [CrossRef]
- Guazzi, M.; Dixon, D.; Labate, V.; Beussink-Nelson, L.; Bandera, F.; Cuttica, M.J.; Shah, S.J. RV contractile function and its coupling to pulmonary circulation in heart failure with preserved ejection fraction: Stratification of clinical phenotypes and outcomes. JACC Cardiovasc. Imaging 2017, 10 Pt. B, 1211–1221. [Google Scholar] [CrossRef]
- Bernardo, R.J.; Haddad, F.; Couture, E.J.; Hansmann, G.; de Jesus Perez, V.A.; Denault, A.Y.; de Man, F.S.; Amsallem, M. Mechanics of right ventricular dysfunction in pulmonary arterial hypertension and heart failure with preserved ejection fraction. Cardiovasc. Diagn. Ther. 2020, 10, 1580–1603. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.N.V.; Kaye, D.M.; Handoko, M.L.; van de Bovenkamp, A.A.; Tedford, R.J.; Keck, C.; Andersen, M.J.; Sharma, K.; Trivedi, R.K.; Carter, R.E.; et al. Diagnosis of heart failure with preserved ejection fraction among patients with unexplained dyspnea. JAMA Cardiol. 2022, 7, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Golla, M.; Golla, S. Heart failure with preserved ejection fraction. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Shah, S.J.; Katz, D.H.; Deo, R.C. Phenotypic spectrum of heart failure with preserved ejection fraction. Heart Fail. Clin. 2014, 10, 407–418. [Google Scholar] [CrossRef]
- Aimo, A.; Januzzi, J.L.; Vergaro, G.; Emdin, M. Prognostic value of high-sensitivity troponins in patients with heart failure with preserved ejection fraction: A systematic review and meta-analysis. Int. J. Cardiol. 2018, 267, 150–155. [Google Scholar] [CrossRef]
- Epelde, F. Impact of Exercise on Physiological, Biochemical, and Analytical Parameters in Patients with Heart Failure with Reduced Ejection Fraction. Medicina 2024, 60, 2017. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Epelde, F. Transforming Diabetes Care: The Expanding Role of DPP-4 Inhibitors in Cardiovascular and Renal Protection. Medicina 2024, 60, 1793. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shah, S.J.; Borlaug, B.A.; Kitzman, D.W.; McCulloch, A.D.; Blaxall, B.C.; Agarwal, R.; Chirinos, J.A.; Collins, S.P.; Deo, R.C.; Gladwin, M.T.; et al. Research priorities for heart failure with preserved ejection fraction: National Heart, Lung, and Blood Institute Working Group Summary. Circulation 2020, 141, 1001–1026. [Google Scholar] [CrossRef]
- Kitzman, D.W.; Shah, S.J.; Borlaug, B.A.; van Heerebeek, L.; Zile, M.R.; Kass, D.A.; Obokata, M.; Lam, C.S.P.; Paulus, W.J. Precision medicine for heart failure with preserved ejection fraction: An overview. J. Cardiovasc. Transl. Res. 2021, 14, 169–180. [Google Scholar]
| Study | Country | N | Phenotyping Method | # of Phenotypes | Key Phenotypes Identified |
|---|---|---|---|---|---|
| Shah et al. (2015) [23] | USA | 419 | Cluster analysis | 3 | Obese–diabetic, older–atrial fibrillation, lean–hypertensive |
| Pieske et al. (2019) [24] | Germany | 1450 | Latent class analysis | 4 | Cardiorenal, metabolic, right-sided HF, low BNP |
| Segar et al. (2020) [9] | USA | 892 | Machine learning (Random Forest) | 5 | Metabolic syndrome, pulmonary HTN, AF-dominant, elderly–frail, high output |
| Banerjee et al. (2023) [25] | Japan | 608 | Hierarchical clustering | 3 | AF with preserved RV function, frail–elderly, low BNP–young |
| Nauta et al. (2020) [26] | Netherlands | 517 | Latent profile analysis | 4 | Younger hypertensive, frail–elderly, obese–metabolic, low output |
| Flint et al. (2020) [27] | Taiwan | 1132 | Unsupervised clustering | 3 | Obese–diabetic, AF-dominant, renal impairment |
| Abdelhamid et al. (2023) [28] | Saudi Arabia | 264 | Logistic regression modeling | 2 | Mild HFpEF vs. severe HFpEF |
| Tromp et al. (2022) [29] | Singapore | 1024 | K-means clustering | 4 | Young–low comorbidity, metabolic, cardiorenal, pulmonary HTN |
| Aimo et al. (2021) [30] | Italy | 684 | Machine learning (SVM) | 5 | Inflammatory, fibrotic, right-heart failure, metabolic, low risk |
| Harada et al. (2022) [31] | Japan | 315 | Echocardiographic pattern recognition | 3 | Exercise-induced HFpEF, invasive-hemodynamics-guided, pulmonary phenotype |
