Paternity After Treatment of Cryptorchidism: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
- Population: Men diagnosed with cryptorchidism during childhood.
- Exposure: Treatment for cryptorchidism before reaching adulthood.
- Outcome: The primary outcome was to determine the chances of paternity among men treated for unilateral versus bilateral cryptorchidism. Secondly, the study should assess how age at the time of treatment for cryptorchidism affects the likelihood of achieving paternity later in life, and the use of ART among men treated for cryptorchidism.
2.2. Information Sources and Search Strategy
2.3. Study Selection and Data Collection
2.4. Data Items
2.5. Risk of Bias Assessment
2.6. Data Synthesis
- (1)
- Quantitative analysis: Studies reporting paternity rates, age at the time of treatment, and the use of ART were assessed. These outcomes were reported either collectively or stratified by unilateral and bilateral cryptorchidism, depending on the individual study. A meta-analysis was not conducted due to considerable heterogeneity across studies in terms of design, outcome definitions, and follow-up periods. Further statistical analyses from the included studies are presented in a separate table.
- (2)
- Visual analysis: Studies reporting paternity rates as percentages were illustrated in a graph grouped by unilateral versus bilateral cryptorchidism. Furthermore, two graphs were constructed to illustrate paternity rates in relation to age at the time of treatment. In studies reporting age, either an interval or mean age was used for illustration. Finally, a graph was included to illustrate the use of ART among men treated for cryptorchidism during childhood.
3. Results
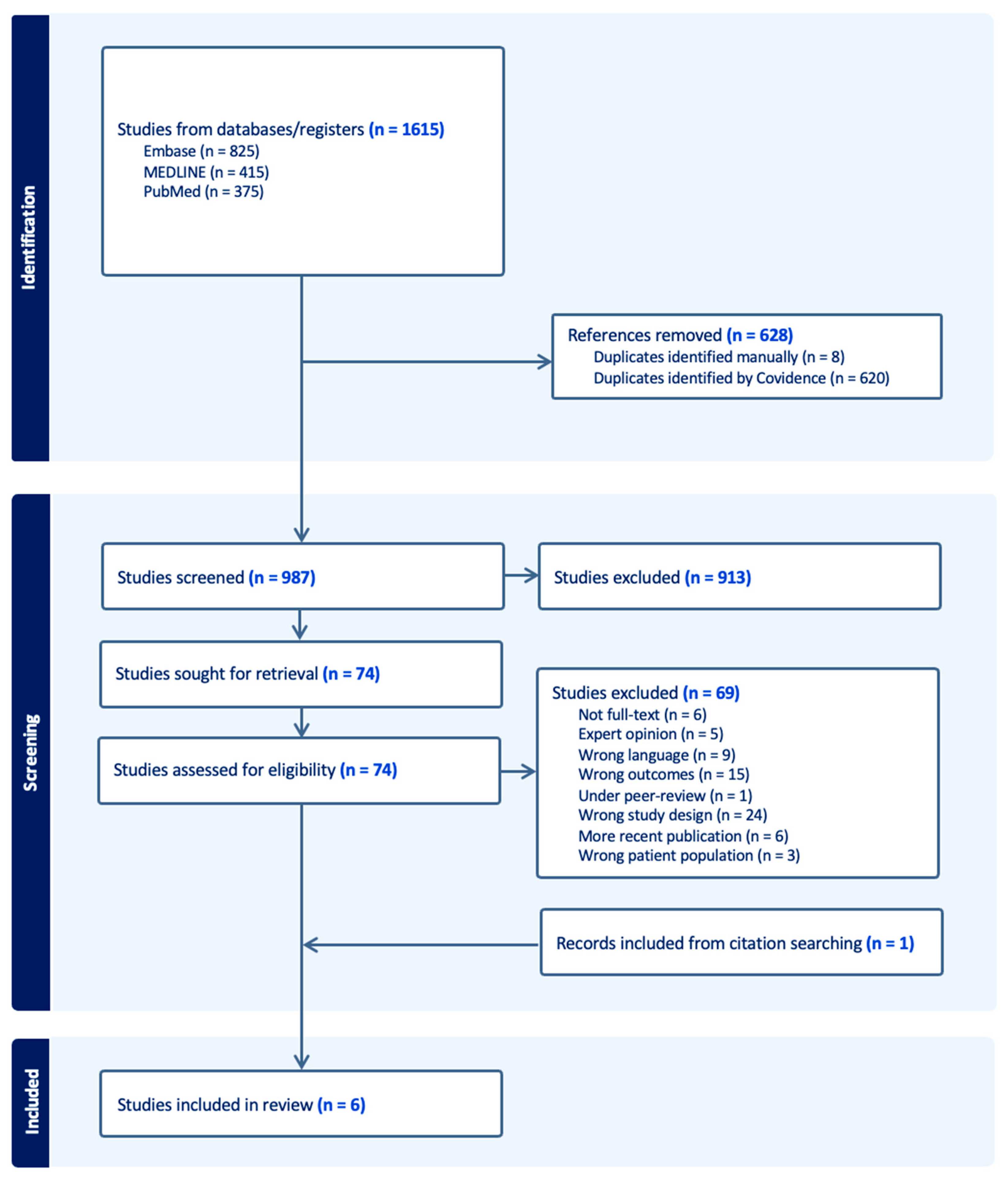
3.1. Study Selection
| Study ID | Design | Sample Size | UDT Group | Controls | Age Groups | Age at Treatment | Groups | Participants | Attempting Paternity | Paternity (%) | No Paternity (%) | Use of ART % | Other Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
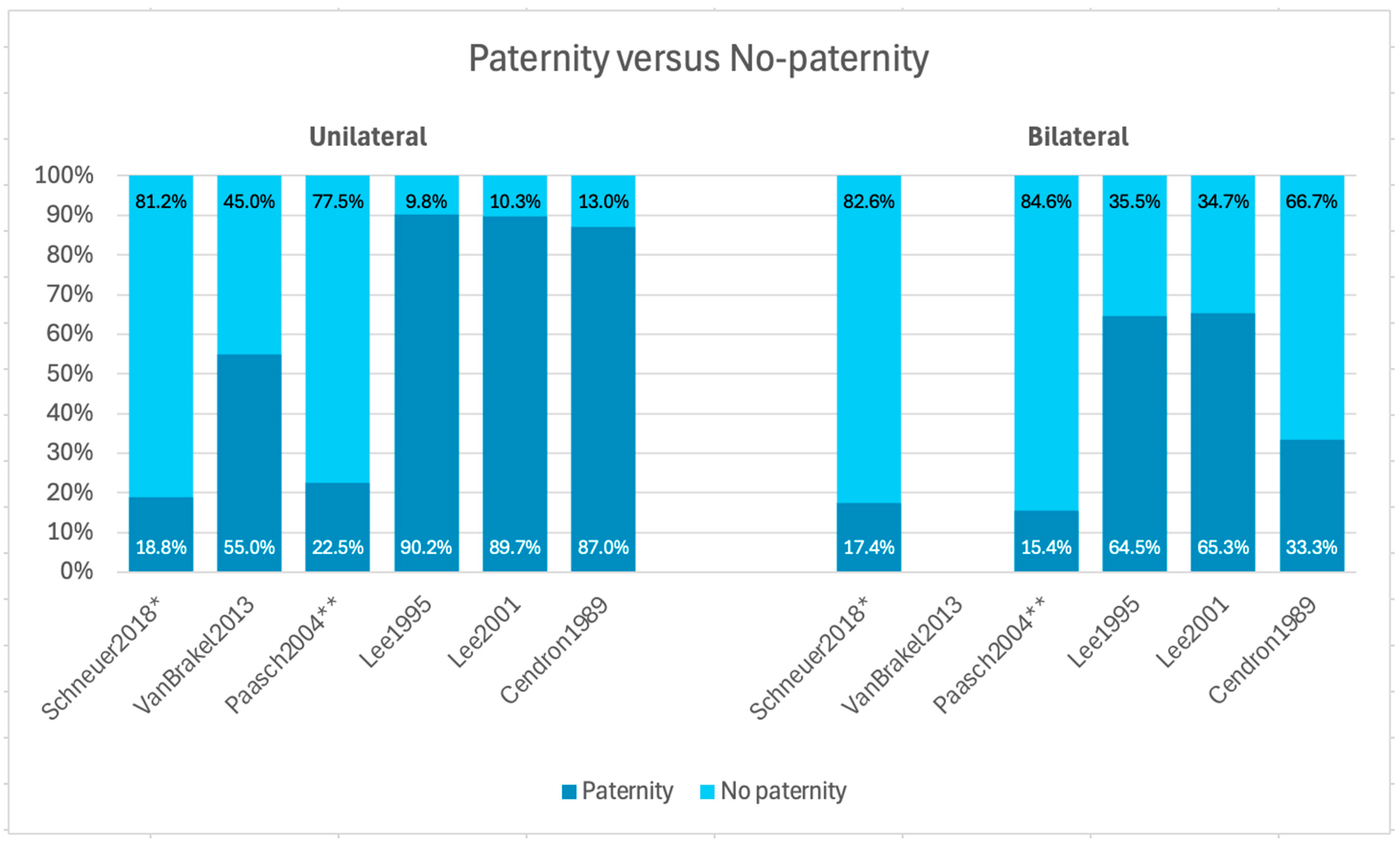
| Schneuer et al. [13] 2018 Australia | Population-based cohort study | 350,835 | 7499 | 341,000 | <18 months | Mean age of UDT: 5.6 years ± 3.9 years | Unilateral | 2765 | - | 519 (18.8) | - (-) | UDT: 0.9 | Risk of testicular cancer, HR for paternity, and RR for the use of ART |
| 18 months–5 years | Bilateral | 351 | - | 61 (17.4) | - (-) | - | |||||||
| ≥6 | Unaffected | 239,239 | - | 49,298 (20.6) | - (-) | Unaffected: 0.3 | |||||||
| Van Brakel et al. [14] 2013 Netherlands | Long-term follow-up study | 225 | 62 | 53 | ≤12 months | Median age of UDT: 3.0 years (0.1–14.6) | Unilateral | 55 | 11 | 6 (55) | 45 (-) | - | Fertility potential, including testis volume, hormone levels, and semen analysis |
| ≤18 months | Unilateral: 2.8 years (0.1–10.3) | Bilateral | 7 | - | - (-) | - (-) | - | ||||||
| ≤24 months | Controls | 53 | 29 | 25 (86) | 14 (-) | - | |||||||
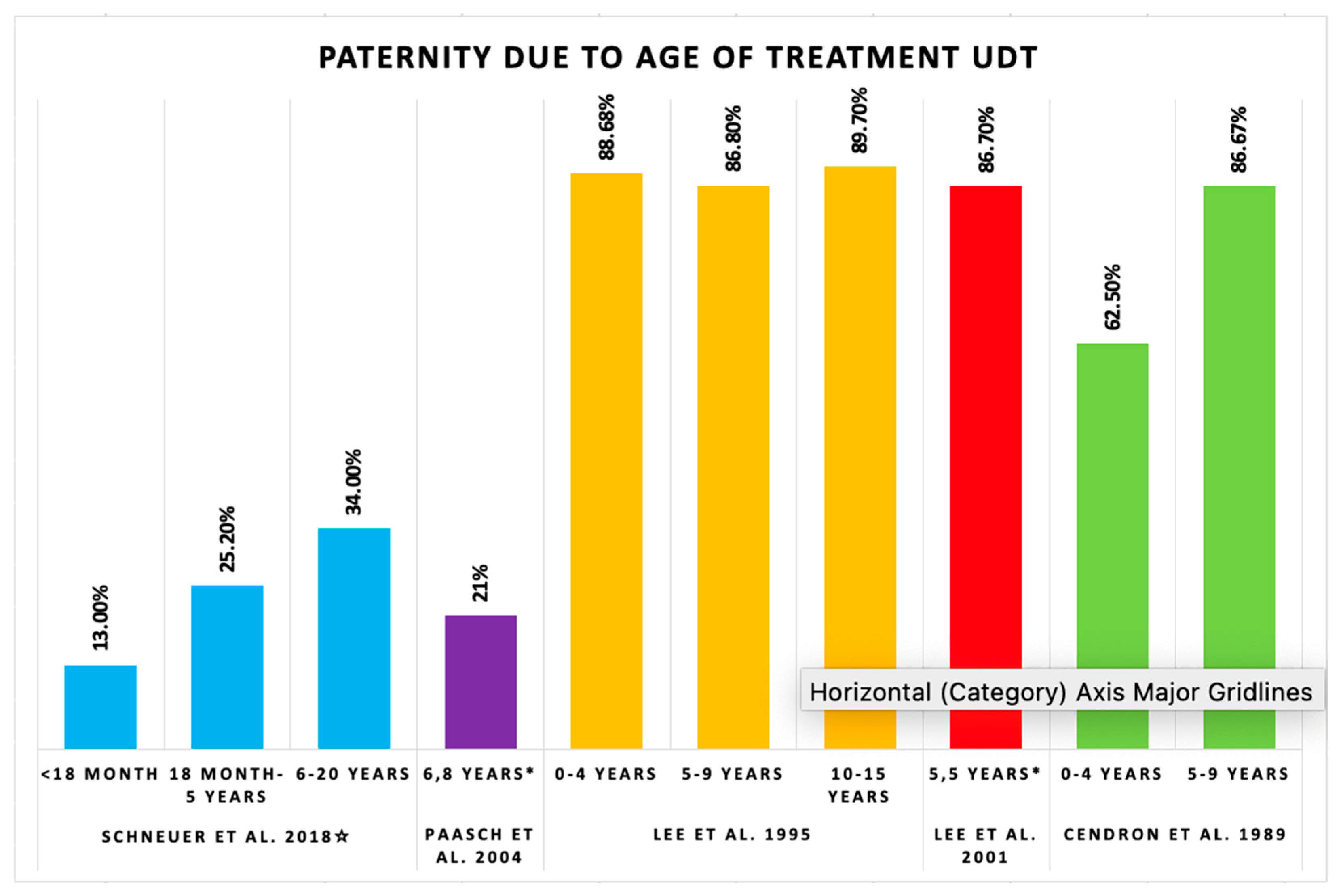
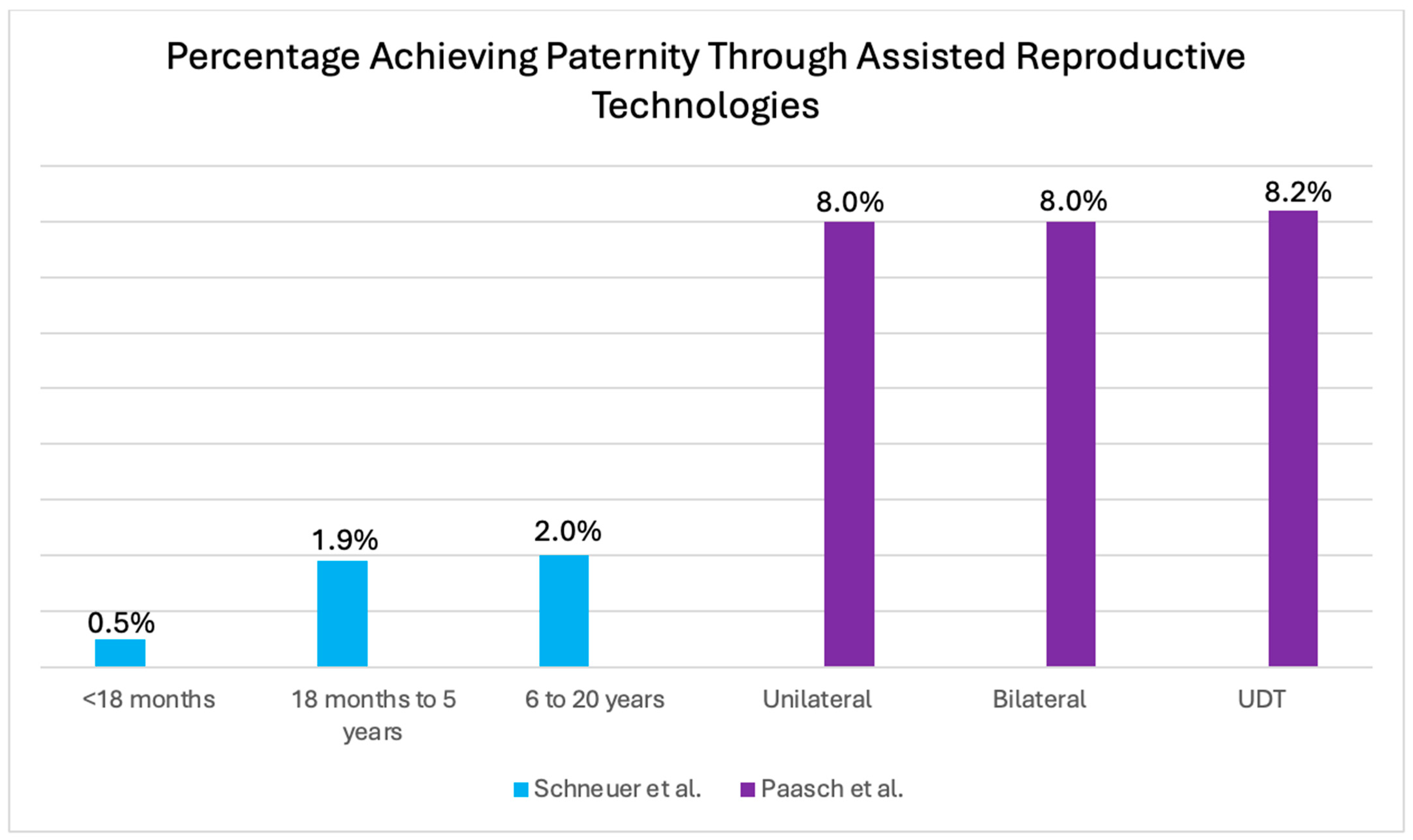
| Paasch et al. [15] 2004 Germany | Clinical study | 1648 | 167 | 374 | Mean age of UDT: 6.8 ± 3.3 years | Unilateral | 130 | 71 | 16 * (22 *) | 78 (-) | IVF: 1, ICSI: 7 | Semen analysis, hormone levels, and conception use | |
| Bilateral | 37 | 26 | 4 * (16 *) | 84 (-) | ICSI: 8 | ||||||||
| Controls | 374 | 176 | 81 * (46.1 *) | 53.9 (-) | IVF: 6.9, ICSI: 3.5, and IUI: 2.8 | ||||||||
