Clinical Predictors of Inpatient Mortality and Poor Postoperative Course After aSAH Microsurgical Clipping: A 10-Year Experience from a Peruvian Tertiary Care Center
Abstract
1. Introduction
2. Materials and Methods
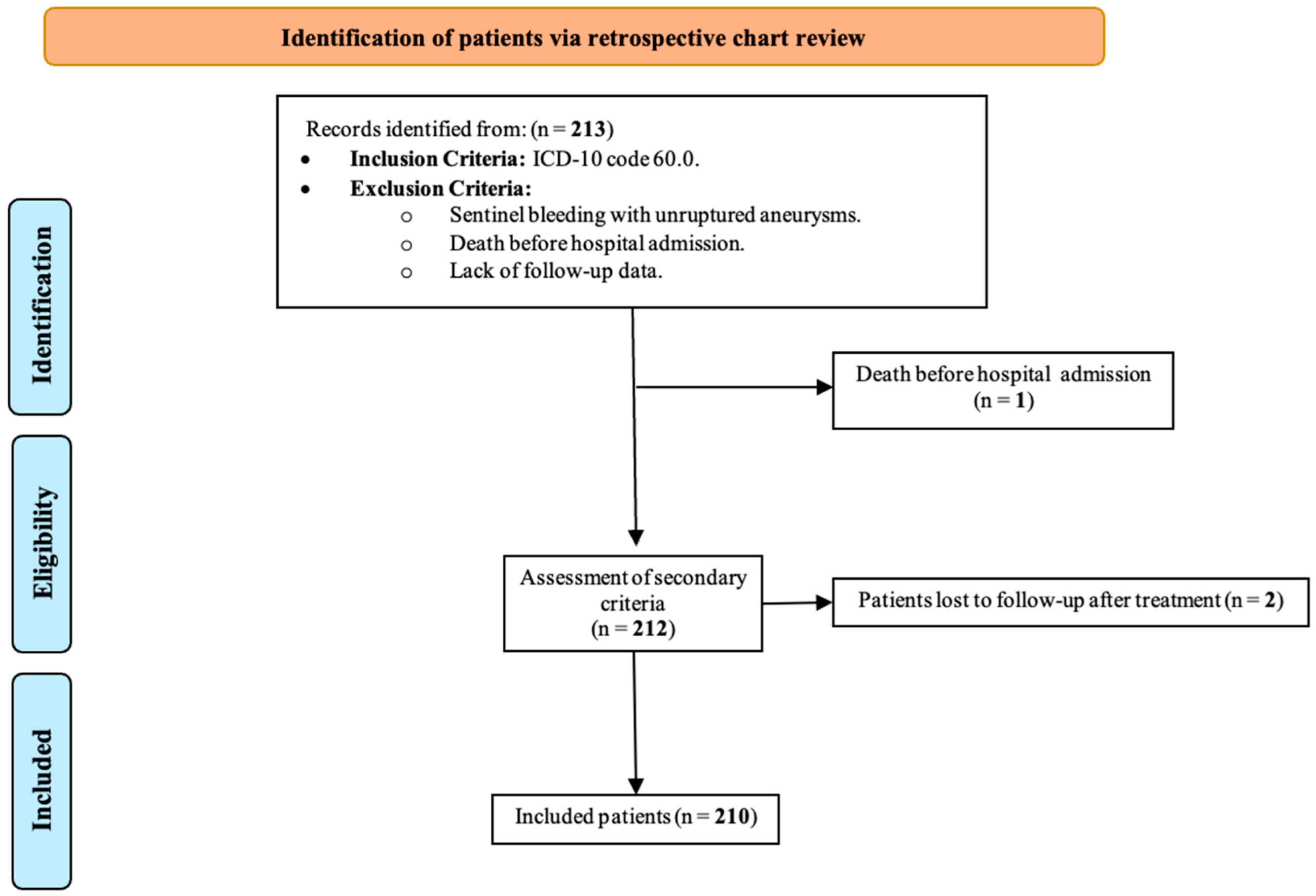
2.1. Study Cohort and Patient Selection
2.2. Demographic and Clinical Characteristics
2.3. Postoperative Outcomes
2.4. Statistical Methods
3. Results
3.1. Baseline Characteristics
3.2. Patient Characteristics According to Mortality and Postoperative Outcomes
3.3. Clinical Predictors of Inpatient Mortality
3.4. Clinical Predictors of Poor Postoperative Course
3.5. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| aSAH | Aneurysmatic subarachnoid hemorrhage |
| IQR | Interquartile range |
| WFNS | World Federation of Neurological Surgeons |
| CI | Confidence interval |
References
- van Donkelaar, C.E.; Bakker, N.A.; Birks, J.; Veeger, N.J.G.M.; Metzemaekers, J.D.M.; Molyneux, A.J.; Groen, R.J.M.; van Dijk, J.M.C. Prediction of Outcome After Aneurysmal Subarachnoid Hemorrhage. Stroke 2019, 50, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Stienen, M.N.; Germans, M.; Burkhardt, J.-K.; Neidert, M.C.; Fung, C.; Bervini, D.; Zumofen, D.; Röthlisberger, M.; Marbacher, S.; Maduri, R.; et al. Predictors of In-Hospital Death After Aneurysmal Subarachnoid Hemorrhage: Analysis of a Nationwide Database (Swiss SOS [Swiss Study on Aneurysmal Subarachnoid Hemorrhage]). Stroke 2018, 49, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Rinkel, G.J.E.; Algra, A. Long-term outcomes of patients with aneurysmal subarachnoid haemorrhage. Lancet Neurol. 2011, 10, 349–356. [Google Scholar] [CrossRef]
- Roquer, J.; Cuadrado-Godia, E.; Guimaraens, L.; Conesa, G.; Rodríguez-Campello, A.; Capellades, J.; García-Arnillas, M.P.; Fernández-Candil, J.L.; Avellaneda-Gómez, C.; Giralt-Steinhauer, E.; et al. Short- and long-term outcome of patients with aneurysmal subarachnoid hemorrhage. Neurology 2020, 95, e1819–e1829. [Google Scholar] [CrossRef]
- Al-Khindi, T.; Macdonald, R.L.; Schweizer, T.A. Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke 2010, 41, e519–e536. [Google Scholar] [CrossRef]
- Etminan, N.; Chang, H.-S.; Hackenberg, K.; de Rooij, N.K.; Vergouwen, M.D.I.; Rinkel, G.J.E.; Algra, A. Worldwide Incidence of Aneurysmal Subarachnoid Hemorrhage According to Region, Time Period, Blood Pressure, and Smoking Prevalence in the Population. JAMA Neurol. 2019, 76, 588–597. [Google Scholar] [CrossRef]
- de Rooij, N.K.; Linn, F.H.H.; van der Plas, J.A.; Algra, A.; Rinkel, G.J.E. Incidence of subarachnoid haemorrhage: A systematic review with emphasis on region, age, gender and time trends. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1365–1372. [Google Scholar] [CrossRef]
- Caranci, F.; Briganti, F.; Cirillo, L.; Leonardi, M.; Muto, M. Epidemiology and genetics of intracranial aneurysms. Eur. J. Radiol. 2013, 82, 1598–1605. [Google Scholar] [CrossRef]
- Mukhtar, T.K.; Molyneux, A.J.; Hall, N.; Yeates, D.R.G.; Goldacre, R.; Sneade, M.; Clarke, A.; Goldacre, M.J. The falling rates of hospital admission, case fatality, and population-based mortality for subarachnoid hemorrhage in England, 1999–2010. J. Neurosurg. 2016, 125, 698–704. [Google Scholar] [CrossRef]
- Vergouwen, M.D.I.; Jong-Tjien-Fa, A.V.; Algra, A.; Rinkel, G.J.E. Time trends in causes of death after aneurysmal subarachnoid hemorrhage: A hospital-based study. Neurology 2016, 86, 59–63. [Google Scholar] [CrossRef]
- Nieuwkamp, D.J.; Setz, L.E.; Algra, A.; Linn, F.H.H.; de Rooij, N.K.; Rinkel, G.J.E. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: A meta-analysis. Lancet Neurol. 2009, 8, 635–642. [Google Scholar] [CrossRef]
- Feigin, V.L.; Rinkel, G.J.E.; Lawes, C.M.M.; Algra, A.; Bennett, D.A.; van Gijn, J.; Anderson, C.S. Risk factors for subarachnoid hemorrhage: An updated systematic review of epidemiological studies. Stroke 2005, 36, 2773–2780. [Google Scholar] [CrossRef]
