Three-Dimensional Transesophageal Echocardiography Is Useful for Preventing Prosthetic-Patient Mismatch After Surgical Aortic Valve Replacement
Abstract
1. Introduction
2. Materials and Methods
2.1. Transthoracic Echocardiography
2.2. Transesophageal Echocardiography
2.3. Operative Procedure
2.4. Statistical Analysis
3. Results
3.1. Preoperative Echocardiographic Parameters
3.2. Types and Size of Implanted Prosthetic Valve
3.3. Surgical Procedures
3.4. Postoperative Echocardiographic Parameters
3.5. Correlations Between EOAi and AV Annulus Diameter Parameters
3.6. Logistic Regression Analysis for Prediction of Severe PPM
4. Discussion
4.1. Incidence of PPM in SAVR
4.2. Risk Factors for PPM After SAVR
4.3. Importance of Preventing PPM in SAVR
4.4. Usefulness of the Recommended Prosthetic AV Size in SAVR
- From our study, it was revealed that use of a valve with a smaller than recommended prosthesis AV size influences postoperative EOAi and may be an independent predictive factor for severe PPM.
- Although the choice of prosthetic valve size is currently determined by the surgeon using a sizer during surgery, size should be preoperatively determined from preoperative imaging in future.
4.5. Application to Mechanical Valves and TAVR
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahimtoola, S.H. The problem of valve prosthesis-patient mismatch. Circulation 1978, 58, 20–24. [Google Scholar] [CrossRef]
- Dahlbacka, S.; Laakso, T.; Kinnunen, E.M.; Moriyama, N.; Laine, M.; Virtanen, M.; Maaranen, P.; Ahvenvaara, T.; Tauriainen, T.; Husso, A.; et al. Patient-Prosthesis Mismatch Worsens Long-Term Survival: Insights From the FinnValve Registry. Ann. Thorac. Surg. 2021, 111, 1284–1290. [Google Scholar] [CrossRef]
- Head, S.J.; Mokhles, M.M.; Osnabrugge, R.L.; Pibarot, P.; Mack, M.J.; Takkenberg, J.J.; Bogers, A.J.; Kappetein, A.P. The impact of prosthesis-patient mismatch on long-term survival after aortic valve replacement: A systematic review and meta-analysis of 34 observational studies comprising 27 186 patients with 133 141 patient-years. Eur. Heart J. 2012, 33, 1518–1529. [Google Scholar] [CrossRef] [PubMed]