| Lee et al. [16] 1995 USA | Epidemiological study | - | 363 | 336 | <1, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, and 15 years | Unilateral interval: 1 month–15 years | Unilateral | 243 | 203 | 183 ** (90.2) | 9.8 (-) | - | Paternity among married or cohabitated men, conception related to time attempting paternity, age at orchiopexy among men attempting paternity within marriage or cohabitation, age at orchiopexy, and time of unprotected intercourse |
| - | Bilateral interval: 1 month–13 years | Bilateral | 38 | 31 | 20 ** (64.5) | 35.5 (-) | - | ||||||
| Controls | 267 | 218 | 203 ** (93.1) | 6.9 (-) | - | ||||||||
| Lee et al. [17] 2001 USA | Cohort study | 1405 | - | 708 | - | - | Unilateral | 609 | 359 | 322 (89.7) | 10.3 (-) | - | Paternity in relation to sperm density, hormone levels, preoperative testis location and size, adult testis volume, and RR of infertility |
| 1.9, 2.7, 2.9, 3.2, 6.0, 7.4, 9.8, and 10.2 years | Bilateral interval: 1.9–10.2 years | Bilateral: | 88 | 49 | 32 (65.3 ***) | 34.7 (-) | - | ||||||
| Controls: | 708 | 443 | 413 (93.2) | 6.8 (-) | - | ||||||||
| Cendron et al. [18] 1989 USA | Critical long-term retrospective analysis | 40 | 40 | - | 0–4 years | Mean age of UDT: 7 years | Unilateral | 30 | 23 | 20 (87) | - (-) | - | Sperm count and paternity, fertility index and paternity |
| 5–9 years | Unilateral interval: 1–14 years | Bilateral | 10 | 9 | 3 (33) | - (-) | - | ||||||
| 10–15 years | Bilateral interval: 1–11 years | Controls | - | - | - (-) | - (-) | - |
| Study ID | Age Groups | Ages | Sample size | Type of Cryptorchidism | Paternity % | Use of ART % | Significance of Age at Orchiopexy |
|---|---|---|---|---|---|---|---|
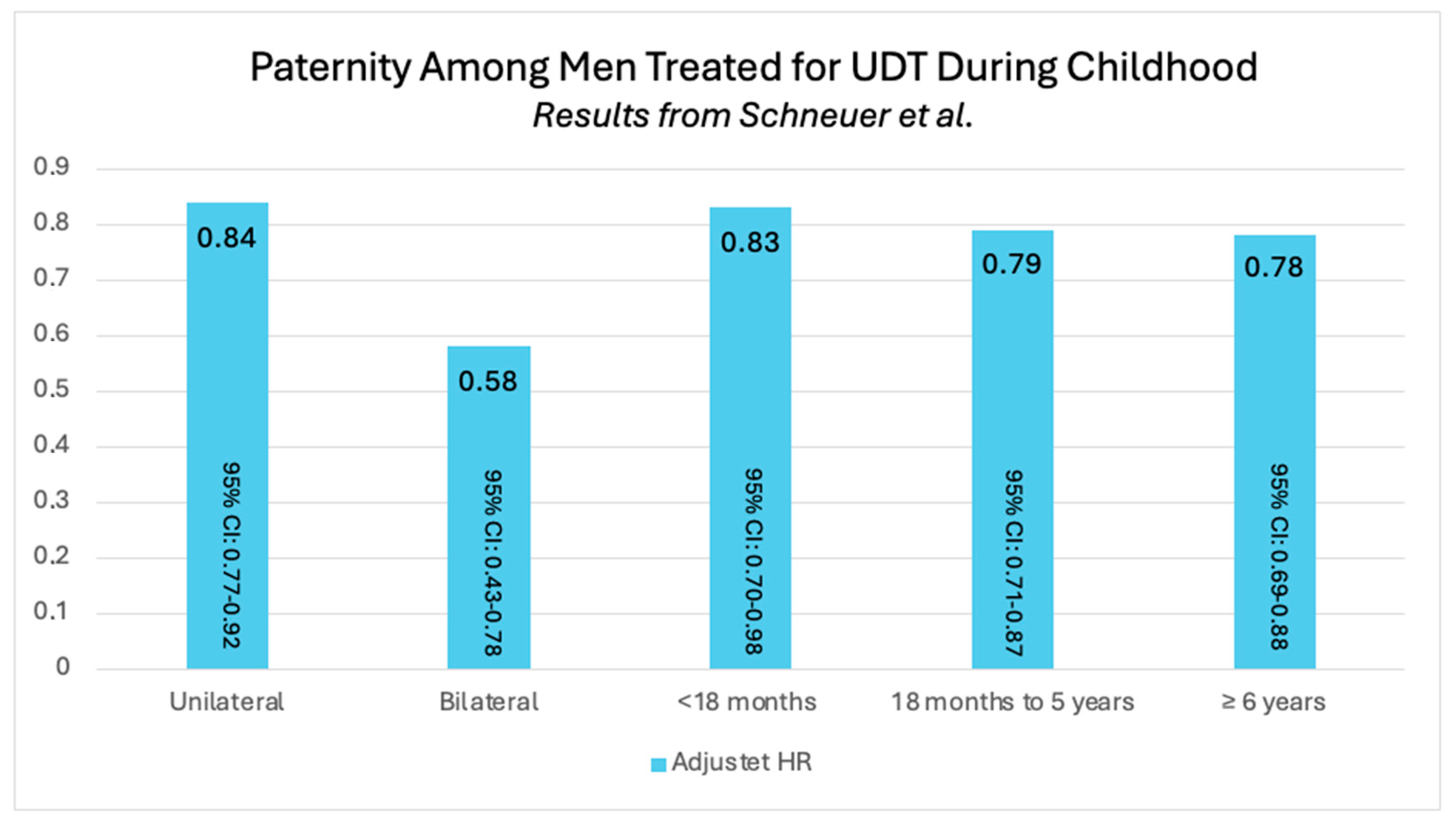
| Schneuer et al. [13] 2018 | Intervals | <18 months | 1202 | UDT | 13.0 | 0.5 | <18 months adjusted HR of 0.83 with 95% CI 0.70–0.98 for paternity |
| 18 months–5 years | 3208 | UDT | 25.2 | 1.9 | 18 months to 5 years adjusted HR of 0.79 with 95% CI 0.71–0.87 for paternity | ||
| 6–20 years | 3049 | UDT | 34.0 | 2.0 | 6 to 20 years adjusted HR of 0.78 with 95% CI 0.69–0.88 for paternity | ||
| Mean age | 5.6 ± 3.9 years | UDT | 26.9 | 1.7 | |||
| Van Brakel et al. [14] 2013 | Intervals | <12 months | 8 | - | - | - | p-value participation rate: 0.248 |
| <18 months | 12 | - | - | - | p-value participation rate: 0.176 | ||
| <24 months | 24 | - | - | - | p-value participation rate: 0.652 | ||
| Median age | 3.0 | 62 | UDT | - | - | ||
| Unilateral median age | 2.8 years (0.1–10.3) | 55 | Unilateral | 55 | - | ||
| Paasch et al. [15] 2004 | Mean age | 6.8 ± 3.3 years | Unilateral | 22 * | IVF: 1 ICSI: 7 | Significant level not reported | |
| Bilateral | 16 * | ICSI: 8 | |||||
| Lee et al., 1995 [16] | Age in years | <1, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, and 15 | Paternity: 184; no paternity: 25 | - | - | - | No significant difference |
| Lee et al., 2001 [17] | Age in years | 1.9, 2.7, 2.9, 3.2, 6.0, 7.4, 9.8, and 10.2 | 8 | Bilateral | 60 | - | Significant level not reported |
| Cendron et al., 1989 [18] | Intervals | 0–4 years | 5 | Unilateral | 80.0 ** | - | Significant level not reported |
| 3 | Bilateral | 33.3 ** | - | ||||
| 5–9 years | 12 | Unilateral | 100 ** | - | |||
| 3 | Bilateral | 33.3 ** | - | ||||
| 10–15 years | 7 | Unilateral | 71.4 ** | ||||
| 3 | Bilateral | 33.3 ** | |||||
| Mean age | 7.0 |
3.2. Level of Study Evidence
3.3. Paternity Rate: Unilateral Versus Bilateral
3.4. Paternity Rate: Age of Treatment
3.5. Paternity Rate: Use of Assisted Reproductive Technology (ART)
4. Discussion
4.1. Explanations and Comparisons with Literature
4.2. Strengths and Limitations
4.3. Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Final Search, Embase Classic + Embase < 1947 to 2025 6 February 2025 > 7 February 2025 | ||
|---|---|---|
| # | Searches | Results |