- Clarke, M. Systematic review of reviews of risk factors for intracranial aneurysms. Neuroradiology 2008, 50, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Osgood, M.L. Aneurysmal Subarachnoid Hemorrhage: Review of the Pathophysiology and Management Strategies. Curr. Neurol. Neurosci. Rep. 2021, 21, 50. [Google Scholar] [CrossRef] [PubMed]
- Garcia, R.M.; Shiraishi-Zapata, C.J.; Zúñiga Vallejos, R.C.; Gil Chiroque, D.P.; Oyanguren Maldonado, M.A.; Paico Palacios, J.C.; Romero, E.E.; Villarreal Álamo, A.H.; Castillo Tovar, J.S.; Aguirre Uribe, S.J.; et al. Surgical care and trauma patients capacity in Perú—Cross-sectional study. Colomb. J. Anesthesiol. 2022, 51, e1058. [Google Scholar] [CrossRef] [PubMed]
- Almubaslat, F.; Sanchez-Boluarte, S.S.; Diaz, M.M. A review of neurological health disparities in Peru. Front. Public Health 2023, 11, 1210238. [Google Scholar] [CrossRef]
- Loconi-Vallejos, A.; Jorge-Dejo, C.; Azurín-Peña, M.; Garcia-Solorzano, F.O. Timing of aneurysm treatment in subarachnoid hemorrhage and grade of functional capacity at discharge: A retrospective cohort study. Egypt. J. Neurol. Psychiatry Neurosurg. 2023, 59, 89. [Google Scholar] [CrossRef]
- Davalos, L.F.; Málaga, G. El accidente cerebrovascular en el Perú: Una enfermedad prevalente olvidada y desatendida. Rev. Peru. Med. Exp. Salud Publica 2014, 31, 400–401. [Google Scholar] [CrossRef]
- Orakdogen, M.; Emon, S.T.; Somay, H.; Engin, T.; Ates, O.; Berkman, M.Z. Prognostic Factors in Patients who Underwent Aneurysmal Clipping due to Spontaneous Subarachnoid Hemorrhage. Turk. Neurosurg. 2016, 26, 840–848. [Google Scholar] [CrossRef]
- Connolly, E.S.; Rabinstein, A.A.; Carhuapoma, J.R.; Derdeyn, C.P.; Dion, J.; Higashida, R.T.; Hoh, B.L.; Kirkness, C.J.; Naidech, A.M.; Ogilvy, C.S.; et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke 2012, 43, 1711–1737. [Google Scholar] [CrossRef]
- Spetzler, R.F.; McDougall, C.G.; Zabramski, J.M.; Albuquerque, F.C.; Hills, N.K.; Russin, J.J.; Partovi, S.; Nakaji, P.; Wallace, R.C. The Barrow Ruptured Aneurysm Trial: 6-year results. J. Neurosurg. 2015, 123, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Zijlstra, I.A.; van der Steen, W.E.; Verbaan, D.; Majoie, C.B.; Marquering, H.A.; Coert, B.A.; Vandertop, W.P.; van den Berg, R. Ruptured middle cerebral artery aneurysms with a concomitant intraparenchymal hematoma: The role of hematoma volume. Neuroradiology 2018, 60, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Abulhasan, Y.B.; Alabdulraheem, N.; Simoneau, G.; Angle, M.R.; Teitelbaum, J. Mortality after Spontaneous Subarachnoid Hemorrhage: Causality and Validation of a Prediction Model. World Neurosurg. 2018, 112, e799–e811. [Google Scholar] [CrossRef] [PubMed]
- Dijkland, S.A.; Roozenbeek, B.; Brouwer, P.A.; Lingsma, H.F.; Dippel, D.W.; Vergouw, L.J.; Vergouwen, M.D.; van der Jagt, M. Prediction of 60-Day Case Fatality After Aneurysmal Subarachnoid Hemorrhage: External Validation of a Prediction Model. Crit. Care Med. 2016, 44, 1523–1529. [Google Scholar] [CrossRef]
- Lantigua, H.; Ortega-Gutierrez, S.; Schmidt, J.M.; Lee, K.; Badjatia, N.; Agarwal, S.; Claassen, J.; Connolly, E.S.; Mayer, S.A. Subarachnoid hemorrhage: Who dies, and why? Crit. Care 2015, 19, 309. [Google Scholar] [CrossRef]
- Shen, J.; Yu, J.; Huang, S.; Mungur, R.; Huang, K.; Pan, X.; Yu, G.; Xie, Z.; Zhou, L.; Liu, Z.; et al. Scoring Model to Predict Functional Outcome in Poor-Grade Aneurysmal Subarachnoid Hemorrhage. Front. Neurol. 2021, 12, 601996. [Google Scholar] [CrossRef]
- Rojas-Panta, G.; Reyes-Narro, G.F.; Toro-Huamanchumo, C.; Choque-Velasquez, J.; Saal-Zapata, G. Prognostic value of scales for aneurysmal subarachnoid hemorrhage: Report of a reference center in Peru. Neurocirugia 2024, 35, 1–5. [Google Scholar] [CrossRef]
