Complicated Diagnosis and Treatment of Rare Painless Acanthamoeba Keratitis
Abstract
1. Introduction
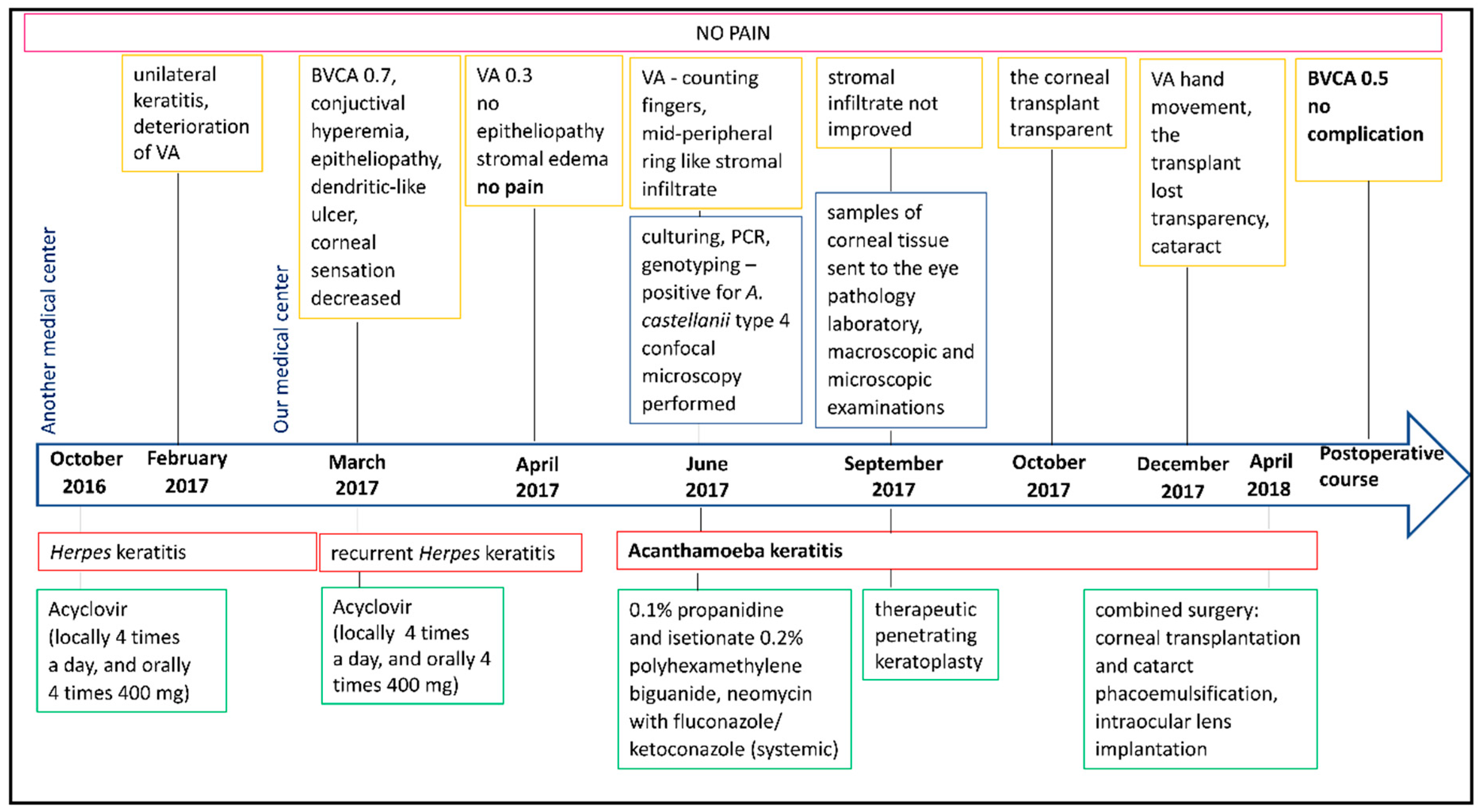
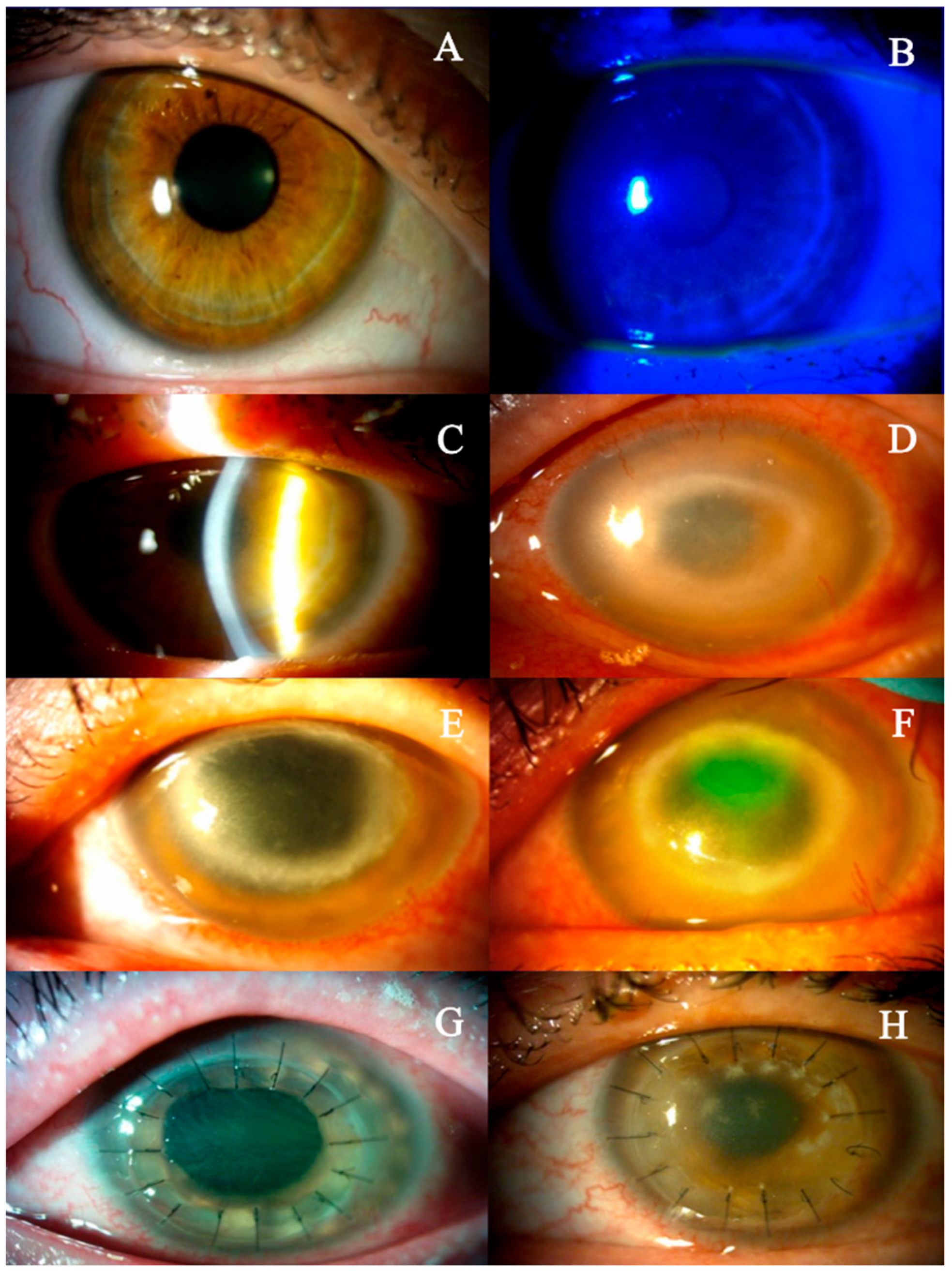
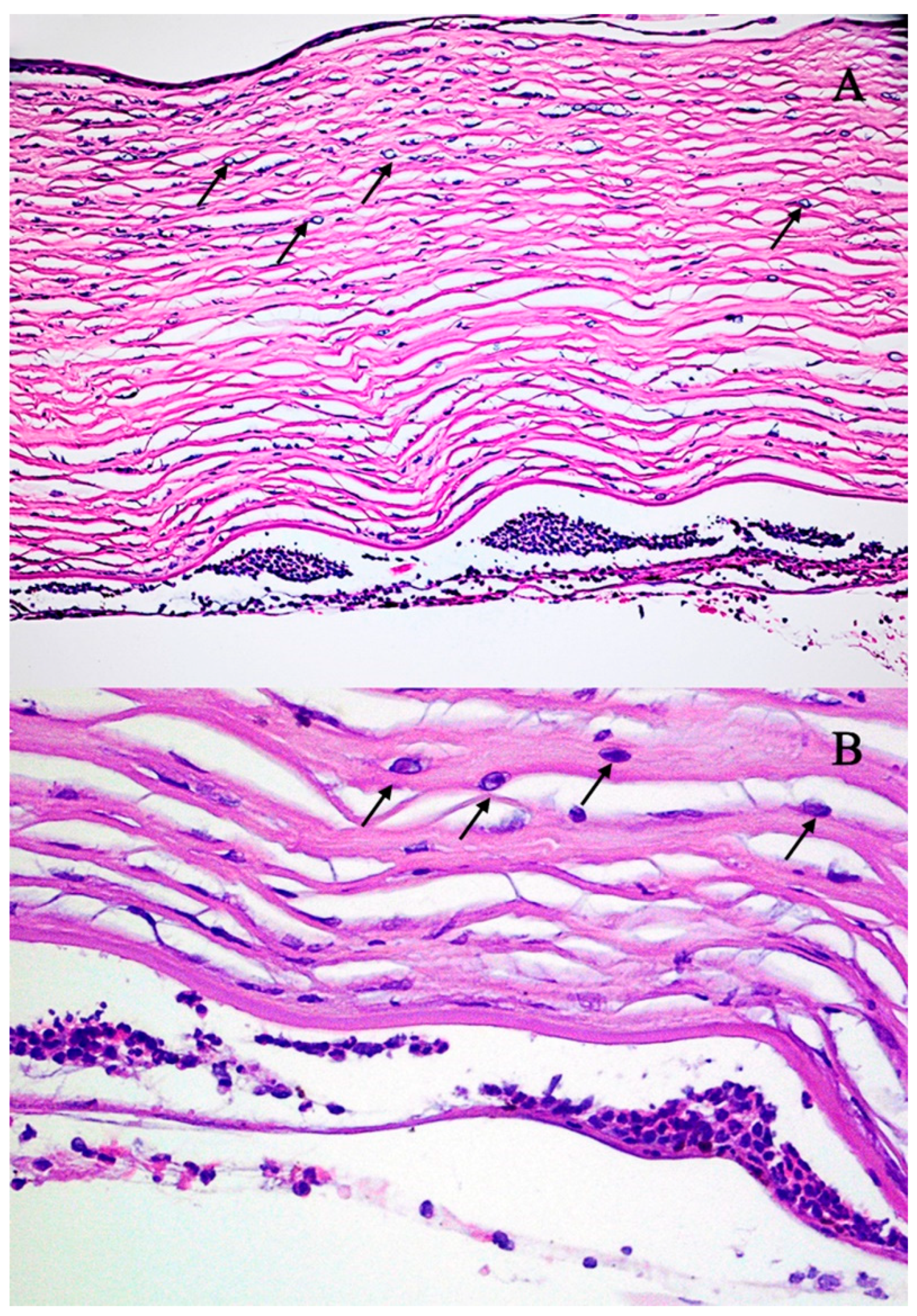
2. Detailed Case Description
3. Discussion and Review
| Reference | Number of Cases | Contact Lens Use | Acanthamoeba castellanii Only | Previous Infections or Coinfections | ||
|---|---|---|---|---|---|---|
| Bacterial | Viral | Fungal | ||||
| Perry et al., 1995 [51] | 6 | + | + | − | − | − |
| + | + | − | − | − | ||
| + | + | − | − | − | ||
| + | + | − | − | − | ||
| − | + | − | − | − | ||
| − | + | − | − | − | ||
| Sharma et al., 2000 [52] | 34 | 34− | 25+ | 9+ | 34− | 34− |
| Roters et al., 2001 [53] | 1 | + | − | + | − | − |
| Tabin et al., 2001 [29] | 2 | − | + | − | − | − |
| − | − | − | Herpes simplex | − | ||
| Georgakopoulos et al., 2006 [54] | 1 | + | + | − | − | − |
| Stemberger et al., 2007 [55] | 1 | + | + | − | − | − |
| Elabjer et al., 2009 [56] | 1 | + | − | + | − | − |
| Shukla et al., 2012 [30] | 5 | + | − | − | Herpes simplex | − |
| + | + | − | − | − | ||
| + | − | + | − | − | ||
| + | − | − | Herpes simplex | − | ||
| + | + | − | − | − | ||
| Kwok et al., 2017 [46] | 1 | + | + | − | − | − |
| Sun et al., 2020 [57] | 1 | + | − | + | Herpes simplex | − |
| Lin et al., 2023 [58] | 1 | + | + | − | − | − |
| 54 | 16 | 38 | 13 | 4 | 0 | |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AK | Acanthamoeba keratitis |
| BCVA | Best corrected visual acuity |
| FK | Fungal keratitis |
| HSK | Herpes simplex keratitis |
| IOP | Intraocular pressure |
| IVCM | In vivo corneal confocal microscopy |
| NK | Neurotrophic keratopathy |
References
- Peguda, H.K.; Lakshminarayanan, R.; Carnt, N.A.; Gu, Z.; Willcox, M.D.P. The Activity of Polyhomoarginine against Acanthamoeba Castellanii. Biology 2022, 11, 1726. [Google Scholar] [CrossRef] [PubMed]
- Chomicz, L.; Szaflik, J.P.; Szostakowska, B.; Izdebska, J.; Baltaza, W.; Łazicka-Gałecka, M.; Kuligowska, A.; Machalińska, A.; Zawadzki, P.J.; Szaflik, J. Successive Acanthamoeba Corneal Isolates Identified in Poland Monitored in Terms of In Vitro Dynamics. Microorganisms 2023, 11, 1174. [Google Scholar] [CrossRef] [PubMed]