| 1 | cryptorchism/ | 17,914 |
| 2 | Cryptochi*.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword heading word, floating subheading word, candidate term word] | 160 |
| 3 | Undescended test*.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword heading word, floating subheading word, candidate term word] | 4822 |
| 4 | Maldescensus testis.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword heading word, floating subheading word, candidate term word] | 94 |
| 5 | Testis, undescended.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword heading word, floating subheading word, candidate term word] | 16 |
| 6 | 1 or 2 or 3 or 4 or 5 | 18,784 |
| 7 | orchidopexy/ | 4836 |
| 8 | Orchidopexi*.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword heading word, floating subheading word, candidate term word] | 252 |
| 9 | Orchiopex*.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword heading word, floating subheading word, candidate term word] | 1998 |
| 10 | surgery/ | 1,027,013 |
| 11 | gonadorelin/ | 42,565 |
| 12 | gonadotropin releasing*.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword heading word, floating subheading word, candidate term word] | 20,815 |
| 13 | gonadorelin.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword heading word, floating subheading word, candidate term word] | 70,900 |
| 14 | GnRh*.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword heading word, floating subheading word, candidate term word] | 37,271 |
| 15 | Hcg.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword heading word, floating subheading word, candidate term word] | 41,969 |
| 16 | Surger*.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword heading word, floating subheading word, candidate term word] | 4,998,509 |
| 17 | 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 | 5,100,952 |
| 18 | paternity/ | 4904 |
| 19 | fertility/ | 79,471 |
| 20 | sperm quality/ | 10,228 |
| 21 | Paternit*.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword heading word, floating subheading word, candidate term word] | 9970 |
| 22 | Fertilit*.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword heading word, floating subheading word, candidate term word] | 186,727 |
| 23 | Sperm quality.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword heading word, floating subheading word, candidate term word] | 14,714 |
| 24 | Semen quality.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword heading word, floating subheading word, candidate term word] | 8610 |
| 25 | live birth/ | 40,609 |
| 26 | Live birth.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword heading word, floating subheading word, candidate term word] | 46,728 |
| 27 | 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 | 246,582 |
| 28 | 6 and 17 and 27 | 1012 |
| 29 | 28 and 1990: 2025.(sa_year). | 825 |
| Final Search, Ovid MEDLINE(R) ALL < 1946 to 6 February 2025 >, 7 February 2025 | ||
|---|---|---|
| Search Strategy: | ||
| # | Searches | Results |
| 1 | Cryptorchism/ | 8788 |
| 2 | Cryptorchi*.mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms, population supplementary concept word, anatomy supplementary concept word] | 11,809 |
| 3 | Undescended test*.mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms, population supplementary concept word, anatomy supplementary concept word] | 3327 |
| 4 | maldescensus testis.mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms, population supplementary concept word, anatomy supplementary concept word] | 54 |
| 5 | testis, undescended.mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms, population supplementary concept word, anatomy supplementary concept word] | 22 |