- Pibarot, P.; Dumesnil, J.G. Hemodynamic and clinical impact of prosthesis-patient mismatch in the aortic valve position and its prevention. J. Am. Coll. Cardiol. 2000, 36, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.G.; Bourne, E.T. Industry-generated charts for the selection of stented aortic valve prostheses: Clinical tool or marketing ploy? Ann. Thorac. Surg. 2011, 91, 1001–1002. [Google Scholar] [CrossRef] [PubMed]
- Head, S.J.; Reardon, M.J.; Deeb, G.M.; Van Mieghem, N.M.; Popma, J.J.; Gleason, T.G.; Williams, M.R.; Radhakrishnan, S.; Fremes, S.; Oh, J.K.; et al. Computed Tomography-Based Indexed Aortic Annulus Size to Predict Prosthesis-Patient Mismatch. Circ. Cardiovasc. Interv. 2019, 12, e007396. [Google Scholar] [CrossRef]
- Khalique, O.K.; Kodali, S.K.; Paradis, J.-M.; Nazif, T.M.; Williams, M.R.; Einstein, A.J.; Pearson, G.D.; Harjai, K.; Grubb, K.; George, I.; et al. Aortic annular sizing using a novel 3-dimensional echocardiographic method: Use and comparison with cardiac computed tomography. Circ. Cardiovasc. Imaging 2014, 7, 155–163. [Google Scholar] [CrossRef]
- Pibarot, P.; Magne, J.; Leipsic, J.; Côté, N.; Blanke, P.; Thourani, V.H.; Hahn, R. Imaging for Predicting and Assessing Prosthesis-Patient Mismatch After Aortic Valve Replacement. JACC Cardiovasc. Imaging 2019, 12, 149–162. [Google Scholar] [CrossRef]
- VARC-3 Writing Committee; Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; et al. Valve Academic Research Consortium 3: Updated endpoint definitions for aortic valve clinical research. Eur. Heart J. 2021, 42, 1825–1857. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Ternacle, J.; Pibarot, P.; Herrmann, H.C.; Kodali, S.; Leipsic, J.; Blanke, P.; Jaber, W.; Mack, M.J.; Clavel, M.-A.; Salaun, E.; et al. Prosthesis-Patient Mismatch After Aortic Valve Replacement in the PARTNER 2 Trial and Registry. JACC Cardiovasc. Interv. 2021, 14, 1466–1477. [Google Scholar] [CrossRef] [PubMed]
- Mylonas, K.S.; Angouras, D.C. Bioprosthetic Valves for Lifetime Management of Aortic Stenosis: Pearls and Pitfalls. J. Clin. Med. 2023, 12, 7063. [Google Scholar] [CrossRef]