- Randag, A.C.; van Rooij, J.; van Goor, A.T.; Verkerk, S.; Wisse, R.P.L.; Saelens, I.E.Y.; Stoutenbeek, R.; van Dooren, B.T.H.; Cheng, Y.Y.Y.; Eggink, C.A. The Rising Incidence of Acanthamoeba Keratitis: A 7-Year Nationwide Survey and Clinical Assessment of Risk Factors and Functional Outcomes. PLoS ONE 2019, 14, e0222092. [Google Scholar] [CrossRef]
- Carnt, N.; Hoffman, J.J.; Verma, S.; Hau, S.; Radford, C.F.; Minassian, D.C.; Dart, J.K.G. Acanthamoeba Keratitis: Confirmation of the UK Outbreak and a Prospective Case-Control Study Identifying Contributing Risk Factors. Br. J. Ophthalmol. 2018, 102, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, D.d.S.; Ferreira, M.d.S.; Guimarães, A.J. Extracellular Vesicles from the Protozoa Acanthamoeba Castellanii: Their Role in Pathogenesis, Environmental Adaptation and Potential Applications. Bioengineering 2019, 6, 13. [Google Scholar] [CrossRef]
- Siddiqui, R.; Khan, N.A. Biology and Pathogenesis of Acanthamoeba. Parasit. Vectors 2012, 5, 6. [Google Scholar] [CrossRef]
- Khan, N.A. Acanthamoeba: Biology and Increasing Importance in Human Health. FEMS Microbiol. Rev. 2006, 30, 564–595. [Google Scholar] [CrossRef]
- Hassan, F.A.M.; Tolba, M.E.M.; Abed, G.H.; Omar, H.M.; Abdel-Hakeem, S.S. Contact Lenses Contamination by Acanthamoeba spp. in Upper Egypt. PLoS ONE 2021, 16, e0259847. [Google Scholar] [CrossRef]
- Alkharashi, M.; Lindsley, K.; Law, H.A.; Sikder, S. Medical Interventions for Acanthamoeba Keratitis. Cochrane Database Syst. Rev. 2015, 2015, CD010792. [Google Scholar] [CrossRef]
- Szentmáry, N.; Daas, L.; Shi, L.; Laurik, K.L.; Lepper, S.; Milioti, G.; Seitz, B. Acanthamoeba Keratitis—Clinical Signs, Differential Diagnosis and Treatment. J. Curr. Ophthalmol. 2019, 31, 16–23. [Google Scholar] [CrossRef]
- Sifaoui, I.; Capote Yanes, E.; Reyes-Batlle, M.; Rodríguez-Expósito, R.L.; Piñero, J.E.; Lorenzo-Morales, J. Combined Amoebicidal Effect of Atorvastatin and Commercial Eye Drops against Acanthamoeba Castellanii Neff: In Vitro Assay Based on Mixture Design. Pathogens 2020, 9, 219. [Google Scholar] [CrossRef] [PubMed]