| 6 | 1 or 2 or 3 or 4 or 5 | 12,751 |
| 7 | orchidopexy/ | 711 |
| 8 | orchidopexi*.mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms, population supplementary concept word, anatomy supplementary concept word] | 181 |
| 9 | Orchiopex*.mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms, population supplementary concept word, anatomy supplementary concept word] | 1870 |
| 10 | General Surgery/ | 41,328 |
| 11 | Surger*.mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms, population supplementary concept word, anatomy supplementary concept word] | 3,278,595 |
| 12 | gonadorelin/ | 30,319 |
| 13 | gonadotropin releasing*.mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms, population supplementary concept word, anatomy supplementary concept word] | 37,791 |
| 14 | gonadorelin.mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms, population supplementary concept word, anatomy supplementary concept word] | 290 |
| 15 | GnRH*.mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms, population supplementary concept word, anatomy supplementary concept word] | 27,327 |
| 16 | Hcg.mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms, population supplementary concept word, anatomy supplementary concept word] | 27,902 |
| 17 | 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 | 3,344,095 |
| 18 | Paternity/ | 3399 |
| 19 | Fertility/ | 46,463 |
| 20 | Semen Analysis/ | 7064 |
| 21 | paternit*.mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms, population supplementary concept word, anatomy supplementary concept word] | 7809 |
| 22 | Fertilit*.mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms, population supplementary concept word, anatomy supplementary concept word] | 137,169 |
| 23 | Sperm quality.mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms, population supplementary concept word, anatomy supplementary concept word] | 6773 |
| 24 | semen quality.mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms, population supplementary concept word, anatomy supplementary concept word] | 6666 |
| 25 | Live Birth/ | 6081 |
| 26 | Live birth.mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms, population supplementary concept word, anatomy supplementary concept word] | 18,077 |
| 27 | 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 | 168,778 |
| 28 | 6 and 17 and 27 | 567 |
| Final Search, PubMed, 7 February 2025 | ||
|---|---|---|
| Search Strategy: | ||
| # | Searches | Results |
| 7 | (((“Cryptorchidism”[Mesh] OR cryptorchi* OR “undescended test*” OR “undescended testes” OR “maldescensus testis” OR “Testis Undescended”)) AND ((“Orchiopexy”[Mesh] OR Orchidopexi* OR “surgical procedures, operative”[Mesh] OR Surgery OR “Gonadotropin-Releasing Hormone”[Mesh] OR “Gonadotropin releasing*” OR Gonadorelin OR GnRH* OR hCG))) AND ((“Paternity”[Mesh] OR “Fertility”[Mesh] OR “Semen analysis”[Mesh] OR “Live birth”[Mesh] OR Paternit* OR Fertilit* OR “Sperm quality” OR “Semen quality” OR “Live Birth”)) Filters: Danish, English, Norwegian, Swedish, Humans from 1990–2025 | 375 |
| 6 | (((“Cryptorchidism”[Mesh] OR cryptorchi* OR “undescended test*” OR “undescended testes” OR “maldescensus testis” OR “Testis Undescended”)) AND ((“Orchiopexy”[Mesh] OR Orchidopexi* OR “surgical procedures, operative”[Mesh] OR Surgery OR “Gonadotropin-Releasing Hormone”[Mesh] OR “Gonadotropin releasing*” OR Gonadorelin OR GnRH* OR hCG))) AND ((“Paternity”[Mesh] OR “Fertility”[Mesh] OR “Semen analysis”[Mesh] OR “Live birth”[Mesh] OR Paternit* OR Fertilit* OR “Sperm quality” OR “Semen quality” OR “Live Birth”)) Filters: Danish, English, Norwegian, Swedish, from 1990–2025 | 490 |
