Prevalence of Electrocardiographic Abnormalities in Patients with Acute Pulmonary Embolism: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Registration
2.2. Search Strategy and Study Selection
2.3. Inclusion Criteria and Outcome of Interest
2.4. Data Extraction and Study Risk of Bias Assessment
2.5. Statistical Analysis
3. Results
3.1. Study Selection and Characteristics of Included Studies
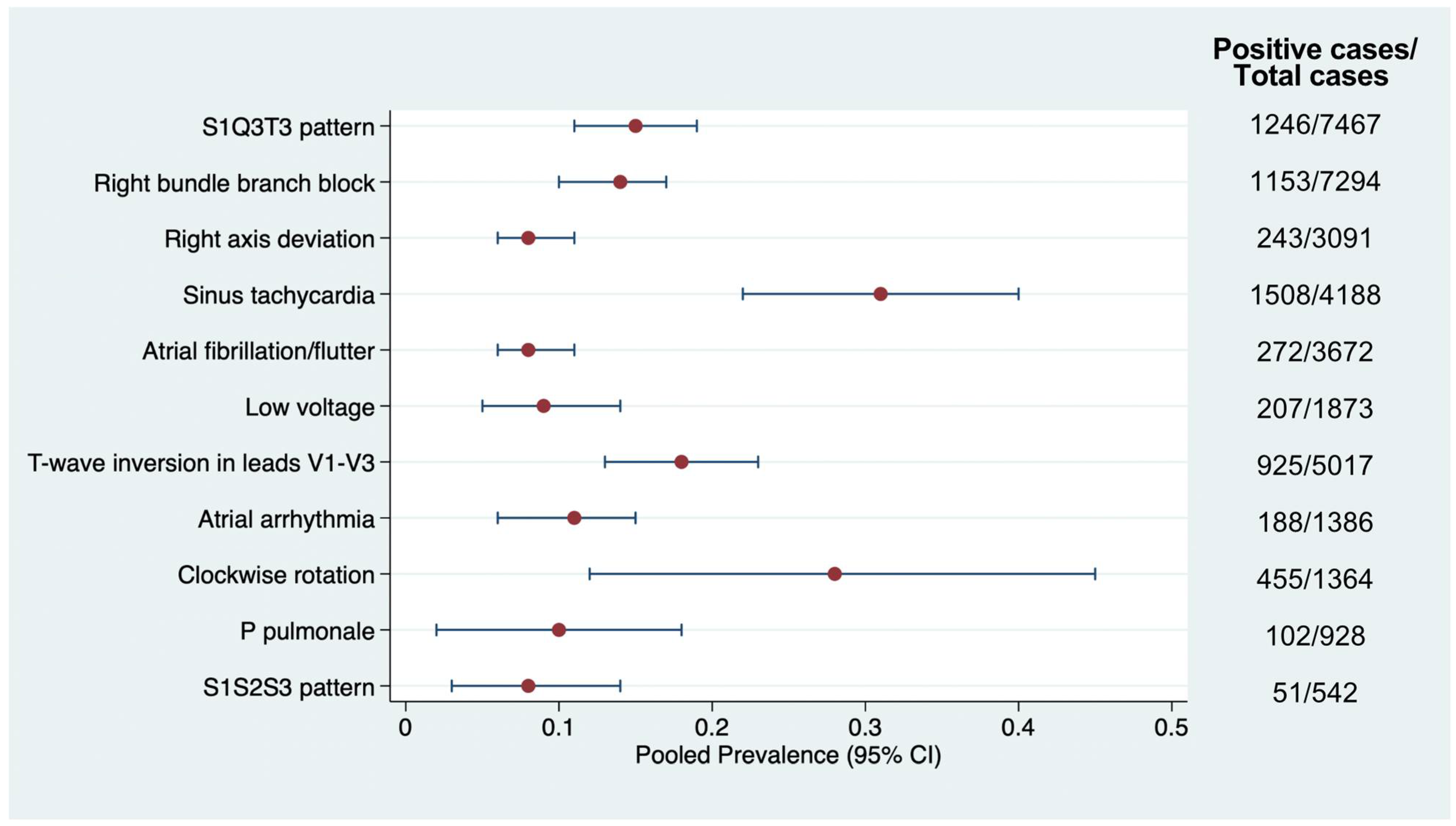
3.2. Prevalence of Electrocardiographic Abnormalities
4. Discussion
4.1. Strengths and Limitations
4.2. Clinical Implications and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CI | Confidence interval |
| CTPA | Computed tomography pulmonary angiography |
| ECG | Electrocardiogram |
| PE | Pulmonary embolism |
| RBBB | Right bundle branch block |
| RV | Right ventricle |
References
- Bĕlohlávek, J.; Dytrych, V.; Linhart, A. Pulmonary Embolism, Part I: Epidemiology, Risk Factors and Risk Stratification, Pathophysiology, Clinical Presentation, Diagnosis and Nonthrombotic Pulmonary Embolism. Exp. Clin. Cardiol. 2013, 18, 129–138. [Google Scholar]
- Barnes, G.D.; Muzikansky, A.; Cameron, S.; Giri, J.; Heresi, G.A.; Jaber, W.; Wood, T.; Todoran, T.M.; Courtney, D.M.; Tapson, V.; et al. Comparison of 4 Acute Pulmonary Embolism Mortality Risk Scores in Patients Evaluated by Pulmonary Embolism Response Teams. JAMA Netw. Open 2020, 3, e2010779. [Google Scholar] [CrossRef]
- van Maanen, R.; Trinks-Roerdink, E.M.; Rutten, F.H.; Geersing, G.J. A Systematic Review and Meta-Analysis of Diagnostic Delay in Pulmonary Embolism. Eur. J. Gen. Pract. 2022, 28, 165–172. [Google Scholar] [CrossRef]
- Mansella, G.; Keil, C.; Nickel, C.H.; Eken, C.; Wirth, C.; Tzankov, A.; Peterson, C.J.; Aujesky, D.; Bingisser, R. Delayed Diagnosis in Pulmonary Embolism: Frequency, Patient Characteristics, and Outcome. Respiration 2020, 99, 589–597. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.-J.; Harjola, V.-P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the Diagnosis and Management of Acute Pulmonary Embolism Developed in Collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef]
- Su, X.-F.; Fan, N.; Yang, X.-M.; Song, J.-M.; Peng, Q.-H.; Liu, X. A Novel Electrocardiography Model for the Diagnosis of Acute Pulmonary Embolism. Front. Cardiovasc. Med. 2022, 9, 825561. [Google Scholar] [CrossRef]
- Geibel, A.; Zehender, M.; Kasper, W.; Olschewski, M.; Klima, C.; Konstantinides, S. V Prognostic Value of the ECG on Admission in Patients with Acute Major Pulmonary Embolism. Eur. Respir. J. 2005, 25, 843–848. [Google Scholar] [CrossRef]
- Co, I.; Eilbert, W.; Chiganos, T. New Electrocardiographic Changes in Patients Diagnosed with Pulmonary Embolism. J. Emerg. Med. 2017, 52, 280–285. [Google Scholar] [CrossRef]