- Magnet, A.; Galván, A.L.; Fenoy, S.; Izquierdo, F.; Rueda, C.; Fernandez Vadillo, C.; Pérez-Irezábal, J.; Bandyopadhyay, K.; Visvesvara, G.S.; da Silva, A.J.; et al. Molecular Characterization of Acanthamoeba Isolated in Water Treatment Plants and Comparison with Clinical Isolates. Parasitol. Res. 2012, 111, 383–392. [Google Scholar] [CrossRef]
- Guimaraes, A.J.; Gomes, K.X.; Cortines, J.R.; Peralta, J.M.; Peralta, R.H.S. Acanthamoeba spp. as a Universal Host for Pathogenic Microorganisms: One Bridge from Environment to Host Virulence. Microbiol. Res. 2016, 193, 30–38. [Google Scholar] [CrossRef]
- Somani, S.N.; Ronquillo, Y.; Moshirfar, M. Acanthamoeba Keratitis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Zhang, Y.; Xu, X.; Wei, Z.; Cao, K.; Zhang, Z.; Liang, Q. The Global Epidemiology and Clinical Diagnosis of Acanthamoeba Keratitis. J. Infect. Public Health 2023, 16, 841–852. [Google Scholar] [CrossRef]
- Azzopardi, M.; Chong, Y.J.; Ng, B.; Recchioni, A.; Logeswaran, A.; Ting, D.S.J. Diagnosis of Acanthamoeba Keratitis: Past, Present and Future. Diagnostics 2023, 13, 2655. [Google Scholar] [CrossRef]
- Chin, J.; Young, A.L.; Hui, M.; Jhanji, V. Acanthamoeba Keratitis: 10-Year Study at a Tertiary Eye Care Center in Hong Kong. Cont. Lens Anterior Eye 2015, 38, 99–103. [Google Scholar] [CrossRef]
- Lorenzo-Morales, J.; Khan, N.A.; Walochnik, J. An Update on Acanthamoeba Keratitis: Diagnosis, Pathogenesis and Treatment. Parasite 2015, 22, 10. [Google Scholar] [CrossRef] [PubMed]
- Illingworth, C.D.; Cook, S.D. Acanthamoeba Keratitis. Surv. Ophthalmol. 1998, 42, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Rosińska, J.; Kozubski, W. Peripheral nervous system involvement in the course of herpes virus infections. Neurol. Neurochir. Pol. 2013, 47, 170–178. [Google Scholar] [CrossRef][Green Version]
- Amponin, D.E.; Przybek-Skrzypecka, J.; Zyablitskaya, M.; Takaoka, A.; Suh, L.H.; Nagasaki, T.; Trokel, S.L.; Paik, D.C. Ex Vivo Anti-Microbial Efficacy of Various Formaldehyde Releasers against Antibiotic Resistant and Antibiotic Sensitive Microorganisms Involved in Infectious Keratitis. BMC Ophthalmol. 2020, 20, 28. [Google Scholar] [CrossRef]
- Marciano-Cabral, F.; Cabral, G. Acanthamoeba spp. as Agents of Disease in Humans. Clin. Microbiol. Rev. 2003, 16, 273–307. [Google Scholar] [CrossRef] [PubMed]