- Dismorr, M.; Glaser, N.; Franco-Cereceda, A.; Sartipy, U. Effect of Prosthesis-Patient Mismatch on Long-Term Clinical Outcomes After Bioprosthetic Aortic Valve Replacement. J. Am. Coll. Cardiol. 2023, 81, 964–975. [Google Scholar] [CrossRef] [PubMed]
- Dayan, V.; Vignolo, G.; Soca, G.; Paganini, J.J.; Brusich, D.; Pibarot, P. Predictors and Outcomes of Prosthesis-Patient Mismatch After Aortic Valve Replacement. JACC Cardiovasc. Imaging 2016, 9, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Sá, M.P.B.D.O.; de Carvalho, M.M.B.; Sobral Filho, D.C.; Cavalcanti, L.R.P.; Rayol, S.D.C.; Diniz, R.G.S.; Menezes, A.M.; Clavel, M.A.; Pibarot, P.; Lima, R.C. Surgical aortic valve replacement and patient-prosthesis mismatch: A meta-analysis of 108 182 patients. Eur. J. Cardiothorac. Surg. 2019, 56, 44–54. [Google Scholar] [CrossRef]
- Bakhtiary, F.; Schiemann, M.; Dzemali, O.; Dogan, S.; Schächinger, V.; Ackermann, H.; Moritz, A.; Kleine, P. Impact of patient-prosthesis mismatch and aortic valve design on coronary flow reserve after aortic valve replacement. J. Am. Coll. Cardiol. 2007, 49, 790–796. [Google Scholar] [CrossRef]
- Flameng, W.; Herregods, M.-C.; Vercalsteren, M.; Herijgers, P.; Bogaerts, K.; Meuris, B. Prosthesis-patient mismatch predicts structural valve degeneration in bioprosthetic heart valves. Circulation 2010, 121, 2123–2129. [Google Scholar] [CrossRef]
- Herrmann, H.C.; Pibarot, P.; Wu, C.; Hahn, R.T.; Tang, G.H.; Abbas, A.E.; Playford, D.; Ruel, M.; Jilaihawi, H.; Sathananthan, J.; et al. Bioprosthetic Aortic Valve Hemodynamics: Definitions, Outcomes, and Evidence Gaps: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 80, 527–544. [Google Scholar] [CrossRef]
- Fazmin, I.T.; Ali, J.M. Prosthesis-Patient Mismatch and Aortic Root Enlargement: Indications, Techniques and Outcomes. J. Cardiovasc. Dev. Dis. 2023, 10, 373. [Google Scholar] [CrossRef]
- Saadi, R.P.; Tagliari, A.P.; Saadi, E.K.; Miglioranza, M.H.; Polanczyck, C.A. Preoperative TAVR Planning: How to Do It. J. Clin. Med. 2022, 11, 2582. [Google Scholar] [CrossRef]
