The Role of Immature Platelet Fraction and Reticulated Platelets in Stroke Monitoring and Outcome Prognosis: A Systematic Review
Abstract
1. Introduction
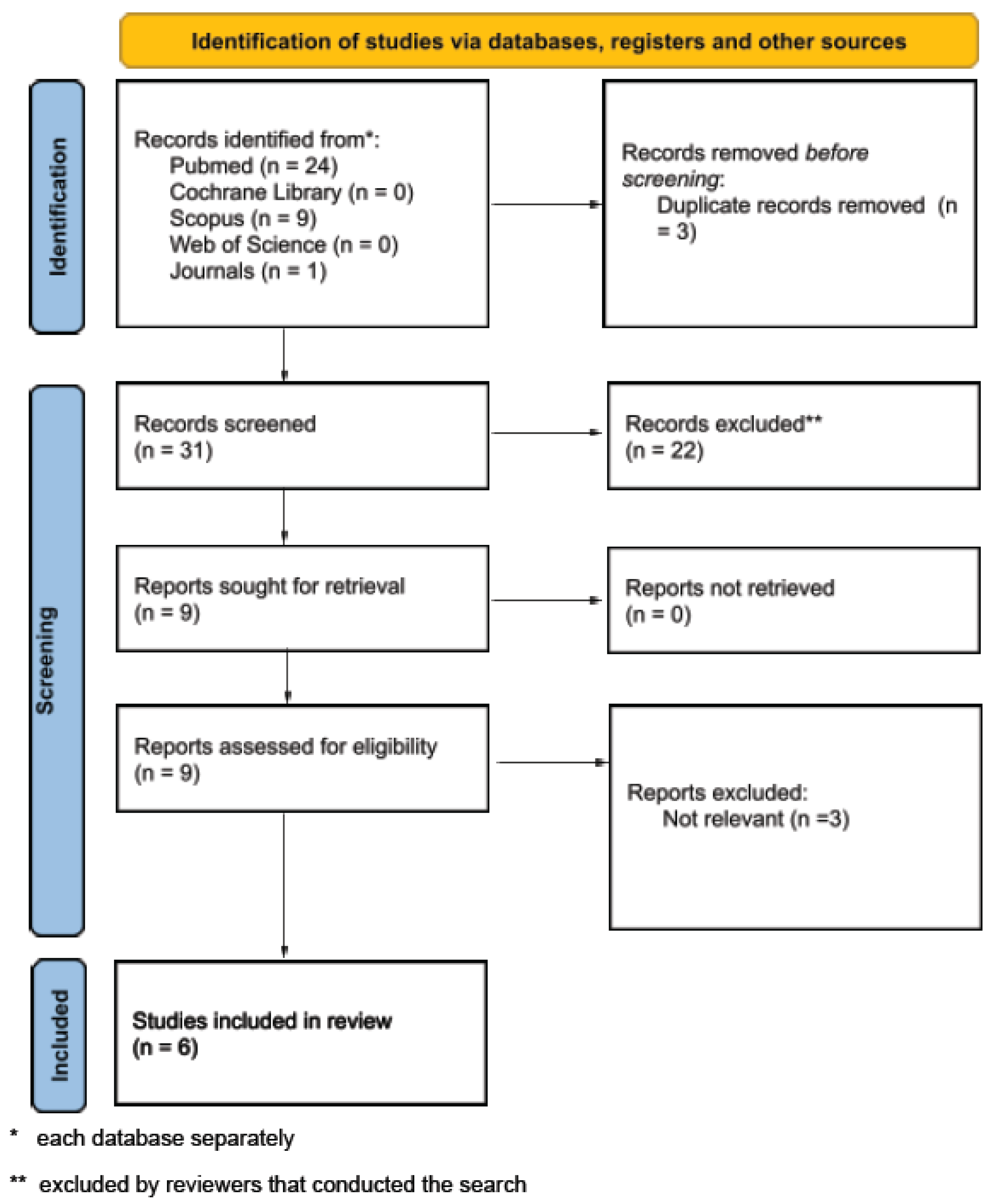
2. Materials and Methods
Study Selection
3. Results
3.1. Included Studies and Characteristics
3.2. Reticulated Platelets in the Acute and Chronic Phases of Stroke
3.3. Association with Stroke Etiology
3.4. Prognostic Value of RP/IPF in Stroke Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Koupenova, M.; Clancy, L.; Corkrey, H.A.; Freedman, J.E. Circulating Platelets as Mediators of Immunity, Inflammation, and Thrombosis. Circ. Res. 2018, 122, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Gremmel, T.; Frelinger, A.L.; Michelson, A.D. Platelet Physiology. Semin. Thromb. Hemost. 2016, 42, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Kaushansky, K. The Molecular Mechanisms That Control Thrombopoiesis. J. Clin. Investig. 2005, 115, 3339–3347. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Roest, M.; de Laat, B.; de Groot, P.G.; Huskens, D. Interplay between Platelets and Coagulation. Blood Rev. 2021, 46, 100733. [Google Scholar] [CrossRef] [PubMed]
- Versteeg, H.H.; Heemskerk, J.W.M.; Levi, M.; Reitsma, P.H. New Fundamentals in Hemostasis. Physiol. Rev. 2013, 93, 327–358. [Google Scholar] [CrossRef] [PubMed]
- Corpataux, N.; Franke, K.; Kille, A.; Valina, C.M.; Neumann, F.-J.; Nührenberg, T.; Hochholzer, W. Reticulated Platelets in Medicine: Current Evidence and Further Perspectives. J. Clin. Med. 2020, 9, 3737. [Google Scholar] [CrossRef] [PubMed]
- Stratz, C.; Bömicke, T.; Younas, I.; Kittel, A.; Amann, M.; Valina, C.M.; Nührenberg, T.; Trenk, D.; Neumann, F.-J.; Hochholzer, W. Comparison of Immature Platelet Count to Established Predictors of Platelet Reactivity During Thienopyridine Therapy. J. Am. Coll. Cardiol. 2016, 68, 286–293. [Google Scholar] [CrossRef]
- Buttarello, M.; Mezzapelle, G.; Freguglia, F.; Plebani, M. Reticulated Platelets and Immature Platelet Fraction: Clinical Applications and Method Limitations. Int. J. Lab. Hematol. 2020, 42, 363–370. [Google Scholar] [CrossRef]
- McBane, R.D.; Gonzalez, C.; Hodge, D.O.; Wysokinski, W.E. Propensity for Young Reticulated Platelet Recruitment into Arterial Thrombi. J. Thromb. Thrombolysis 2014, 37, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Italiano, J.E.; Shivdasani, R.A. Megakaryocytes and Beyond: The Birth of Platelets. J. Thromb. Haemost. 2003, 1, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Hedley, B.; Llewellyn-Smith, N.; Lang, S.; Hsia, C.C.; MacNamara, N.; Rosenfeld, D.; Keeney, M. Combined Accurate Platelet Enumeration and Reticulated Platelet Determination by Flow Cytometry. Cytom. B Clin. Cytom. 