- Mastropasqua, L.; Massaro-Giordano, G.; Nubile, M.; Sacchetti, M. Understanding the Pathogenesis of Neurotrophic Keratitis: The Role of Corneal Nerves. J. Cell Physiol. 2017, 232, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.J.; Marfurt, C.F.; Kruse, F.; Tervo, T.M.T. Corneal Nerves: Structure, Contents and Function. Exp. Eye Res. 2003, 76, 521–542. [Google Scholar] [CrossRef] [PubMed]
- Meng, I.D.; Kurose, M. The Role of Corneal Afferent Neurons in Regulating Tears under Normal and Dry Eye Conditions. Exp. Eye Res. 2013, 117, 79–87. [Google Scholar] [CrossRef]
- Duncan, N.W.; Houser, K.H.; Khandelwal, S.S. Neurotrophic Keratitis: A Review. Touchreviews Ophthalmol. 2022, 16, 67–71. [Google Scholar] [CrossRef]
- Kurbanyan, K.; Hoesl, L.M.; Schrems, W.A.; Hamrah, P. Corneal Nerve Alterations in Acute Acanthamoeba and Fungal Keratitis: An in Vivo Confocal Microscopy Study. Eye 2012, 26, 126–132. [Google Scholar] [CrossRef]
- Dart, J.K.G.; Saw, V.P.J.; Kilvington, S. Acanthamoeba Keratitis: Diagnosis and Treatment Update 2009. Am. J. Ophthalmol. 2009, 148, 487–499.e2. [Google Scholar] [CrossRef]
- Tabin, G.; Taylor, H.; Snibson, G.; Murchison, A.; Gushchin, A.; Rogers, S. Atypical Presentation of Acanthamoeba Keratitis. Cornea 2001, 20, 757–759. [Google Scholar] [CrossRef]
- Shukla Kent, S.; Robert, M.-C.; Tokarewicz, A.C.; Mather, R. Painless Acanthamoeba Keratitis. Can. J. Ophthalmol. 2012, 47, 383–384. [Google Scholar] [CrossRef]
- Bonini, S.; Rama, P.; Olzi, D.; Lambiase, A. Neurotrophic Keratitis. Eye 2003, 17, 989–995. [Google Scholar] [CrossRef]
- Cerva, L. Amoebic Meningoencephalitis: Axenic Culture of Naegleria. Science 1969, 163, 576. [Google Scholar] [CrossRef] [PubMed]
- Page, F. An Illustrated Key to Freshwater and Soil Amoebae; Freshwater Biological Association: Ambleside, UK, 1967; Volume 34. [Google Scholar]
- Schroeder, J.M.; Booton, G.C.; Hay, J.; Niszl, I.A.; Seal, D.V.; Markus, M.B.; Fuerst, P.A.; Byers, T.J. Use of Subgenic 18S Ribosomal DNA PCR and Sequencing for Genus and Genotype Identification of Acanthamoebae from Humans with Keratitis and from Sewage Sludge. J. Clin. Microbiol. 2001, 39, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Derda, M.; Solarczyk, P.; Cholewiński, M.; Hadaś, E. Genotypic Characterization of Amoeba Isolated from Acanthamoeba Keratitis in Poland. Parasitol. Res. 2015, 114, 1233–1237. [Google Scholar] [CrossRef]