- Joury, A.; Duran, A.; Stewart, M.; Gilliland, Y.E.; Spindel, S.M.; Qamruddin, S. Prosthesis-patient mismatch following aortic and mitral valves replacement—A comprehensive review. Prog. Cardiovasc. Dis. 2022, 72, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.; Webb, J.G.; Willson, A.B.; Wheeler, M.; Blanke, P.; Moss, R.R.; Thompson, C.R.; Munt, B.; Norgaard, B.L.; Yang, T.-H.; et al. Multidetector CT predictors of prosthesis-patient mismatch in transcatheter aortic valve replacement. J. Cardiovasc. Comput. Tomogr. 2013, 7, 248–255. [Google Scholar] [CrossRef] [PubMed]

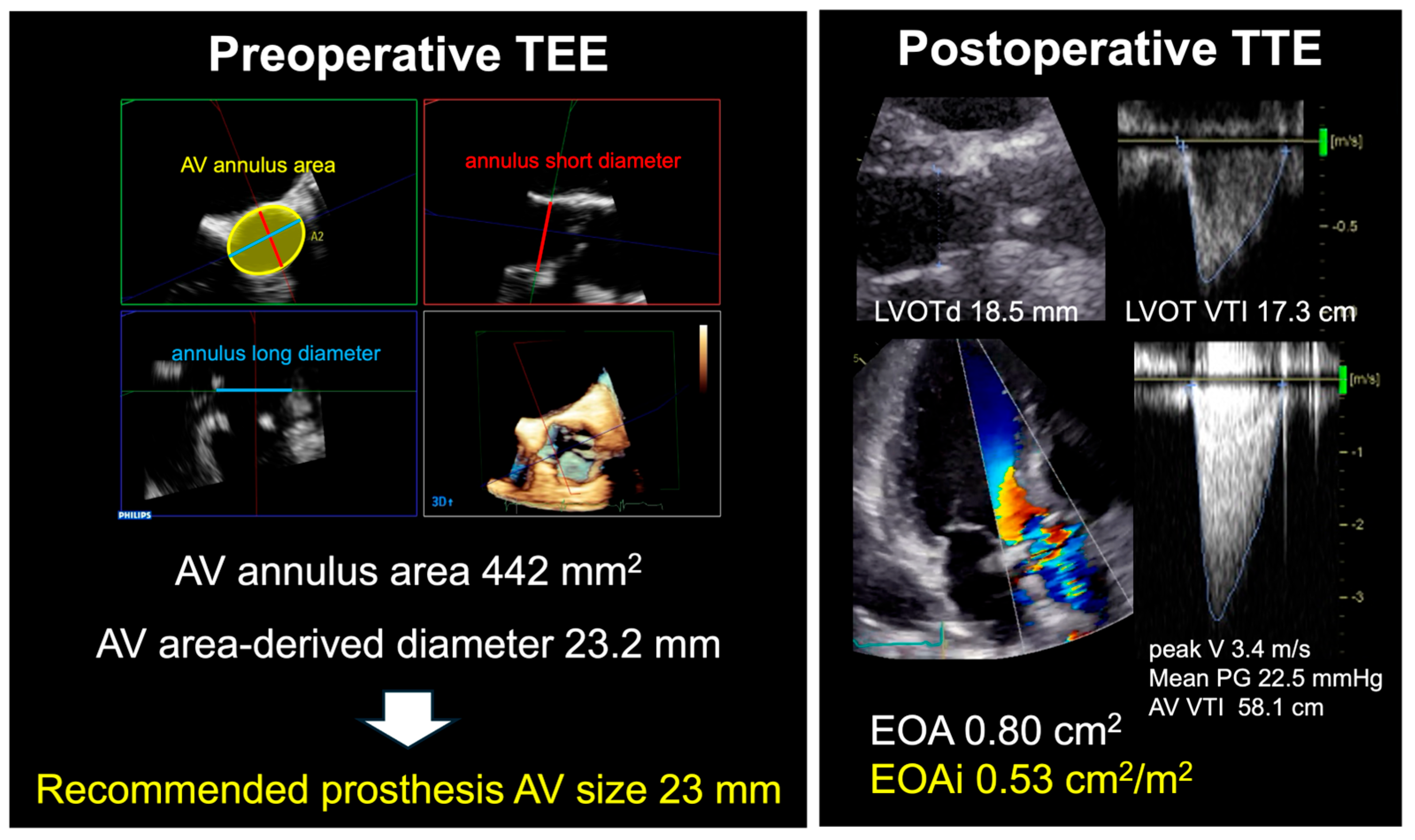
| 3D TEE-Derived Annulus Diameter (mm) | Recommended Prosthesis AV Size (mm) |
|---|---|
| ≤20.9 | 19 |
| 21.0–22.9 | 21 |
| 23.0–24.9 | 23 |
| 25.0–26.9 | 25 |
| 27.0–28.9 | 27 |
| 29.0≤ | 29 |
| Severe PPM Group (n = 6) | Without Severe PPM Group (n = 199) | p Value | |
|---|---|---|---|
| Age, years | 66 ± 7 | 73 ± 7 | 0.040 |
| Men, n (%) | 4 (67) | 125 (63) | 1.000 |
| Body weight, Kg | 56 ± 14 | 57 ± 12 | 0.828 |
| Body height, cm | 161 ± 10 | 161 ± 10 | 0.932 |
| Body surface area, m2 | 1.64 ± 0.22 | 1.58 ± 0.19 | 0.456 |
| Hypertension, n (%) | 4 (67) | 151 (76) | 0.635 |
| Diabetes mellitus, n (%) | 1 (2) | 36 (18) | 1.000 |
| Chronic kidney disease, n (%) | 4 (67) | 102 (51) | 0.684 |
| Atrial fibrillation, n (%) | 2 (33) | 59 (30) | 1.000 |
| Coronary artery disease, n (%) | 2 (33) | 51 (26) | 0.650 |
| Severe PPM Group (n = 6) | Without Severe PPM Group (n = 199) | p Value | |
|---|---|---|---|
| Transthoracic echocardiography | |||
| LAVI (mL/m2) | 55.2 ± 30.4 | 47.9 ± 35.7 | 0.620 |
| LVEDD (mm) | 58.2 ± 12.0 | 53.6 ± 10.3 | 0.281 |
| LVESD (mm) | 42.5 ± 10.0 | 38.8 ± 11.0 | 0.415 |
| LVEF (%) | 43.9 ± 18.5 | 54.8 ± 13.5 | 0.054 |
| AV annulus diameter (mm) | 21.5 ± 2.4 | 22.2 ± 2.6 | 0.502 |
| Valvular disease types | 0.783 | ||
| Aortic stenosis | 3 (50.0) | 75 (37.7) | |
| Aortic regurgitation | 3 (50.0) | 112 (56.3) | |
| Aortic stenosis and regurgitation | 0 (0) | 11 (5.5) | |
| Transesophageal echocardiography | |||