- Sharp, S.J.; Poulaliou, M.; Thompson, S.G.; White, I.R.; Wood, A.M. A review of published analyses of case-cohort studies and recommendations for future reporting. PLoS ONE 2014, 9, e101176. [Google Scholar] [CrossRef]
- Hoh, B.L.; Ko, N.U.; Amin-Hanjani, S.; Chou, S.H.-Y.; Cruz-Flores, S.; Dangayach, N.S.; Derdeyn, C.P.; Du, R.; Hänggi, D.; Hetts, S.W.; et al. 2023 Guideline for the Management of Patients with Aneurysmal Subarachnoid Hemorrhage: A Guideline from the American Heart Association/American Stroke Association. Stroke 2023, 54, e314–e370. [Google Scholar] [CrossRef]
- Deutsch, B.C.; Neifert, S.N.; Caridi, J.M. No Disparity in outcomes between surgical clipping and endovascular coiling after aneurysmal subarachnoid hemorrhage. World Neurosurg. 2018, 120, e318–e325. [Google Scholar] [CrossRef]
- Ikawa, F.; Michihata, N.; Matsushige, T.; Abiko, M.; Ishii, D.; Oshita, J.; Okazaki, T.; Sakamoto, S.; Kurogi, R.; Iihara, K.; et al. In-hospital mortality and poor outcome after surgical clipping and endovascular coiling for aneurysmal subarachnoid hemorrhage using nationwide databases: A systematic review and meta-analysis. Neurosurg. Rev. 2020, 43, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Mahlamäki, K.; Rautalin, I.; Korja, M. Case Fatality Rates of Subarachnoid Hemorrhage Are Decreasing with Substantial between-Country Variation: A Systematic Review of Population-Based Studies between 1980 and 2020. Neuroepidemiology 2022, 56, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Odensass, S.; Gümüs, M.; Said, M.; Rodemerk, J.; Darkwah Oppong, M.; Li, Y.; Ahmadipour, Y.; Dammann, P.; Wrede, K.H.; Sure, U.; et al. Predictors of survival after aneurysmal subarachnoid hemorrhage: The long-term observational cohort study. Clin. Neurol. Neurosurg. 2024, 247, 108605. [Google Scholar] [CrossRef]
- Said, M.; Gümüs, M.; Darkwah Oppong, M.; Dömer, P.; Helgers, S.O.A.; Dammann, P.; Wrede, K.H.; Woitzik, J.; Sure, U.; Jabbarli, R. Risk Score for Early Prediction of In-Hospital Mortality After Aneurysmal Subarachnoid Hemorrhage: Pooled Analysis with Score Construction and Validation. World Neurosurg. 2024, 194, 123426. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.A.; Mai, T.D.; Vu, L.D.; Dao, C.X.; Ngo, H.M.; Hoang, H.B.; Tran, T.A.; Pham, T.Q.; Pham, D.T.; Nguyen, M.H.; et al. Validation of the accuracy of the modified World Federation of Neurosurgical Societies subarachnoid hemorrhage grading scale for predicting the outcomes of patients with aneurysmal subarachnoid hemorrhage. PLoS ONE 2023, 18, e0289267. [Google Scholar] [CrossRef]
- Turek, G.; Lewszuk, A.; Kochanowicz, J.; Lyson, T.; Zielinska-Turek, J.; Gorbacz, K.; Mariak, Z. Early outcomes and perioperative complications of endovascular embolization in patients with aneurysmal SAH. Neurol. Neurochir. Pol. 2016, 50, 342–348. [Google Scholar] [CrossRef]
- Bae, I.-S.; Chun, H.-J.; Choi, K.-S.; Yi, H.-J. Modified Glasgow coma scale for predicting outcome after subarachnoid hemorrhage surgery. Medicine 2021, 100, e25815. [Google Scholar] [CrossRef]
- Brawanski, N.; Dubinski, D.; Bruder, M.; Berkefeld, J.; Hattingen, E.; Senft, C.; Seifert, V.; Konczalla, J. Poor grade subarachnoid hemorrhage: Treatment decisions and timing influence outcome. Should we, and when should we treat these patients? Brain Hemorrhages 2021, 2, 29–33. [Google Scholar] [CrossRef]
- Díaz-Ruiz, R.; Vargas-Fernández, R.; Rojas-Roque, C.; Hernández-Vásquez, A. Socioeconomic inequalities in the use of medical consultation services in Peru, 2019. Int. J. Equity Health 2024, 23, 10. [Google Scholar] [CrossRef]
- Casamassa, S. The Poor Majority: Inequality in Mexico and Peru. Glob. Major. E-J. 2021, 12, 4–22. [Google Scholar]
- Resolución Ministerial, N. 546-2011-Ministerio de Salud del Perú (MINSA). Available online: https://www.gob.pe/institucion/minsa/normas-legales/243402-546-2011-minsa (accessed on 14 February 2025).