- Bahreini, Z.; Kamali, M.; Kheshty, F.; Bazrafshan Drissi, H.; Boogar, S.S.; Bazrafshan, M. Differentiating Electrocardiographic Indications of Massive and Submassive Pulmonary Embolism: A Cross-Sectional Study in Southern Iran from 2015 to 2020. Clin. Cardiol. 2024, 47, e24252. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef]
- Munn, Z.; Moola, S.; Lisy, K.; Riitano, D.; Tufanaru, C. Chapter 5: Systematic Reviews of Prevalence and Incidence; Aromataris, E., Munn, Z., Eds.; JBI Manual for Evidence Synthesis; Faculty of Health and Medical Sciences, The University of Adelaide: Adelaide, Australia, 2020. [Google Scholar]
- Fletcher, J. What Is Heterogeneity and Is It Important? BMJ 2007, 334, 94–96. [Google Scholar] [CrossRef]
- Rodger, M.; Makropoulos, D.; Turek, M.; Quevillon, J.; Raymond, F.; Rasuli, P.; Wells, P.S. Diagnostic Value of the Electrocardiogram in Suspected Pulmonary Embolism. Am. J. Cardiol. 2000, 86, 807–809. [Google Scholar] [CrossRef]
- Wang, F.; Wang, L.; Yan, C.; Chang, X.; Wang, H.; Zhu, K.; Xu, Y.; Xu, D. Algorithms of Electrocardiographic Changes for Quantitative and Localization Analysis of Thrombus Burden in Patients with Acute Pulmonary Thromboembolism. Rev. Cardiovasc. Med. 2023, 24, 281. [Google Scholar] [CrossRef]
- Zhan, Z.Q.; Wang, C.Q.; Nikus, K.C.; He, C.R.; Wang, J.; Mao, S.; Dong, X.J. Electrocardiogram Patterns during Hemodynamic Instability in Patients with Acute Pulmonary Embolism. Ann. Noninvasive Electrocardiol. 2014, 19, 543–551. [Google Scholar] [CrossRef]
- Weekes, A.J.; Raper, J.D.; Thomas, A.M.; Lupez, K.; Cox, C.A.; Esener, D.; Boyd, J.S.; Nomura, J.T.; Davison, J.; Ockerse, P.M.; et al. Electrocardiographic Findings Associated with Early Clinical Deterioration in Acute Pulmonary Embolism. Acad. Emerg. Med. 2022, 29, 1185–1196. [Google Scholar] [CrossRef]
- Kukla, P.; Długopolski, R.; Krupa, E.; Furtak, R.; Mirek, E.; Bryniarska; Jastrzębski, M.; Wańczura, P.; Bryniarski, L. The Prognostic Value of ST−segment Elevation in the Lead AVR in Patients with Acute Pulmonary Embolism. Kardiol. Pol. 2011, 69, 649–654. [Google Scholar]
- Park, S.J.; Kwon, C.H.; Bae, B.J.; Kim, B.S.; Kim, S.H.; Kim, H.J.; Hwang, H.K.; Chung, S.M. Diagnostic Value of the Corrected QT Difference between Leads V1 and V6 in Patients with Acute Pulmonary Thromboembolism. Medicine 2017, 96, e8430. [Google Scholar] [CrossRef]
- Witting, M.D.; Mattu, A.; Rogers, R.; Halvorson, C. Simultaneous T-Wave Inversions in Anterior and Inferior Leads: An Uncommon Sign of Pulmonary Embolism. J. Emerg. Med. 2012, 43, 228–235. [Google Scholar] [CrossRef]
- Yan, X.X.; Zhang, H.D.; Peng, F.H.; Ding, L.; Mi, L.J.; Zhang, A.K.; Hua, L.; Tang, M. Prognostic Significance of Quantitative Electrocardiographic Parameters in Patients with Non-High-Risk Pulmonary Embolism. Eur. J. Intern. Med. 2024, 120, 46–51. [Google Scholar] [CrossRef]