| AV annulus area (mm2) | 492 ± 93 | 451 ± 89 | 0.265 |
| AV annulus area-derived diameter (mm) | 24.9 ± 2.4 | 23.9 ± 2.4 | 0.272 |
| Recommended prosthesis AV size (mm) | 24.3 ± 2.1 | 22.9 ± 2.4 | 0.141 |
| Severe PPM Group (n = 6) | Without Severe PPM Group (n = 199) | p Value | |
|---|---|---|---|
| Valve type | 0.251 | ||
| Inspris RESILIA | 4 (66.7) | 159 (79.9) | |
| CEP Magna Ease | 2 (33.3) | 24 (12.1) | |
| Avalus | 0 (0) | 15 (7.5) | |
| Crown | 0 (0) | 1 (0.5) | |
| Valve size | |||
| 19 | 1 (17.7) | 20 (10.1) | 0.843 |
| 21 | 1 (17.7) | 42 (21.1) | |
| 23 | 3 (50.0) | 64 (32.2) | |
| 25 | 1 (17.7) | 56 (28.1) | |
| 27 | 0 (0) | 17 (8.5) | |
| Mean valve size (mm) | 22.3 ± 2.1 | 23.1 ± 2.2 | 0.419 |
| Severe PPM Group (n = 6) | Without Severe PPM Group (n = 199) | p Value | |
|---|---|---|---|
| Minimally invasive approach | 0 (0) | 14 (7.0) | 1.000 |
| Suture annular position | 0.703 | ||
| Supra | 3 (50.0) | 78 (39.2) | |
| Intra | 3 (50.0) | 119 (59.8) | |
| Para | 0 (0) | 2 (1.0) | |
| Bentall procedure | 0 (0) | 21 (10.6) | 1.000 |
| Concomitant procedure | |||
| Mitral valve surgery | 1 (16.7) | 47 (23.6) | 1.000 |
| Tricuspid valve surgery | 1 (16.7) | 16 (8.0) | 1.000 |
| Severe PPM Group (n = 6) | Without Severe PPM Group (n = 199) | p Value | |
|---|---|---|---|
| Transthoracic echocardiography | |||
| LAVI (mL/m2) | 37.7 ± 7.4 | 36.7 ± 19.7 | 0.905 |
| LVEDD (mm) | 51.7 ± 8.0 | 48.2 ± 8.9 | 0.349 |
| LVESD (mm) | 37.8 ± 6.5 | 35.6 ± 10.1 | 0.597 |
| LVEF (%) | 49.6 ± 14.2 | 51.0 ± 14.2 | 0.816 |
| EOAI (cm2/m2) | 0.61 ± 0.04 | 1.14 ± 0.30 | <0.001 |
| Peak velocity (m/s) | 2.9 ± 0.7 | 2.1 ± 0.4 | <0.001 |
| Mean PG (mmHg) | 17.9 ± 8.0 | 9.6 ± 4.1 | <0.001 |
| Transesophageal echocardiography | |||
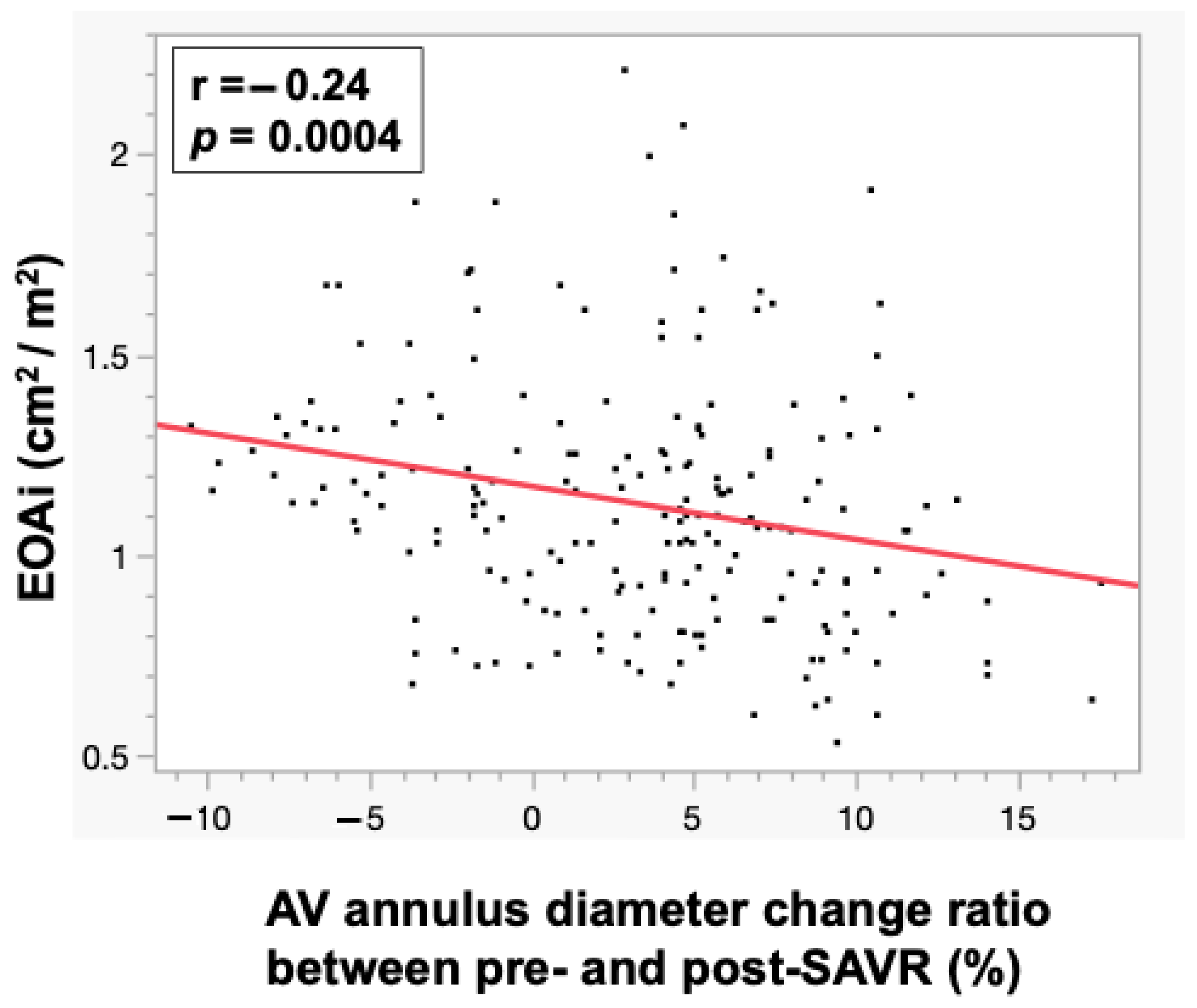
| AV annulus diameter change ratio between pre- and post-SAVR (%) | 10.4 ± 3.6 | 3.0 ± 5.6 | 0.002 |
| Use of a valve with a smaller than recommended prosthesis AV size (%) | 5 (83.3) | 41 (20.6) | <0.001 |
| Variable | Univariate | Multivariable | ||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Age | 0.91 (0.83–0.99) | 0.048 | 0.91 (0.82–1.01) | 0.071 |