2015, 88, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, D.; Han, J.; Klug, M.; Kirmes, K.; Viggiani, G.; von Scheidt, M.; Schreiner, N.; Condorelli, G.; Laugwitz, K.-L.; Bernlochner, I. Role of Reticulated Platelets in Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- McCabe, D.J.; Harrison, P.; Sidhu, P.S.; Brown, M.M.; Machin, S.J. Circulating Reticulated Platelets in the Early and Late Phases after Ischaemic Stroke and Transient Ischaemic Attack. Br. J. Haematol. 2004, 126, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.T.; Tobin, W.O.; Murphy, S.; Kinsella, J.A.; Smith, D.R.; Lim, S.Y.; Murphy, S.M.; Coughlan, T.; Collins, D.R.; O’Neill, D.; et al. Profile of reticulated platelets in the early, subacute and late phases after transient ischemic attack or ischemic stroke. Platelets 2022, 33, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Uchiyama, S.; Yamazaki, M.; Okubo, K.; Takakuwa, Y.; Iwata, M. Flow Cytometric Analysis of Reticulated Platelets in Patients with Ischemic Stroke. Thromb. Res. 2002, 106, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.J.X.; Lim, S.T.; Kinsella, J.A.; Murphy, D.; Enright, H.M.; McCabe, D.J.H. Increased Platelet Count and Reticulated Platelets in Recently Symptomatic versus Asymptomatic Carotid Artery Stenosis and in Cerebral Microembolic Signal-Negative Patient Subgroups: Results from the HaEmostasis in Carotid STenosis (HEIST) Study. J. Neurol. 2018, 265, 1037–1049. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.-H.; Choa, K.H.; Yub, S. Immature Platelet Fraction is Associated with Early Neurological Deterioration in Acute Ischemic Stroke. J. Neurol. Sci. 2021, 429, 118764. [Google Scholar] [CrossRef]
- Smith, N.M.; Pathansali, R.; Bath, P.M. Altered Megakaryocyte-Platelet-Haemostatic Axis in Patients with Acute Stroke. Platelets 2002, 13, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Jakubiak, G.K.; Pawlas, N.; Lejawa, M.; Morawiecka-Pietrzak, M.; Zalejska-Fiolka, J.; Stanek, A.; Cieślar, G. Relationship of Thyroid Volume and Function with Carotid and Femoral Intima-Media Thickness in Euthyroid People Aged 18–65 Taking into Account the Impact of Diabetes, Hypertension, and Excess Body Mass. J. Clin. Med. 2025, 14, 604. [Google Scholar] [CrossRef] [PubMed]

| Aspect | Reticulated Platelets | Immature Platelet Fraction |
|---|---|---|
| Definition | Subset of young platelets | Percentage of immature platelets to total platelet count |
| Measuring method | Manual or semi-automated flow cytometry | Fully automated hematology analyzers |
| Staining | Thiazole orange (or any other RNA-binding dye) combined with CD41/42b for platelet gating | RNA-sensitive fluorescent dye (analyzer reagent) |
| Specificity | High | High |