- Doukas, A.; Barth, H.; Petridis, K.A.; Mehdorn, M.; von der Brelie, C. Misdiagnosis of acute subarachnoid hemorrhage in the era of multimodal diagnostic options. Am. J. Emerg. Med. 2019, 37, 2079–2083. [Google Scholar] [CrossRef] [PubMed]
- Bentancourt, V.; Jaume, A.; Aboal, C. Errores diagnósticos en la hemorragia subaracnoidea aneurismática. Rev. Urug. Med. Int. 2016, 1, 62–68. [Google Scholar]
- Gonzalo Rojas, D.; Juan Garay, H.; Wesley Alaba, G.; César Rodriguez, C.; Rolando Lovaton, E.; Relix Huaman, H. Definitive treatment of cerebral aneurysms at the Cayetano Heredia National Hospital in Lima Peru: A case series results. Peruv. J. Neurosurg. 2021, 3. [Google Scholar] [CrossRef]
- Ayala-García, R.; Álvarez-Carranza, J. Hemorragia subaracnoidea aneurismática en un centro privado de Lima, Perú. Interciencia Méd. 2023, 13, 7–13. [Google Scholar] [CrossRef]
- Belavadi, R.; Gudigopuram, S.V.R.; Raguthu, C.C.; Gajjela, H.; Kela, I.; Kakarala, C.L.; Hassan, M.; Sange, I. Surgical Clipping Versus Endovascular Coiling in the Management of Intracranial Aneurysms. Cureus 2021, 13, e20478. [Google Scholar] [CrossRef] [PubMed]
- Brinjikji, W.; Rabinstein, A.A.; Lanzino, G.; Kallmes, D.F.; Cloft, H.J. Effect of age on outcomes of treatment of unruptured cerebral aneurysms: A study of the National Inpatient Sample 2001–2008. Stroke 2011, 42, 1320–1324. [Google Scholar] [CrossRef]
- Ahmed, S.I.; Javed, G.; Bareeqa, S.B.; Samar, S.S.; Shah, A.; Giani, A.; Aziz, Z.; Tasleem, A.; Humayun, S.H. Endovascular Coiling Versus Neurosurgical Clipping for Aneurysmal Subarachnoid Hemorrhage: A Systematic Review and Meta-analysis. Cureus 2019, 11, e4320. [Google Scholar] [CrossRef] [PubMed]
- Harker, P.; Quitián-Reyes, H.; Feo-Lee, O.H.; Iragorri, N.; Puentes, J.C.; Harker, P.; Quitián-Reyes, H.; Feo-Lee, O.H.; Iragorri, N.; Puentes, J.C. Cost-Effectiveness Analysis Applied to the Treatment of Unruptured Anterior Circulation Aneurysms in a Middle-Income Country. Univ. Medica 2020, 61, 3–10. [Google Scholar] [CrossRef]
- Won, Y.D.; Cheong, J.W.; Park, Y.K.; Yoon, B.-H.; Kim, J.H.; Kang, H.I. Shunt-dependent hydrocephalus and shunt failure rate in patients with aneurysmal subarachnoid hemorrhage in Korea. J. Neurosurg. 2024, 141, 1254–1261. [Google Scholar] [CrossRef]
- Khanafer, A.; Bhogal, P.; Hellstern, V.; Harmening, C.; Bäzner, H.; Ganslandt, O.; Henkes, H. Vasospasm-Related Death after Aneurysmal Subarachnoid Hemorrhage: A Retrospective Case-Control Study. J. Clin. Med. 2022, 11, 4642. [Google Scholar] [CrossRef]
| Baseline Characteristics | ||
|---|---|---|
| Gender, n (%) | Female | 66 (31.4) |
| Male | 144 (68.6) | |
| Age median (IQR), years | 52.5 (22) | |
| Age group, n (%) | ≤65 yo | 178 (84.8) |
| >65 yo | 32 (15.2) | |