| 5 | (((“Cryptorchidism”[Mesh] OR cryptorchi* OR “undescended test*” OR “undescended testes” OR “maldescensus testis” OR “Testis Undescended”)) AND ((“Orchiopexy”[Mesh] OR Orchidopexi* OR “surgical procedures, operative”[Mesh] OR Surgery OR “Gonadotropin-Releasing Hormone”[Mesh] OR “Gonadotropin releasing*” OR Gonadorelin OR GnRH* OR hCG))) AND ((“Paternity”[Mesh] OR “Fertility”[Mesh] OR “Semen analysis”[Mesh] OR “Live birth”[Mesh] OR Paternit* OR Fertilit* OR “Sperm quality” OR “Semen quality” OR “Live Birth”)) Filters: Danish, English, Norwegian, from 1990–2025 | 490 |
| 4 | (((“Cryptorchidism”[Mesh] OR cryptorchi* OR “undescended test*” OR “undescended testes” OR “maldescensus testis” OR “Testis Undescended”)) AND ((“Orchiopexy”[Mesh] OR Orchidopexi* OR “surgical procedures, operative”[Mesh] OR Surgery OR “Gonadotropin-Releasing Hormone”[Mesh] OR “Gonadotropin releasing*” OR Gonadorelin OR GnRH* OR hCG))) AND ((“Paternity”[Mesh] OR “Fertility”[Mesh] OR “Semen analysis”[Mesh] OR “Live birth”[Mesh] OR Paternit* OR Fertilit* OR “Sperm quality” OR “Semen quality” OR “Live Birth”)) Filters: Danish, English, from 1990–2025 | 490 |
| 3 | (((“Cryptorchidism”[Mesh] OR cryptorchi* OR “undescended test*” OR “undescended testes” OR “maldescensus testis” OR “Testis Undescended”)) AND ((“Orchiopexy”[Mesh] OR Orchidopexi* OR “surgical procedures, operative”[Mesh] OR Surgery OR “Gonadotropin-Releasing Hormone”[Mesh] OR “Gonadotropin releasing*” OR Gonadorelin OR GnRH* OR hCG))) AND ((“Paternity”[Mesh] OR “Fertility”[Mesh] OR “Semen analysis”[Mesh] OR “Live birth”[Mesh] OR Paternit* OR Fertilit* OR “Sperm quality” OR “Semen quality” OR “Live Birth”)) Filters: English, from 1990–2025 | 490 |
| 2 | (((“Cryptorchidism”[Mesh] OR cryptorchi* OR “undescended test*” OR “undescended testes” OR “maldescensus testis” OR “Testis Undescended”)) AND ((“Orchiopexy”[Mesh] OR Orchidopexi* OR “surgical procedures, operative”[Mesh] OR Surgery OR “Gonadotropin-Releasing Hormone”[Mesh] OR “Gonadotropin releasing*” OR Gonadorelin OR GnRH* OR hCG))) AND ((“Paternity”[Mesh] OR “Fertility”[Mesh] OR “Semen analysis”[Mesh] OR “Live birth”[Mesh] OR Paternit* OR Fertilit* OR “Sperm quality” OR “Semen quality” OR “Live Birth”)) Filters: from 1990–2025 | 550 |
| 1 | (((“Cryptorchidism”[Mesh] OR cryptorchi* OR “undescended test*” OR “undescended testes” OR “maldescensus testis” OR “Testis Undescended”)) AND ((“Orchiopexy”[Mesh] OR Orchidopexi* OR “surgical procedures, operative”[Mesh] OR Surgery OR “Gonadotropin-Releasing Hormone”[Mesh] OR “Gonadotropin releasing*” OR Gonadorelin OR GnRH* OR hCG))) AND ((“Paternity”[Mesh] OR “Fertility”[Mesh] OR “Semen analysis”[Mesh] OR “Live birth”[Mesh] OR Paternit* OR Fertilit* OR “Sperm quality” OR “Semen quality” OR “Live Birth”)) | 759 |
Appendix B
| Selection | Comparability | Outcome | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study ID | 1.1 | 1.2 | 1.3 | 1.4 | 2.a | 2.b | 3.1 | 3.2 | 3.3 | AHRQ |
| Schneuer et al. [13] | All liveborn boys with UDT in Western Australia | All liveborn boys in Western Australia | Hospital data or WARDA | Outcome not demonstrated at the baseline | Unilateral and bilateral UDTs were analyzed separately, but age was assessed for both groups combined | Adjusted for maternal age, birth weight, socioeconomic disadvantage, and hospital stay in first 15 years | Record linkage | Born in 1970 to 1999 and followed until 31 December 2016 | The loss of 4726 participants (1.33%) occurred from the total of 353,336 | Selection: 4 ☆ Comparability: 2 ☆ Outcome: 2 ☆ Overall: good |