| Upadhya et al. (2019) [32] | USA | 723 | PCA + cluster analysis | 4 | Inflammatory–metabolic, fibrotic, low-risk–young, frailty-dominant |
| Schelbert et al. (2017) [33] | USA | 1048 | Decision tree classification | 3 | Diabetic–hypertensive, atrial fibrillation, preserved renal function |
| Tanaka et al. (2020) [34] | Japan | 506 | Latent class modeling | 4 | Young female-dominant, obese–diabetic, sarcopenic elderly, low output |
| Chung et al. (2021) [35] | South Korea | 634 | Bayesian clustering | 3 | High-output HF, cardiorenal phenotype, mild functional class |
| Lim et al. (2022) [36] | Malaysia | 478 | Neural-network-based clustering | 5 | AF and RV dysfunction, metabolic–inflammatory, renal-impaired, low congestion, mixed type |
| Jasinska-Piadlo et al. (2023) [37] | USA | 501 | Echocardiographic-guided subgrouping | 3 | Exercise-limited, metabolic comorbidity, preserved functional class |
| Nouraei et al. (2021) [38] | Japan | 687 | Recursive partitioning | 4 | Pulmonary HTN, metabolic–high BMI, mild HFpEF, AF–elderly |
| Hegde et al. (2019) [39] | India | 732 | Clinical severity index | 3 | Low risk, moderate risk, high symptom burden |
| Gori et al. (2014) [40] | Italy | 298 | Comorbidity clustering | 4 | Renal–metabolic, frail–female, pulmonary–HFpEF, younger males |
| Borlaug et al. (2015) [41] | USA | 423 | Invasive hemodynamic profiling | 2 | Normal PA pressure, elevated PA pressure with RV dysfunction |
| Phenotype | Key Characteristics | Prognostic Implication | Potential Treatment Focus |
|---|---|---|---|
| Metabolic–Obese | Obesity, diabetes, hypertension, systemic inflammation, high BMI | High rehospitalization, modest response to SGLT2i | SGLT2 inhibitors, weight loss, metabolic modulation |
| Frail–Elderly | Advanced age, sarcopenia, cognitive impairment, polypharmacy | Poor quality of life, highest mortality risk | Geriatric care, exercise rehab, palliative focus |
| Atrial-Fibrillation-Dominant | History of atrial fibrillation, enlarged LA, high NT-proBNP | Variable outcomes, challenging management | Rate/rhythm control, anticoagulation, ablation consideration |
| Cardiorenal | CKD, anemia, volume overload, diuretic resistance | High mortality, poor response to conventional therapy | Aggressive volume management, renal support |
| Right Heart/Pulmonary | Pulmonary hypertension, RV dysfunction, systemic congestion | Worst exercise capacity, high hospitalization risk | Investigational pulmonary vasodilators, RV protection |
| Younger Hypertensive | Middle-aged, mild symptoms, preserved function, low comorbidity burden | Generally favorable prognosis, often undertreated | Lifestyle modification, close follow-up |
| Inflammatory | Elevated CRP/IL-6, high WBC, systemic inflammation profile | Unknown therapeutic response, elevated systemic risk | Anti-inflammatory therapies (under investigation) |
| Fibrotic | LV fibrosis, diastolic stiffness, abnormal strain imaging | Progressive remodeling, risk of transition to HFrEF | Antifibrotic drugs, ARNI, strain monitoring |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Epelde, F. Heterogeneity in Heart Failure with Preserved Ejection Fraction: A Systematic Review of Phenotypic Classifications and Clinical Implications. J. Clin. Med. 2025, 14, 4820. https://doi.org/10.3390/jcm14144820
Epelde F. Heterogeneity in Heart Failure with Preserved Ejection Fraction: A Systematic Review of Phenotypic Classifications and Clinical Implications. Journal of Clinical Medicine. 2025; 14(14):4820. https://doi.org/10.3390/jcm14144820
Chicago/Turabian StyleEpelde, Francisco. 2025. "Heterogeneity in Heart Failure with Preserved Ejection Fraction: A Systematic Review of Phenotypic Classifications and Clinical Implications" Journal of Clinical Medicine 14, no. 14: 4820. https://doi.org/10.3390/jcm14144820
APA StyleEpelde, F. (2025). Heterogeneity in Heart Failure with Preserved Ejection Fraction: A Systematic Review of Phenotypic Classifications and Clinical Implications. Journal of Clinical Medicine, 14(14), 4820. https://doi.org/10.3390/jcm14144820