| Comorbidities, n (%) | None | 78 (47.3) |
| ≥1 | 87 (52.7) | |
| Familiar aneurysm history, n (%) | No | 162 (98.8) |
| Yes | 2 (1.2) | |
| Thunderclap headache | No | 2 (1.0) |
| Yes | 207 (99.0) | |
| aSAH-to-admission median (IQR), days | 2 (6.42) | |
| Glasgow scale on admission | Mild (13–15) | 181 (86.6) |
| Moderate (9–12) | 18 (8.6) | |
| Severe (<9) | 10 (4.8) | |
| WFNS SAH scale on admission | Mild (1–3) | 184 (87.6) |
| Severe (4–5) | 26 (12.4) | |
| aSAH-to-surgery, median (IQR), days | 10 (13) | |
| aSAH-to-surgery, median (IQR), days | ≤3 days | 43 (21.2) |
| 4–10 days | 68 (33.5) | |
| >10 days | 92 (45.3) | |
| Diagnostic test, n (%) | CT angiography | 168 (80.4) |
| Angiography | 18 (8.6) | |
| Both | 23 (11.0) | |
| Fisher CT scale on admission | Class I | 9 (4.3) |
| Class II | 20 (9.6) | |
| Class III | 121 (57.9) | |
| Class IV | 59 (28.2) | |
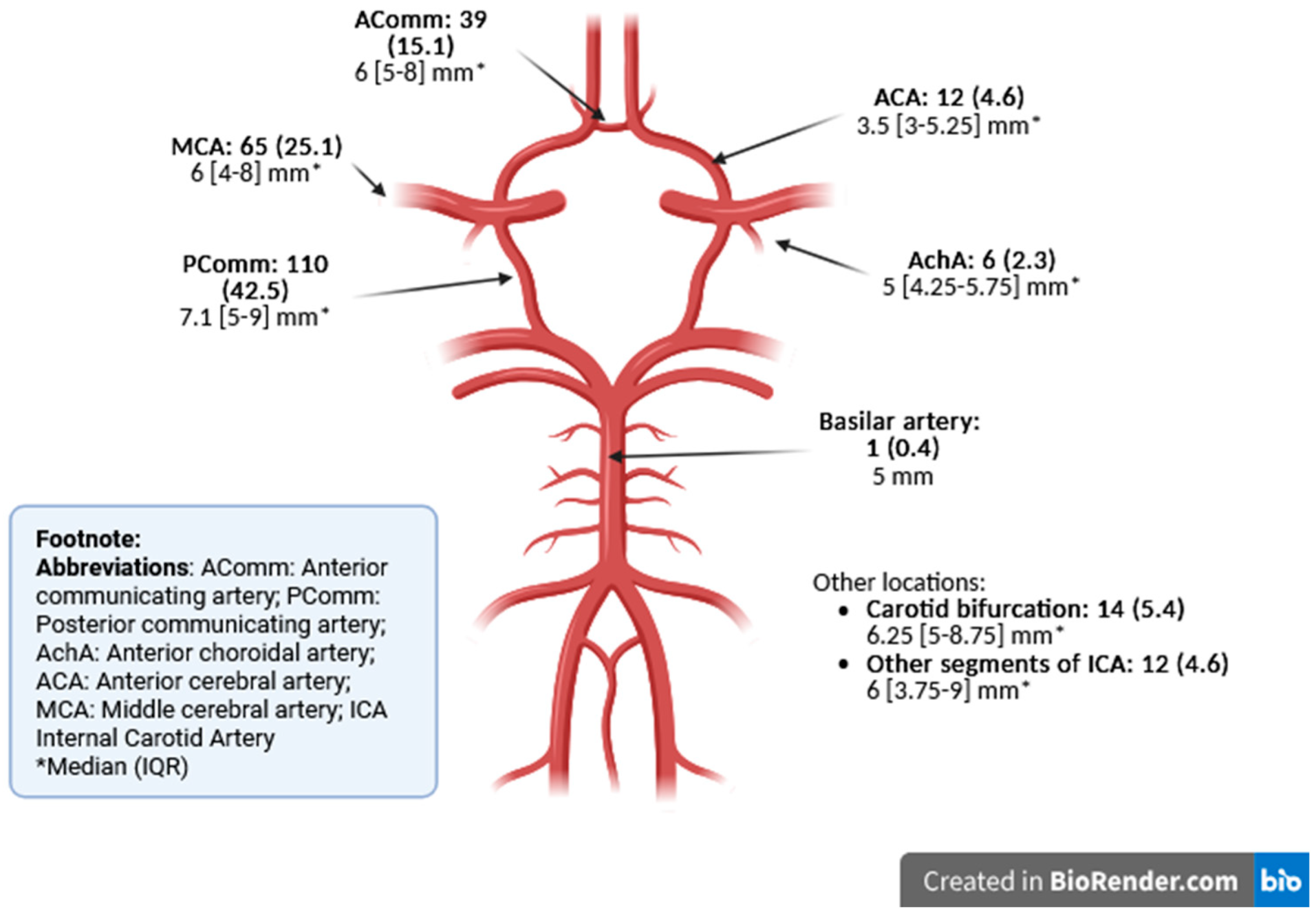
| Aneurysms per patient, median (IQR) | 1 (0) | |
| Characteristics | Aneurysm Location (n = 259) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Anterior Circulation (n = 258) | Posterior Circulation (n = 1) | ||||||||
| AComm (n = 39) | ACA (n = 12) | MCA (n = 65) | AchA (n = 6) | Carotid Bifurcation (n = 14) | ICA (n = 12) | PComm (n = 110) | Basilar Artery (n = 1) | ||
| Maximum diameter | Small/medium (<15 mm) | 39 (100) 6 [5,6,7,8] * | 12 (100) 3.5 [3–5.25] * | 63 (96.9) 6 [4,5,6,7,8] * | 6 (100) 5 [4.25–5.75] * | 13 (92.9) 6 [5,6,7,8] * | 11 (91.7) 6 [3.5–8.5] * | 108 (98.2) 7 [5,6,7,8,9] * | 1 (100) 5 [5] * |
| Large (15–25 mm) | - | - | 2 (3.1) 18 [16,17,18,19,20] * | - | 1 (7.1) 20 [20] * | 1 (8.3) 16 [16] * | 2 (1.8) 17.5 [15,16,17,18,19,20] * | - | |
| Giant (>25 mm) | - | - | - | - | - | - | - | - | |
| Microsurgical clipping | 38 (16.4) | 12 (5.2) | 55 (23.7) | 6 (2.6) | 12 (5.2) | 8 (3.5) | 101 (43.5) | - | |
| Clipping Characteristics | |
|---|---|
| Craniotomy approach, n (%) | |
| Mini-pterional | 184 (87.2) |
| Lateral supraorbital | 10 (4.7) |
| Frontal | 10 (4.7) |
| Decompressive hemicraniectomy | 7 (3.3) |
| Transitory clipping in parenteral artery | 104 (40.2) |
| Transitory clipping time in 1st aneurysm | |
| <5 min | 30 (29.41) |
| 5–10 min | 45 (44.12) |
| >10 min (prolonged) | 27 (26.47) |
| Transitory clipping time in 2nd aneurysm | |
| <5 min | 8 (36.36) |
| 5–10 min | 10 (45.45) |
| >10 min (prolonged) | 4 (18.18) |
| Transitory clipping time in 3rd aneurysm | |
| <5 min | 4 (100) |
| 5–10 min | 0 |
| >10 min (prolonged) | 0 |
| Transitory clipping time in 4th aneurysm | |
| <5 min | 1 (100) |
| 5–10 min | 0 |
| >10 min (prolonged) | 0 |
| Characteristics | Mortality | p | mRS | p | |||
|---|---|---|---|---|---|---|---|
| No (n = 186), n (%) | Yes (n = 24), n (%) | No/Mild Disability (n = 154), n (%) | Moderate/Severe Disability (n = 56), n (%) | ||||