- Visvesvara, G.S.; Shoff, M.E.; Sriram, R.; Booton, G.C.; Crary, M.; Fuerst, P.A.; Hanley, C.S.; Garner, M.M. Isolation, Morphologic, Serologic and Molecular Identification of Acanthamoeba T4 Genotype from the Liver of a Temminck’s Tragopan (Tragopan Temminckii). Vet. Parasitol. 2010, 170, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Mafra, C.S.P.; Carrijo-Carvalho, L.C.; Chudzinski-Tavassi, A.M.; Taguchi, F.M.d.C.; Foronda, A.S.; Carvalho, F.R.d.S.; de Freitas, D. Antimicrobial Action of Biguanides on the Viability of Acanthamoeba Cysts and Assessment of Cell Toxicity. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6363–6372. [Google Scholar] [CrossRef]
- Corsaro, D.; Feroldi, V.; Saucedo, G.; Ribas, F.; Loret, J.-F.; Greub, G. Novel Chlamydiales Strains Isolated from a Water Treatment Plant. Environ. Microbiol. 2009, 11, 188–200. [Google Scholar] [CrossRef]
- Yera, H.; Zamfir, O.; Bourcier, T.; Viscogliosi, E.; Noël, C.; Dupouy-Camet, J.; Chaumeil, C. The Genotypic Characterisation of Acanthamoeba Isolates from Human Ocular Samples. Br. J. Ophthalmol. 2008, 92, 1139–1141. [Google Scholar] [CrossRef]
- Yera, H.; Zamfir, O.; Bourcier, T.; Ancelle, T.; Batellier, L.; Dupouy-Camet, J.; Chaumeil, C. Comparison of PCR, Microscopic Examination and Culture for the Early Diagnosis and Characterization of Acanthamoeba Isolates from Ocular Infections. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 221–224. [Google Scholar] [CrossRef]
- Daas, L.; Szentmáry, N.; Eppig, T.; Langenbucher, A.; Hasenfus, A.; Roth, M.; Saeger, M.; Nölle, B.; Lippmann, B.; Böhringer, D.; et al. The German Acanthamoeba keratitis register: Initial results of a multicenter study. Ophthalmologe 2015, 112, 752–763. [Google Scholar] [CrossRef]
- Szentmáry, N.; Goebels, S.; Matoula, P.; Schirra, F.; Seitz, B. Acanthamoeba keratitis--a rare and often late diagnosed disease. Klin. Monbl Augenheilkd. 2012, 229, 521–528. [Google Scholar] [CrossRef]