- Bolt, L.; Lauber, S.; Limacher, A.; Samim, D.; Löwe, A.; Tritschler, T.; Baumgartner, C.; Aujesky, D. Prognostic Value of Electrocardiography in Elderly Patients with Acute Pulmonary Embolism. Am. J. Med. 2019, 132, e835–e843. [Google Scholar] [CrossRef]
- Vanni, S.; Polidori, G.; Vergara, R.; Pepe, G.; Nazerian, P.; Moroni, F.; Garbelli, E.; Daviddi, F.; Grifoni, S. Prognostic Value of ECG Among Patients with Acute Pulmonary Embolism and Normal Blood Pressure. Am. J. Med. 2009, 122, 257–264. [Google Scholar] [CrossRef]
- Casazza, F.; Pacchetti, I.; Rulli, E.; Roncon, L.; Zonzin, P.; Zuin, M.; Becattini, C.; Bongarzoni, A.; Pignataro, L. Prognostic Significance of Electrocardiogram at Presentation in Patients with Pulmonary Embolism of Different Severity. Thromb. Res. 2018, 163, 123–127. [Google Scholar] [CrossRef]
- Cetin, M.S.; Ozcan Cetin, E.H.; Arisoy, F.; Kuyumcu, M.S.; Topaloglu, S.; Aras, D.; Temizhan, A. Fragmented QRS Complex Predicts In-Hospital Adverse Events and Long-Term Mortality in Patients with Acute Pulmonary Embolism. Ann. Noninvasive Electrocardiol. 2016, 21, 470–478. [Google Scholar] [CrossRef]
- Pourafkari, L.; Ghaffari, S.; Tajlil, A.; Akbarzadeh, F.; Jamali, F.; Nader, N.D. Clinical Significance of ST Elevation in Lead AVR in Acute Pulmonary Embolism. Ann. Noninvasive Electrocardiol. 2017, 22, e12368. [Google Scholar] [CrossRef]
- Kukla, P.; Długopolski, R.; Krupa, E.; Furtak, R.; Szełemej, R.; Mirek-Bryniarska, E.; Jastrzebski, M.; Nowak, J.; Wańczura, P.; Bryniarski, L. Electrocardiography and Prognosis of Patients with Acute Pulmonary Embolism. Cardiol. J. 2011, 18, 648–653. [Google Scholar] [CrossRef]
- Kusayama, T.; Furusho, H.; Kinoshita, M.; Kaneko, S.; Usuda, K.; Takamura, M. Characteristics of Synthesized Right-Sided Chest Electrocardiograms in Patients with Acute Pulmonary Embolism. J. Cardiol. 2019, 73, 313–317. [Google Scholar] [CrossRef]
- Obradović, S.; Džudović, B.; Rusović, S.; Subota, V.; Obradović, D. Gender-Related Differences in Clinical Presentation, Electrocardiography, Laboratory Markers and Outcome in Patients with Acute Embolism. Vojnosanit. Pregl. 2016, 73, 844–849. [Google Scholar] [CrossRef]
- Richman, P.B.; Loutfi, H.; Lester, S.J.; Cambell, P.; Matthews, J.; Friese, J.; Wood, J.; Kasper, D.; Chen, F.; Mandell, M. Electrocardiographic Findings in Emergency Department Patients with Pulmonary Embolism. J. Emerg. Med. 2004, 27, 121–126. [Google Scholar] [CrossRef]
- Çağdaş, M.; Karakoyun, S.; Rencüzoğulları, İ.; Karabağ, Y.; Artaç, İ.; İliş, D.; Hamideyin, Ş.; Karayol, S.; Çiftçi, H.; Çınar, T. Diagnostic Value of QRS and S Wave Variation in Patients with Suspicion of Acute Pulmonary Embolism. Am. J. Emerg. Med. 2018, 36, 2197–2202. [Google Scholar] [CrossRef]