| Gender | 0.800 a | 0.638 a | |||||
| Female | 59 (89.4) | 7 (10.6) | 47 (71.2) | 19 (28.8) | |||
| Male | 127 (88.2) | 17 (11.8) | 107 (74.3) | 37 (25.7) | |||
| Age | 0.418 a | 0.018 a | |||||
| ≤65 years old | 159 (89.3) | 19 (10.7) | 136 (76.4) | 42 (23.6) | |||
| >65 years old | 27 (84.4) | 5 (15.6) | 18 (56.3) | 14 (43.7) | |||
| History of any chronic disease | 0.993 a | 0.426 a | |||||
| None | 69 (88.5) | 9 (11.5) | 59 (75.6) | 19 (24.4) | |||
| ≥1 | 77 (88.5) | 10 (11.5) | 61 (70.1) | 26 (29.9) | |||
| Time from stroke to admission (in days), median (IQR) | 2 (6.5) | 5.5 (7.0) | 0.060 b | 2 (6.5) | 2.5 (6) | 0.743 b | |
| Glasgow scale score on admission, categorized | <0.001 a | 0.009 a | |||||
| Mild (15–13) | 166 (91.7) | 15 (8.3) | 144 (79.6) | 37 (20.4) | |||
| Moderate (12–9) | 17 (94.4) | 1 (5.6) | 10 (55.6) | 8 (44.4) | |||
| Severe (<9) | 3 (30.0) | 7 (70.0) | 0 (0.0) | 10 (100.0) | |||
| WFNS clinical scale score on admission | <0.001 a | <0.001 a | |||||
| Mild (1 to 3) | 169 (91.8) | 15 (8.1) | 145 (78.8) | 39 (21.2) | |||
| Severe (4 to 5) | 17 (65.4) | 9 (34.6) | 9 (34.6) | 17 (65.4) | |||
| Fisher tomographic scale score on admission | 0.516 a | 0.260 a | |||||
| I—no evidence of bleeding in cisterns or ventricles. | 9 (100.0) | 0 (0.0) | 9 (100.0) | 0 (0.0) | |||
| II—thin diffuse blood, with a layer of < 1 mm in cisterns measured vertically | 19 (95.0) | 1 (5.0) | 16 (80.0) | 4 (20.0) | |||
| III—thick cisternal clot of >1 mm in cisterns, measured vertically | 107 (88.4) | 14 (11.6) | 87 (71.9) | 34 (28.1) | |||
| IV—intraparenchymal hematoma, intraventricular hemorrhage, and +/− diffuse bleeding | 51 (86.4) | 8 (13.6) | 42 (71.2) | 17 (28.8) | |||
| Time from stroke to operation (in days), median (IQR) | 9 (12.0) | 10 (23.0) | 0.480 b | 9 (12.0) | 10 (12.0) | 0.708 b | |
| Need for transient clipping in parenteral artery | 0.161 a | 0.041 a | |||||
| No | 98 (91.6) | 9 (8.4) | 85 (79.4) | 22 (20.6) | |||
| Yes | 88 (85.4) | 15 (14.6) | 69 (77.0) | 34 (33.0) | |||
| Intraoperative neurosurgical complications | 0.022 a | 0.049 a | |||||
| None | 132 (92.3) | 11 (7.7) | 111 (77.6) | 32 (22.4) | |||
| ≥1 | 49 (81.7) | 11 (18.3) | 42 (64.6) | 23 (35.4) | |||
| Postoperative neurosurgical complications | <0.001 a | <0.001 a | |||||
| None | 146 (95.4) | 7 (4.6) | 128 (83.7) | 25 (16.3) | |||
| 1 | 32 (76.2) | 10 (23.8) | 22 (52.4) | 20 (47.6) | |||
| ≥2 | 6 (46.1) | 7 (53.8) | 3 (23.1) | 10 (76.9) | |||
| Specific postoperative neurosurgical complications | <0.001 a | <0.001 a | |||||
| No complications | 146 (95.4) | 7 (4.6) | 128 (52.0) | 25 (47.9) | |||
| ≥1 cerebral infarction and/or vasospasm | 33 (73.3) | 12 (26.7) | 23 (51.1) | 22 (48.9) | |||