| Van Brakel et al. [14] | The study includes men operated on before age of 2 and boys from a previous placebo-controlled trial | Control group from another study | No description | Outcome not demonstrated at the baseline | Age at orchiopexy and UDT laterality did not significantly differ between participants and non-participants | Adjusts for background characteristics in the unilateral UDT and control groups | Self-reported | UDT age: 20.1–37.9 years Control age: 23.0–49.8 years | A loss of 163 participants (72.4%) occurred from the total of 225 | Selection: 2 ☆ Comparability: 2 ☆ Outcome: 0 ☆ Overall: poor |
| Paasch et al. [15] | UDT group with infertility | Infertile men attempting paternity without UDT | Winsperm 2000 database | Outcome not demonstrated at the baseline | Correlates for unilateral and bilateral UDTs, reporting only the mean age | Adjusts for use of fertility treatment | Self-reported | Age of 19–62 years | A loss of 268 participants (49.5%) occurred from a total of 541 | Selection: 3 ☆ Comparability: 2 ☆ Outcome:0 ☆ Overall: poor |
| Lee et al. [16] | Men who had orchiopexy at the Children’s Hospital of Pittsburgh (1955–1969) | Controls matched on age, hospital, and surgery date for an unrelated minor problem | Medical records | Outcome not demonstrated at the baseline | Unilateral and bilateral UDTs were analyzed separately, but age was assessed for both groups combined | Adjusts for men attempting paternity, marital status, and time to conceive | Self-reported | Follow-up after orchiopexy is 26 to 40 years. No age of participants at participation | No statement | Selection: 4 ☆ Comparability: 2 ☆ Outcome: 0 ☆ Overall: poor |
| Lee et al. [17] | Men who had orchiopexy at the Children’s Hospital of Pittsburgh (1955–1975) | Controls matched on age, hospital, and surgery date for an unrelated minor problem | Medical records | Outcome not demonstrated at the baseline | Unilateral and bilateral UDTs were analyzed separately, but age was assessed for bilateral UDT | Adjusts for men attempting paternity | Self-reported | Follow-up after orchiopexy is 26 to 46 years. No age of participants at participation | No statement | Selection: 4 ☆ Comparability: 2 ☆ Outcome: 0 ☆ Overall: poor |
| Cendron et al. [18] | Men with UDT and orchiopexy with biopsy at the Children’s Hospital of Philadelphia | None | Hospital records | Outcome not demonstrated at the baseline | Unilateral and bilateral UDTs and age were analyzed separately | Adjusts for attempting paternity | Self-reported | Follow-up after biopsy is 30 to 40 years. No age of participants at participation | A loss of 9 participants (18.4%) occurred from a total of 49 | Selection: 2 ☆ Comparability: 2 ☆ Outcome: 1 ☆ Overall: poor |
Appendix C
| Study | Additionally Statistical Results |
|---|---|
| Schneuer et al. [13] | Unilateral UDT adjusted HR of 0.84 with 95% CI: 0.77–0.92 for paternity |
| Bilateral UDT adjusted HR of 0.58 with 95% CI: 0.43–0.78 for paternity | |
| Paternity rates are lower in men with undescended testes, and delayed orchidopexy (>18 months) increases ART use | |
| A 6-month delay in orchidopexy raises ART risk by 5% and reduces paternity by 1% | |
| UDT had a two-fold increase in the risk of the future use of ART (adjusted RR of 2.26, 95% CI: 1.58–3.25) | |
| <18-month adjusted RR of 1.33 with 95% CI: 0.43–4.13 for the use of ART | |
| 18 months to 5 years adjusted RR of 2.74 with 95% CI: 1.72–4.37 for the use of ART | |
| 6 to 20 years adjusted RR of 2.16 with 95% CI: 1.16–4.03 for the use of ART | |