| Availability | Usually limited to research centers | Widely available |
| Analysis time | 1–2 h | Rapid (within minutes) |
| Limitations | Requires expertise, lack of standardization, and sample aging affects results | Lower resolution for identifying platelet subtypes, dependent on proprietary algorithms |
| Study | Year | Type of Study | Sample Size | Type of Stroke | Grouping Criteria |
|---|---|---|---|---|---|
| McCabe et al. [14] | 2004 | Observational | 176 | Ischemic or TIA | Early phase (1–27 days, n = 79), Late phase (79–725 days, n = 70), and Control (n = 27) |
| Lim et al. [15] | 2020 | Observational | 244 | Ischemic or TIA | Early (<4 weeks, n = 210), with follow up (n = 182), Late (>90 days, n = 145), and Control group (n = 34) |
| Nakamura et al. [16] | 2002 | Observational | 208 | Ischemic | Acute (1–7 days, n = 10), Subacute (8–30 days, n = 12), Chronic (>31 days, n = 46) and Control (n = 140) |
| Murphy et al. [17] | 2018 | Observational | 114 | LLA type or TIA | Early (n = 43), Late (n = 37) and Asymptomatic (n = 34) |
| Cho et al. [18] | 2021 | Observational | 16,551 | Ischemic | Subgroup (n = 72, 4.4%) from a stroke registry of 1655 patients |
| Smith et al. [19] | 2002 | Observational | 38 | Ischemic or Hemorrhagic | Acute (n = 24) and Control group (n = 14) |
| Study | Methodology |
|---|---|
| McCabe et al. [14] | Sysmex XE-2100 Hematology Analyzer and Coulter EPICS XL-MCL flow cytometer (with thiazole orange staining and CD42b antibody) |
| Lim et al. [15] | Sysmex XE-2100 Hematology Analyzer and Beckman Coulter XL MCL flow cytometer (with thiazole orange and CD42b antibody conjugated to PE) |
| Nakamura et al. [16] | Coulter Profile II flow cytometer (with thiazole orange for CD41 antibody) |
| Murphy et al. [17] | Sysmex XE-2100 Hematology Analyzer and Beckman Coulter flow cytometer (with thiazole orange and CD42b antibody) |
| Cho et al. [18] | Sysmex XE-2100 Hematology Analyzer |
| Smith et al. [19] | Sysmex series 9000 Hematology Analyzer |
| Study | Outcomes | Antithrombotic Therapy | Limitations |
|---|---|---|---|
| McCabe et al. [14] | No significant increase in unadjusted %RP in early or late-phase stroke/TIA patients compared to controls. (only after age and PVD adjustment). No significant effect of aspirin dose escalation | Aspirin monotherapy (early: 70%, late: 64%). Some on combinations (Aspirin + dipyridamole or clopidogrel) Controls untreated (85%). | Small sample size in the aspirin escalation group. |
| Lim et al. [15] | %RP was significantly increased in the late phase. SVD had a significantly higher %RP at baseline and 90 days | Dynamic regimens over time: Baseline: 76% on aspirin monotherapy, 22% no antiplatelet 14 days: 47% on aspirin + dipyridamole, 34% clopidogrel, 19% aspirin alone 90 days: 52% Aspirin + dipyridamole, 33% clopidogrel, 15% aspirin alone | Selection bias due to the two included studies. Variations of inclusion time for the acute phase. |