| Other complications | 5 (50.0) | 5 (50.0) | 2 (20.0) | 8 (80.0) | |||
| Postoperative clinical complications | 0.026 a | <0.001 a | |||||
| None | 118 (92.9) | 9 (7.1) | 111 (87.4) | 16 (12.6) | |||
| 1 | 36 (80.0) | 9 (20.0) | 25 (55.6) | 20 (44.4) | |||
| ≥2 | 25 (80.6) | 6 (19.3) | 13 (41.9) | 18 (58.1) | |||
| Variable | Crude Model | Adjusted Model * | |||||
|---|---|---|---|---|---|---|---|
| RR | 95% CI | p | RR | 95% CI | p | ||
| Age | |||||||
| ≤65 years old | Ref. | Ref. | |||||
| >65 years old | 1.46 | 0.59–3.65 | 0.413 | 0.68 | 0.27–1.71 | 0.410 | |
| Glasgow scale score on admission, categorized | |||||||
| Mild (15–13) | Ref. | Ref. | |||||
| Moderate (12–9) | 0.67 | 0.09–4.81 | 0.691 | - | - | - | |
| Severe (<9) | 8.45 | 4.48–15.92 | <0.001 | - | - | - | |
| WFNS clinical scale score on admission | |||||||
| Mild (1 to 3) | Ref. | Ref. | |||||
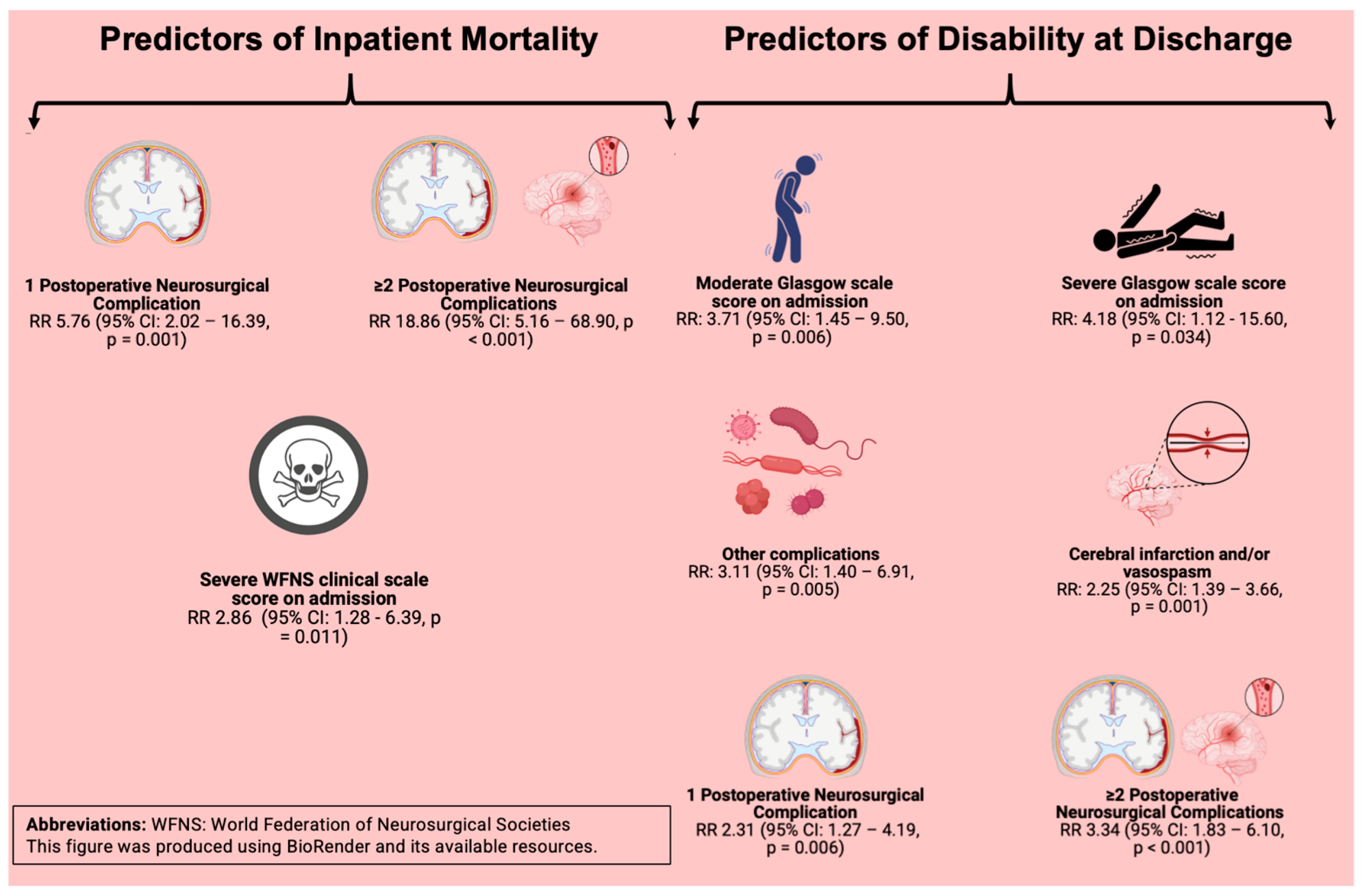
| Severe (4 to 5) | 4.25 | 2.07–8.71 | <0.001 | 2.86 | 1.28–6.39 | 0.011 | |
| Intraoperative neurosurgical complications | |||||||
| None | Ref. | Ref. | |||||
| ≥1 | 2.4 | 1.12–5.16 | 0.025 | 1.40 | 0.63–3.40 | 0.404 | |
| Postoperative neurosurgical complications | |||||||
| None | Ref. | Ref. | |||||
| 1 | 5.2 | 2.1–12.87 | <0.001 | 5.76 | 2.02–16.39 | 0.001 | |
| ≥2 | 11.77 | 4.86–28.48 | <0.001 | 18.86 | 5.16–68.90 | <0.001 | |
| Specific postoperative neurosurgical complications | |||||||
| No complications | Ref. | Ref. | |||||
| At least cerebral infarction and/or vasospasm | 5.83 | 2.43–13.95 | <0.001 | 0.47 | 0.20–1.11 | 0.086 | |
| Other complications | 10.93 | 4.20–28.40 | <0.001 | - | - | - | |
| Reported postoperative clinical complications | |||||||
| None | Ref. | Ref. | |||||
| 1 | 2.82 | 1.19–6.68 | 0.018 | 1.90 | 0.80–4.53 | 0.146 | |
| ≥2 | 2.73 | 1.05–7.12 | 0.040 | 2.23 | 0.79–6.31 | 0.131 | |
| Variable | mRS (Disability) | |||||||
|---|---|---|---|---|---|---|---|---|
| Crude Model | Adjusted Model ** | |||||||
| RR | 95% CI | p | RR | 95% CI | p | |||
| Age | ||||||||
| ≤65 years old | Ref. | Ref. | ||||||
| >65 years old | 1.85 | 1.15–2.98 | 0.011 | 1.26 | 0.72–2.20 | 0.423 | ||
| Glasgow scale score on admission, categorized | ||||||||
| Mild (15–13) | Ref. | Ref. | ||||||
| Moderate (12–9) | 2.17 | 1.20–3.93 | 0.010 | 3.71 | 1.45–9.50 | 0.006 | ||
| Severe (<9) | 4.89 | 3.67–6.53 | <0.001 | 4.18 | 1.12–15.60 | 0.034 | ||
| WFNS clinical scale score on admission | ||||||||
| Mild (1 to 3) | Ref. | Ref. | ||||||
| Severe (4 to 5) | 3.08 | 2.08–4.58 | <0.001 | 0.39 | 0.11–1.35 | 0.137 | ||
| Need for transient clipping in parenteral artery | ||||||||
| No | Ref. | |||||||
| Yes | 1.61 | 1.01–2.55 | 0.046 | 1.44 | 0.85–2.41 | 0.173 | ||
| Intraoperative neurosurgical complications | ||||||||