- Raghavan, A.; Baidwal, S.; Venkatapathy, N.; Rammohan, R. The Acanthamoeba-Fungal Keratitis Study. Am. J. Ophthalmol. 2019, 201, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Osato, M.; Wilhalmus, K. Infectious Keratitis. In Cornea; Little, Brown: Boston, MA, USA, 1994; pp. 202–266. [Google Scholar]
- Bacon, A.S.; Frazer, D.G.; Dart, J.K.; Matheson, M.; Ficker, L.A.; Wright, P. A Review of 72 Consecutive Cases of Acanthamoeba Keratitis, 1984–1992. Eye 1993, 7 Pt 6, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Kwok, P.W.R.; Kam, K.W.; Jhanji, V.; Young, A.L. Painless Acanthamoeba Keratitis with Normal Vision. Optom. Vis. Sci. 2017, 94, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Johns, K.J.; O’Day, D.M.; Head, W.S.; Neff, R.J.; Elliott, J.H. Herpes Simplex Masquerade Syndrome: Acanthamoeba Keratitis. Curr. Eye Res. 1987, 6, 207–212. [Google Scholar] [CrossRef]
- Maycock, N.J.R.; Jayaswal, R. Update on Acanthamoeba Keratitis: Diagnosis, Treatment, and Outcomes. Cornea 2016, 35, 713–720. [Google Scholar] [CrossRef]
- Pettit, D.A.; Williamson, J.; Cabral, G.A.; Marciano-Cabral, F. In Vitro Destruction of Nerve Cell Cultures by Acanthamoeba spp.: A Transmission and Scanning Electron Microscopy Study. J. Parasitol. 1996, 82, 769–777. [Google Scholar] [CrossRef]
- Kaiserman, I.; Bahar, I.; McAllum, P.; Srinivasan, S.; Elbaz, U.; Slomovic, A.R.; Rootman, D.S. Prognostic Factors in Acanthamoeba Keratitis. Can. J. Ophthalmol. 2012, 47, 312–317. [Google Scholar] [CrossRef]
- Perry, H.D.; Donnenfeld, E.D.; Foulks, G.N.; Moadel, K.; Kanellopoulos, A.J. Decreased Corneal Sensation as an Initial Feature of Acanthamoeba Keratitis. Ophthalmology 1995, 102, 1565–1568. [Google Scholar] [CrossRef]
- Sharma, S.; Garg, P.; Rao, G.N. Patient Characteristics, Diagnosis, and Treatment of Non-Contact Lens Related Acanthamoeba Keratitis. Br. J. Ophthalmol. 2000, 84, 1103–1108. [Google Scholar] [CrossRef]
- Roters, S.; Aisenbrey, S.; Severin, M.; Konen, W.; Seitz, H.M.; Krieglstein, G.K. Painless acanthamoeba keratitis. Klin. Monbl Augenheilkd. 2001, 218, 570–573. [Google Scholar] [CrossRef]
- Georgakopoulos, C.D.; Exarchou, A.M.; Gartaganis, S.P. Unusual Case of Acanthamoeba Keratitis in a Contact Lens Wearer. Eye Contact Lens 2006, 32, 166–167. [Google Scholar] [CrossRef] [PubMed]