| Van Brakel et al. [14] | p-value for the comparison of paternity rates between unilateral UDT participants and controls: 0.08 |
| Paasch et al. [15] | The conception rate for patients with bilateral cryptorchidism was not significantly lower than the conception rate for patients with unilateral cryptorchidism |
| p-value for spontaneous conception rate in the CG was significantly lower than in the NCG: <0.01 | |
| p-value for total conception rate in the CG was significantly lower than in the NCG: <0.05 | |
| p-value for non-conception rate in the CG was significantly lower than in the NCG: <0.01 | |
| p-value for conception after IVF in the CG was significantly higher than in the NCG: <0.05 | |
| Lee et al. [16] | No significant difference between the proportion of unilateral UDT and control married men who had fathered children |
| Significant difference between the bilateral UDT group with children compared to married controls with children. p-value < 0.005 | |
| Significant difference between men with bilateral UDT who were married and had attempted compared to the control group. p-value < 0.001 | |
| Lee et al. [17] | Rates of paternity for those who have attempted paternity show a significantly lower rate for the bilateral group than for both the unilateral (p-value < 0.001) and control (p-value < 0.001) groups |
| The RR for infertility is 5.3 in the bilateral group, with a p-value < 0.0001 | |
| Cendron et al. [18] | Statistical results not reported |
References
- Schmidt, L. Infertility and assisted reproduction in Denmark. Epidemiology and psychosocial consequences. Dan. Med. Bull. 2006, 53, 390–417. [Google Scholar] [PubMed]
- Eisenberg, M.L.; Esteves, S.C.; Lamb, D.J.; Hotaling, J.M.; Giwercman, A.; Hwang, K.; Cheng, Y.S. Male infertility. Nat. Rev. Dis. Primers 2023, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, H.E.; Bjerknes, R.; Cortes, D.; Jørgensen, N.; Rajpert-De Meyts, E.; Thorsson, A.V.; Thorup, J.; Main, K.M. Cryptorchidism: Classification, prevalence and long-term consequences. Acta Paediatr. 2007, 96, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Niedzielski, J.K.; Oszukowska, E.; Slowikowska-Hilczer, J. Undescended testis—Current trends and guidelines: A review of the literature. Arch. Med. Sci. 2016, 12, 667–677. [Google Scholar] [CrossRef]
- Boisen, K.A.; Kaleva, M.M.; Main, K.M.; Virtanen, H.E.; Haavisto, A.-M.; Schmidt, I.M.; Chellakooty, M.; Damgaard, I.N.; Mau Kai, C.; Reunanen, M.; et al. Høj og stigende forekomst af kryptorkisme i Danmark. Ugeskr. Læger 2004, 166, 4372–4375. [Google Scholar]
- Berkowitz, G.S.; Lapinski, R.H.; Gazella, J.G.; Dolgin, S.E.; Bodian, C.A.; Holzman, I.R. Prevalence and natural history of cryptorchidism. Pediatrics 1993, 92, 44–49. [Google Scholar] [CrossRef]
- Chan, E.; Wayne, C.; Nasr, A.; FRCSC for the Canadian Association of Pediatric Surgeon Evidence-Based Resource. Ideal timing of orchiopexy: A systematic review. Pediatr. Surg. Int. 2014, 30, 87–97. [Google Scholar] [CrossRef]
- Gracia, J.; Zalabardo, J.S.; García, J.S.; García, C.; Ferrández, A. Clinical, physical, sperm and hormonal data in 251 adults operated on for cryptorchidism in childhood. BJU Int. 2000, 85, 1100–1103. [Google Scholar] [CrossRef]
- Cortes, D.; Thorup, J.M.; Visfeldt, J. Cryptorchidism: Aspects of fertility and neoplasms. A study including data of 1,335 consecutive boys who underwent testicular biopsy simultaneously with surgery for cryptorchidism. Horm. Res. 2001, 55, 21–27. [Google Scholar]
- Leslie, S.W.; Soon-Sutton, T.L.; Khan, M.A.B. Male Infertility; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2021. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 5 May 2025).