| None | Ref. | Ref. | ||||||
| ≥1 | 1.58 | 1.01–2.48 | 0.046 | - | - | - | ||
| Postoperative neurosurgical complications | ||||||||
| None | Ref. | Ref. | ||||||
| 1 | 1.49 | 0.93–2.39 | 0.098 | 1.06 | 0.61–1.82 | 0.844 | ||
| ≥2 | 2.68 | 1.23–5.85 | 0.013 | 1.50 | 0.58–3.90 | 0.405 | ||
| Specific postoperative neurosurgical complications | ||||||||
| No complications | Ref. | Ref. | ||||||
| Cerebral infarction and/or vasospasm | 2.99 | 1.87–4.78 | <0.001 | 2.25 | 1.39–3.66 | 0.001 | ||
| Other complications | 4.90 | 3.04–7.87 | <0.001 | 3.11 | 1.40–6.91 | 0.005 | ||
| Postoperative clinical complications | ||||||||
| None | Ref. | Ref. | ||||||
| 1 | 3.53 | 2.01–6.20 | <0.001 | 2.31 | 1.27–4.19 | 0.006 | ||
| ≥2 | 4.61 | 2.66–7.98 | <0.001 | 3.34 | 1.83–6.10 | <0.001 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terry, F.; Enríquez-Marulanda, A.; Chinchihualpa-Paredes, N.; Carbajal-Galarza, M.; Vidal-Cuellar, C.L.; Mas-Ubillus, G.; Diaz-Llanes, B.; Quispe-Vicuña, C.; Pacheco-Barrios, N.; Arbulu-Zuazo, R.; et al. Clinical Predictors of Inpatient Mortality and Poor Postoperative Course After aSAH Microsurgical Clipping: A 10-Year Experience from a Peruvian Tertiary Care Center. J. Clin. Med. 2025, 14, 4799. https://doi.org/10.3390/jcm14134799
Terry F, Enríquez-Marulanda A, Chinchihualpa-Paredes N, Carbajal-Galarza M, Vidal-Cuellar CL, Mas-Ubillus G, Diaz-Llanes B, Quispe-Vicuña C, Pacheco-Barrios N, Arbulu-Zuazo R, et al. Clinical Predictors of Inpatient Mortality and Poor Postoperative Course After aSAH Microsurgical Clipping: A 10-Year Experience from a Peruvian Tertiary Care Center. Journal of Clinical Medicine. 2025; 14(13):4799. https://doi.org/10.3390/jcm14134799
Chicago/Turabian StyleTerry, Fernando, Alejandro Enríquez-Marulanda, Nathaly Chinchihualpa-Paredes, Meiling Carbajal-Galarza, Claudia L Vidal-Cuellar, Guiliana Mas-Ubillus, Bruno Diaz-Llanes, Carlos Quispe-Vicuña, Niels Pacheco-Barrios, Rommel Arbulu-Zuazo, and et al. 2025. "Clinical Predictors of Inpatient Mortality and Poor Postoperative Course After aSAH Microsurgical Clipping: A 10-Year Experience from a Peruvian Tertiary Care Center" Journal of Clinical Medicine 14, no. 13: 4799. https://doi.org/10.3390/jcm14134799
APA StyleTerry, F., Enríquez-Marulanda, A., Chinchihualpa-Paredes, N., Carbajal-Galarza, M., Vidal-Cuellar, C. L., Mas-Ubillus, G., Diaz-Llanes, B., Quispe-Vicuña, C., Pacheco-Barrios, N., Arbulu-Zuazo, R., Moses, Z. B., Sequeiros, J., Luther, E., Starke, R. M., Taussky, P., & Lopez-Calle, J. (2025). Clinical Predictors of Inpatient Mortality and Poor Postoperative Course After aSAH Microsurgical Clipping: A 10-Year Experience from a Peruvian Tertiary Care Center. Journal of Clinical Medicine, 14(13), 4799. https://doi.org/10.3390/jcm14134799