- Stemberger, K.; Dick, B.; Kramann, C.; Thieme, H.; Weber, A.; Petry, F.; Pfeiffer, N. Painless acanthamoeba keratitis. Ophthalmologe 2007, 104, 415–417. [Google Scholar] [CrossRef]
- Elabjer, B.K.; Busić, M.; Sviben, M.; Elabjer, E.; Predović, J. Painless Acanthamoeba Keratitis in a Soft Contact Lens Wearer—Case Report. Coll. Antropol. 2009, 33, 951–954. [Google Scholar]
- Sun, Y.; Li, W.; Wang, M.; Xing, Q.; Sun, X. Clinical Diagnosis and Treatment of Rare Painless Keratitis Caused by Three Pathogens: Clinical Practice and Experiential Discussion. J. Int. Med. Res. 2020, 48, 300060519895671. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Khaliddin, N. Different Outcomes of Acanthamoeba Keratitis: A Case Series. Cureus 2023, 15, e48129. [Google Scholar] [CrossRef] [PubMed]
- List, W.; Glatz, W.; Riedl, R.; Mossboeck, G.; Steinwender, G.; Wedrich, A. Evaluation of Acanthamoeba Keratitis Cases in a Tertiary Medical Care Centre over 21 Years. Sci. Rep. 2021, 11, 1036. [Google Scholar] [CrossRef]
- Lim, N.; Goh, D.; Bunce, C.; Xing, W.; Fraenkel, G.; Poole, T.R.G.; Ficker, L. Comparison of Polyhexamethylene Biguanide and Chlorhexidine as Monotherapy Agents in the Treatment of Acanthamoeba Keratitis. Am. J. Ophthalmol. 2008, 145, 130–135. [Google Scholar] [CrossRef]
- Cohen, E.J.; Parlato, C.J.; Arentsen, J.J.; Genvert, G.I.; Eagle, R.C.; Wieland, M.R.; Laibson, P.R. Medical and Surgical Treatment of Acanthamoeba Keratitis. Am. J. Ophthalmol. 1987, 103, 615–625. [Google Scholar] [CrossRef]
- Bouheraoua, N.; Gaujoux, T.; Goldschmidt, P.; Chaumeil, C.; Laroche, L.; Borderie, V.M. Prognostic Factors Associated with the Need for Surgical Treatments in Acanthamoeba Keratitis. Cornea 2013, 32, 130–136. [Google Scholar] [CrossRef]
- Robaei, D.; Carnt, N.; Minassian, D.C.; Dart, J.K.G. Therapeutic and Optical Keratoplasty in the Management of Acanthamoeba Keratitis: Risk Factors, Outcomes, and Summary of the Literature. Ophthalmology 2015, 122, 17–24. [Google Scholar] [CrossRef]
- Roozbahani, M.; Hammersmith, K.M.; Rapuano, C.J.; Nagra, P.K.; Zhang, Q. Therapeutic Penetrating Keratoplasty for Acanthamoeba Keratitis: A Review of Cases, Complications and Predictive Factors. Int. Ophthalmol. 2019, 39, 2889–2896. [Google Scholar] [CrossRef] [PubMed]