- Stein, P.D.; Matta, F.; Sabra, M.J.; Treadaway, B.; Vijapura, C.; Warren, R.; Joshi, P.; Sadiq, M.; Kofoed, J.T.; Hughes, P.; et al. Relation of Electrocardiographic Changes in Pulmonary Embolism to Right Ventricular Enlargement. Am. J. Cardiol. 2013, 112, 1958–1961. [Google Scholar] [CrossRef]
- Novicic, N.; Dzudovic, B.; Subotic, B.; Shalinger-Martinovic, S.; Obradovic, S. Electrocardiography Changes and Their Significance during Treatment of Patients with Intermediate-High and High-Risk Pulmonary Embolism. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 271–278. [Google Scholar] [CrossRef]
- Thomson, D.; Kourounis, G.; Trenear, R.; Messow, C.-M.; Hrobar, P.; Mackay, A.; Isles, C. ECG in Suspected Pulmonary Embolism. Postgrad. Med. J. 2019, 95, 12–17. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, G.; Wang, S.; Du, W.; Lv, P.; Guo, H.; Sun, Q.; Liu, Y.; Qi, X. The Electrocardiographic Characteristics of an Acute Embolism in the Pulmonary Trunk and the Main Pulmonary Arteries. Am. J. Emerg. Med. 2016, 34, 212–217. [Google Scholar] [CrossRef]
- Qaddoura, A.; Digby, G.C.; Kabali, C.; Kukla, P.; Zhan, Z.-Q.; Baranchuk, A.M. The Value of Electrocardiography in Prognosticating Clinical Deterioration and Mortality in Acute Pulmonary Embolism: A Systematic Review and Meta-Analysis. Clin. Cardiol. 2017, 40, 814–824. [Google Scholar] [CrossRef]
- Wells, P.S.; Anderson, D.R.; Rodger, M.; Stiell, I.; Dreyer, J.F.; Barnes, D.; Forgie, M.; Kovacs, G.; Ward, J.; Kovacs, M.J. Excluding Pulmonary Embolism at the Bedside without Diagnostic Imaging: Management of Patients with Suspected Pulmonary Embolism Presenting to the Emergency Department by Using a Simple Clinical Model and d-Dimer. Ann. Intern. Med. 2001, 135, 98–107. [Google Scholar] [CrossRef]
- Le Gal, G.; Righini, M.; Roy, P.-M.; Sanchez, O.; Aujesky, D.; Bounameaux, H.; Perrier, A. Prediction of Pulmonary Embolism in the Emergency Department: The Revised Geneva Score. Ann. Intern. Med. 2006, 144, 165–171. [Google Scholar] [CrossRef]
- Kim, J.H.; Noseworthy, P.A.; McCarty, D.; Yared, K.; Weiner, R.; Wang, F.; Wood, M.J.; Hutter, A.M.; Picard, M.H.; Baggish, A.L. Significance of Electrocardiographic Right Bundle Branch Block in Trained Athletes. Am. J. Cardiol. 2011, 107, 1083–1089. [Google Scholar] [CrossRef]
- Arrigo, M.; Price, S.; Harjola, V.-P.; Huber, L.C.; Schaubroeck, H.A.I.; Vieillard-Baron, A.; Mebazaa, A.; Masip, J. Diagnosis and Treatment of Right Ventricular Failure Secondary to Acutely Increased Right Ventricular Afterload (Acute Cor Pulmonale): A Clinical Consensus Statement of the Association for Acute CardioVascular Care of the European Society of Cardiology. Eur. Heart J. Acute Cardiovasc. Care 2024, 13, 304–312. [Google Scholar] [CrossRef]