| Male sex | 0.78 (0.39–1.56) | 0.477 | ||
| Body surface area | 1.18 (0.77–1.82) | 0.459 | ||
| Postoperative AV annulus diameter | 0.86 (0.59–1.24) | 0.418 | ||
| Post operative AV annulus diameter/BSA | 0.63 (0.34–1.09) | 0.105 | ||
| AV annulus diameter change ratio between pre- and post-SAVR | 1.36 (1.13–1.72) | <0.001 | ||
| Use of a valve with a smaller than recommended prosthesis AV size | 19.27 (2.19–169) | 0.008 | 19.3 (2.14–175) | 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, K.; Sasaki, H.; Takaoka, H.; Matsumoto, M.; Nishikawa, Y.; Noguchi, Y.; Aoki, S.; Suzuki, K.; Yashima, S.; Kinoshita, M.; et al. Three-Dimensional Transesophageal Echocardiography Is Useful for Preventing Prosthetic-Patient Mismatch After Surgical Aortic Valve Replacement. J. Clin. Med. 2025, 14, 4762. https://doi.org/10.3390/jcm14134762
Yoshida K, Sasaki H, Takaoka H, Matsumoto M, Nishikawa Y, Noguchi Y, Aoki S, Suzuki K, Yashima S, Kinoshita M, et al. Three-Dimensional Transesophageal Echocardiography Is Useful for Preventing Prosthetic-Patient Mismatch After Surgical Aortic Valve Replacement. Journal of Clinical Medicine. 2025; 14(13):4762. https://doi.org/10.3390/jcm14134762
Chicago/Turabian StyleYoshida, Kazuki, Haruka Sasaki, Hiroyuki Takaoka, Moe Matsumoto, Yusei Nishikawa, Yoshitada Noguchi, Shuhei Aoki, Katsuya Suzuki, Satomi Yashima, Makiko Kinoshita, and et al. 2025. "Three-Dimensional Transesophageal Echocardiography Is Useful for Preventing Prosthetic-Patient Mismatch After Surgical Aortic Valve Replacement" Journal of Clinical Medicine 14, no. 13: 4762. https://doi.org/10.3390/jcm14134762
APA StyleYoshida, K., Sasaki, H., Takaoka, H., Matsumoto, M., Nishikawa, Y., Noguchi, Y., Aoki, S., Suzuki, K., Yashima, S., Kinoshita, M., Suzuki-Eguchi, N., Takanashi, S., Matsushita, K., Matsumiya, G., & Kobayashi, Y. (2025). Three-Dimensional Transesophageal Echocardiography Is Useful for Preventing Prosthetic-Patient Mismatch After Surgical Aortic Valve Replacement. Journal of Clinical Medicine, 14(13), 4762. https://doi.org/10.3390/jcm14134762