- Schneuer, F.J.; Milne, E.; Jamieson, S.E.; Pereira, G.; Hansen, M.; Barker, A.; Holland, A.J.A.; Bower, C.; Nassar, N. Association between male genital anomalies and adult male reproductive disorders: A population-based data linkage study spanning more than 40 years. Lancet Child. Adolesc. Health 2018, 2, 736–743. [Google Scholar] [CrossRef] [PubMed]
- van Brakel, J.; Kranse, R.; de Muinck Keizer-Schrama, S.M.P.F.; Hendriks, A.E.J.; de Jong, F.H.; Bangma, C.H.; Hazebroek, F.W.J.; Dohle, G.R. Fertility potential in men with a history of congenital undescended testes: A long-term follow-up study. Andrology 2013, 1, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Paasch, U.; Thieme, C.; Grunewald, S.; Glander, H.-J. Electronic data base systems support the evaluation of male infertility factors, example cryptorchidism. Urol. Int. 2004, 72, 154–161. [Google Scholar] [CrossRef]
- Lee, P.; O’Leary, L.; Songer, N.; Bellinger, M.; Laporte, R. Paternity after cryptorchidism: Lack of correlation with age at orchidopexy. Br. J. Urol. 1995, 75, 704–707. [Google Scholar] [CrossRef]
- Lee, P.A.; Coughlin, M.T. Fertility after bilateral cryptorchidism. Evaluation by paternity, hormone, and semen data. Horm. Res. Paediatr. 2001, 55, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Cendron, M.; Keating, M.A.; Huff, D.S.; Koop, C.; Snyder, H.M.; Duckett, J.W. Cryptorchidism, orchiopexy and infertility: A critical long-term retrospective analysis. J. Urol. 1989, 142 Pt 2, 559–562, discussion 572. [Google Scholar] [CrossRef]
- Statistik, D. FOD11: Gennemsnitsalder for Fødende Kvinder og Nybagte Fædre. 2024. Available online: https://www.statistikbanken.dk/FOD11 (accessed on 11 May 2025).
- Goldman, B. Fathers of American Newborns Keep Getting Older. 2017. Available online: https://med.stanford.edu/news/all-news/2017/08/fathers-of-american-newborns-keep-getting-older.html (accessed on 1 May 2025).
- Rohayem, J.; Luberto, A.; Nieschlag, E.; Zitzmann, M.; Kliesch, S. Delayed treatment of undescended testes may promote hypogonadism and infertility. Endocrine 2017, 55, 914–924. [Google Scholar] [CrossRef]
- Bartoletti, R.; Pastore, A.L.; Fabris, F.M.; Di Vico, T.; Morganti, R.; Mogorovich, A.; Morelli, G.; Peroni, D.; Al Salhi, Y.; Zucchi, A. 16 years follow-up evaluation of immediate vs delayed vs. combined hormonal therapy on fertility of patients with cryptorchidism: Results of a longitudinal cohort study. Reprod. Biol. Endocrinol. 2022, 20, 102. [Google Scholar] [CrossRef]
- Hadziselimovic, F.; Hoecht, B. Testicular histology related to fertility outcome and postpubertal hormone status in cryptorchidism. Klin. Padiatr. 2008, 220, 302–307. [Google Scholar] [CrossRef]
- Batra, N.V.; DeMarco, R.T.; Bayne, C.E. A narrative review of the history and evidence-base for the timing of orchidopexy for cryptorchidism. J. Pediatr. Urol. 2021, 17, 239–245. [Google Scholar] [CrossRef]
- Fedder, J.; Boesen, M. Effect of a combined GnRH/hCG therapy in boys with undescended testicles: Evaluated in relation to testicular localization within the first week after birth. Arch. Androl. 1998, 40, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Fedder, J. Prevalence of small testicular hyperechogenic foci in subgroups of 382 non-vasectomized, azoospermic men: A retrospective cohort study. Andrology 2017, 5, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Fedder, J. History of cryptorchidism and ejaculate volume as simple predictors for the presence of testicular sperm. Syst. Biol. Reprod. Med. 2011, 57, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Thorup, J.; Sun, C.; Cortes, D.; Southwell, B.; Hutson, J. Immunofluorescent analysis of testicular biopsies with germ cell and Sertoli cell markers shows significant MVH negative germ cell depletion with older age at orchiopexy. J. Urol. 2014, 191, 458–464. [Google Scholar] [CrossRef]
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef]
| Newcastle–Ottawa Quality Assessment Scale | Score | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Included Studies | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Total Number of Stars | |
| Schneuer et al. [13] | a ☆ | a ☆ | a ☆ | a ☆ | a☆ | b ☆ | b ☆ | b | b ☆ | 8 |
| Van Brakel et al. [14] | b ☆ | b | d | a ☆ | a☆ | b ☆ | c | b | c | 4 |
| Paasch et al. [15] | c | a ☆ | a ☆ | a ☆ | - | b ☆ | c | b | c | 4 |
| Lee et al. [16] | b ☆ | a ☆ | a ☆ | a ☆ | a☆ | b ☆ | c | b | d | 6 |
| Lee et al. [17] | b ☆ | a ☆ | a ☆ | a ☆ | a☆ | b ☆ | c | b | d | 6 |
| Cendron et al. [18] | c | - | a ☆ | a ☆ | a ☆ | b ☆ | c | b | b☆ | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henriksen, A.L.; Poulsen, I.-M.; Sørensen, F.; Fedder, J. Paternity After Treatment of Cryptorchidism: A Systematic Review. J. Clin. Med. 2025, 14, 4768. https://doi.org/10.3390/jcm14134768
Henriksen AL, Poulsen I-M, Sørensen F, Fedder J. Paternity After Treatment of Cryptorchidism: A Systematic Review. Journal of Clinical Medicine. 2025; 14(13):4768. https://doi.org/10.3390/jcm14134768
Chicago/Turabian StyleHenriksen, Anna Lund, Ida-Marie Poulsen, Freja Sørensen, and Jens Fedder. 2025. "Paternity After Treatment of Cryptorchidism: A Systematic Review" Journal of Clinical Medicine 14, no. 13: 4768. https://doi.org/10.3390/jcm14134768
APA StyleHenriksen, A. L., Poulsen, I.-M., Sørensen, F., & Fedder, J. (2025). Paternity After Treatment of Cryptorchidism: A Systematic Review. Journal of Clinical Medicine, 14(13), 4768. https://doi.org/10.3390/jcm14134768