| Nakamura et al. [16] | %RP was significantly higher in patients with cardioembolic stroke and lower in patients under antithrombotic treatments | No antithrombotic (n = 8) Antiplatelet therapy (n = 50) Anticoagulant therapy (n = 16) | Small sample size to correlate with stroke phases and treatment response |
| Murphy et al. [17] | Automated %RPF and #RP were significantly higher in early symptomatic than asymptomatic MES−ve patients. No significant differences in patients on specific antiplatelet regimens | Aspirin monotherapy (64.7%) Combination therapies No antiplatelet therapy (Asymptomatic, 2.9%). Median daily aspirin dose elevated in early symptomatic patients | Small sample size for recurrent event prediction or event prognosis. |
| Cho et al. [18] | High IPF was an independent predictor of the prevalence of END. | No mention of antithrombotic therapy | Single-center study with retrospective design. Low prevalence of END may limit statistical power. |
| Smith et al. [19] | A trend towards an increase in %RP was found in stroke patients | No mention of antithrombotic therapy | Small sample size with incomplete matching of all vascular risk factors. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsankof, A.; Tsakiris, D.A.; Skoura, L.; Tsiatsiou, P.; Ztriva, E.; Ntaios, G.; Savopoulos, C.; Kaiafa, G. The Role of Immature Platelet Fraction and Reticulated Platelets in Stroke Monitoring and Outcome Prognosis: A Systematic Review. J. Clin. Med. 2025, 14, 4760. https://doi.org/10.3390/jcm14134760
Tsankof A, Tsakiris DA, Skoura L, Tsiatsiou P, Ztriva E, Ntaios G, Savopoulos C, Kaiafa G. The Role of Immature Platelet Fraction and Reticulated Platelets in Stroke Monitoring and Outcome Prognosis: A Systematic Review. Journal of Clinical Medicine. 2025; 14(13):4760. https://doi.org/10.3390/jcm14134760
Chicago/Turabian StyleTsankof, Alexandra, Dimitrios A. Tsakiris, Lemonia Skoura, Panagiota Tsiatsiou, Eleftheria Ztriva, Georgios Ntaios, Christos Savopoulos, and Georgia Kaiafa. 2025. "The Role of Immature Platelet Fraction and Reticulated Platelets in Stroke Monitoring and Outcome Prognosis: A Systematic Review" Journal of Clinical Medicine 14, no. 13: 4760. https://doi.org/10.3390/jcm14134760
APA StyleTsankof, A., Tsakiris, D. A., Skoura, L., Tsiatsiou, P., Ztriva, E., Ntaios, G., Savopoulos, C., & Kaiafa, G. (2025). The Role of Immature Platelet Fraction and Reticulated Platelets in Stroke Monitoring and Outcome Prognosis: A Systematic Review. Journal of Clinical Medicine, 14(13), 4760. https://doi.org/10.3390/jcm14134760