- Claerhout, I.; Goegebuer, A.; Van Den Broecke, C.; Kestelyn, P. Delay in Diagnosis and Outcome of Acanthamoeba Keratitis. Graefes Arch. Clin. Exp. Ophthalmol. 2004, 242, 648–653. [Google Scholar] [CrossRef] [PubMed]
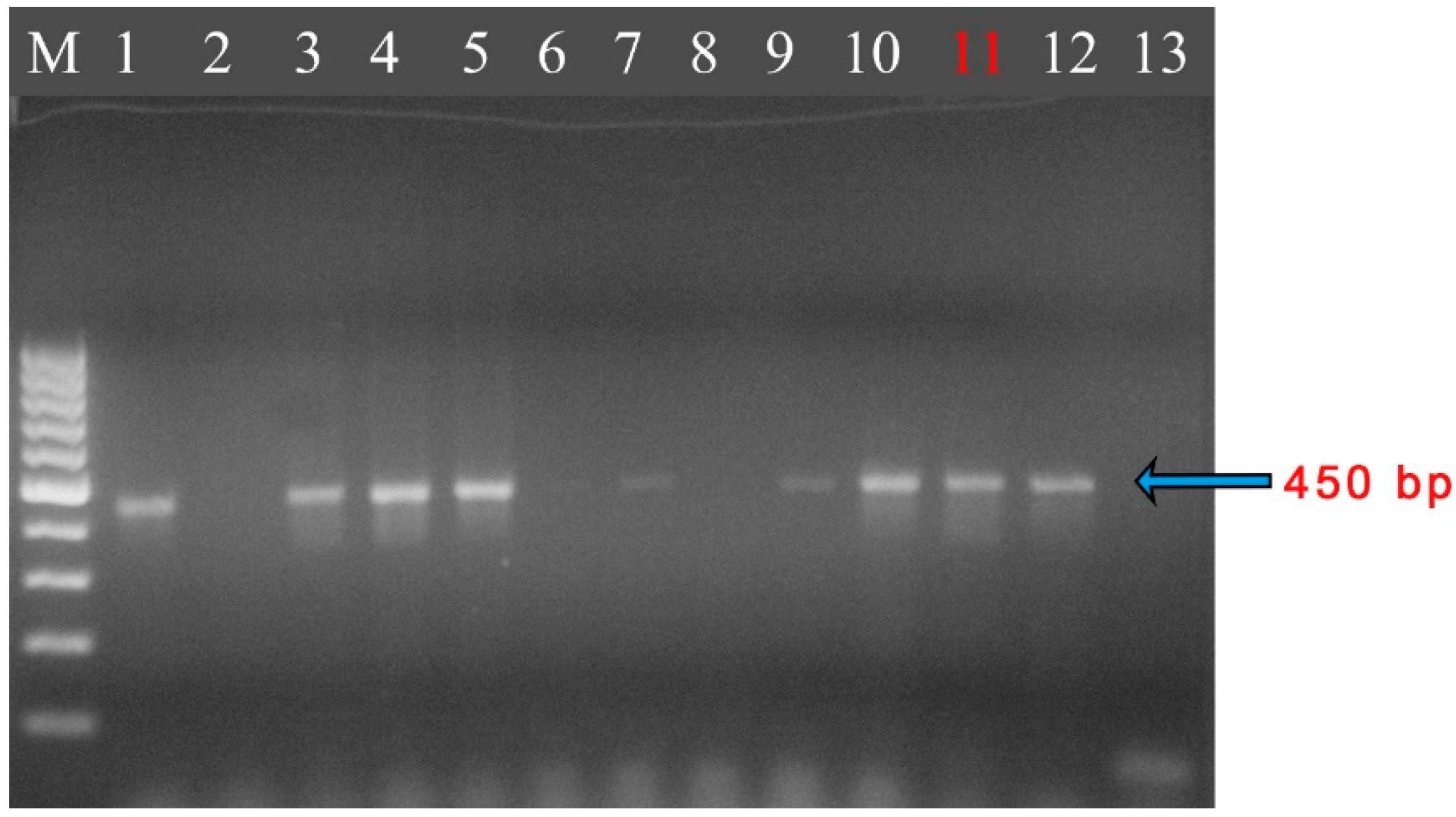

| Sampling | Isolate, Accession No. | Published Sequences in GeneBank | |||
|---|---|---|---|---|---|
| Accession No. | Sampling, Isolate | Region of Origin | References | ||
| Corneal scraping | Ac55, KP120880 | GQ889265 | Liver of a Temminck’s tragopan, Acanthamoeba sp., genotype: T4 CDCV600 | USA | Visvesvara et al., 2010 [36] |
| KF318460 | Corneal surface tissue, Acanthamoeba sp., 1 FRC-2013 | Brazil | Mafra et al., 2013 [37] | ||
| EU377583 | Biofilm, Acanthamoeba sp., CRIB53 | Switzerland | Corsaro et al., 2009 [38] | ||
| DQ087296 | Contact lenses and contact lens case, Acanthamoeba sp., S6 | France | Yera et al., 2008 [39] | ||
| DQ087297 | Corneal scraping, Acanthamoeba sp., 222BAL | France | Yera et al., 2007 [40] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wróbel-Dudzińska, D.; Ziaja-Sołtys, M.; Rymgayłło-Jankowska, B.; Derda, M.; Klepacz, R.; Zalewski, D.; Żarnowski, T.; Bogucka-Kocka, A. Complicated Diagnosis and Treatment of Rare Painless Acanthamoeba Keratitis. J. Clin. Med. 2025, 14, 4763. https://doi.org/10.3390/jcm14134763
Wróbel-Dudzińska D, Ziaja-Sołtys M, Rymgayłło-Jankowska B, Derda M, Klepacz R, Zalewski D, Żarnowski T, Bogucka-Kocka A. Complicated Diagnosis and Treatment of Rare Painless Acanthamoeba Keratitis. Journal of Clinical Medicine. 2025; 14(13):4763. https://doi.org/10.3390/jcm14134763
Chicago/Turabian StyleWróbel-Dudzińska, Dominika, Marta Ziaja-Sołtys, Beata Rymgayłło-Jankowska, Monika Derda, Robert Klepacz, Daniel Zalewski, Tomasz Żarnowski, and Anna Bogucka-Kocka. 2025. "Complicated Diagnosis and Treatment of Rare Painless Acanthamoeba Keratitis" Journal of Clinical Medicine 14, no. 13: 4763. https://doi.org/10.3390/jcm14134763
APA StyleWróbel-Dudzińska, D., Ziaja-Sołtys, M., Rymgayłło-Jankowska, B., Derda, M., Klepacz, R., Zalewski, D., Żarnowski, T., & Bogucka-Kocka, A. (2025). Complicated Diagnosis and Treatment of Rare Painless Acanthamoeba Keratitis. Journal of Clinical Medicine, 14(13), 4763. https://doi.org/10.3390/jcm14134763