- Laurentius, A.; Ariani, R. Diagnostic Comparison of Anterior Leads T-Wave Inversion and McGinn-White Sign in Suspected Acute Pulmonary Embolism: A Systematic Review and Meta-Analysis. Hong Kong J. Emerg. Med. 2023, 30, 54–60. [Google Scholar] [CrossRef]
- Scridon, A. Autonomic Imbalance and Atrial Ectopic Activity-a Pathophysiological and Clinical View. Front. Physiol. 2022, 13, 1058427. [Google Scholar] [CrossRef]
- Bayés de Luna, A.; Carrió, I.; Subirana, M.T.; Torner, P.; Cosín, J.; Sagués, F.; Guindo, J. Electrophysiological Mechanisms of the SI SII SIII Electrocardiographic Morphology. J. Electrocardiol. 1987, 20, 38–44. [Google Scholar] [CrossRef]
- Shopp, J.D.; Stewart, L.K.; Emmett, T.W.; Kline, J.A. Findings From 12-Lead Electrocardiography That Predict Circulatory Shock From Pulmonary Embolism: Systematic Review and Meta-Analysis. Acad. Emerg. Med. 2015, 22, 1127–1137. [Google Scholar] [CrossRef]
- Digby, G.C.; Kukla, P.; Zhan, Z.-Q.; Pastore, C.A.; Piotrowicz, R.; Schapachnik, E.; Zareba, W.; Bayés de Luna, A.; Pruszczyk, P.; Baranchuk, A.M. The Value of Electrocardiographic Abnormalities in the Prognosis of Pulmonary Embolism: A Consensus Paper. Ann. Noninvasive Electrocardiol. 2015, 20, 207–223. [Google Scholar] [CrossRef]
- Ryu, H.M.; Lee, J.H.; Kwon, Y.S.; Lee, S.H.; Bae, M.H.; Lee, J.H.; Yang, D.H.; Park, H.S.; Cho, Y.; Chae, S.C.; et al. Electrocardiography Patterns and the Role of the Electrocardiography Score for Risk Stratification in Acute Pulmonary Embolism. Korean Circ. J. 2010, 40, 499–506. [Google Scholar] [CrossRef]
- Al-Zaiti, S.S.; Martin-Gill, C.; Zègre-Hemsey, J.K.; Bouzid, Z.; Faramand, Z.; Alrawashdeh, M.O.; Gregg, R.E.; Helman, S.; Riek, N.T.; Kraevsky-Phillips, K.; et al. Machine Learning for ECG Diagnosis and Risk Stratification of Occlusion Myocardial Infarction. Nat. Med. 2023, 29, 1804–1813. [Google Scholar] [CrossRef]
- Tuminah, S.; Indrawati, L.; Riyadina, W.; Wurisastuti, T.; Letelay, A.M.; Sitorus, N.; Putri, A.S.; Isfandari, S.; Irmansyah, I. Number of Comorbidities and the Risk of Delay in Seeking Treatment for Coronary Heart Disease: A Longitudinal Study in Bogor City, Indonesia. Osong Public Health Res. Perspect. 2024, 15, 201–211. [Google Scholar] [CrossRef]
- Kraik, K.; Dykiert, I.A.; Niewiadomska, J.; Ziemer-Szymańska, M.; Mikołajczak, K.; Kreń, M.; Kukiełka, P.; Martuszewski, A.; Harych, T.; Poręba, R.; et al. The Most Common Errors in Automatic ECG Interpretation. Front. Physiol. 2025, 16, 1590170. [Google Scholar] [CrossRef]


| First Author, Year | Country | n | Age (Year), mean ± SD | %Male | Design | Inclusion Criteria | ECG Findings of Interest |
|---|---|---|---|---|---|---|---|
| Wang, 2023 [14] | China | 341 | 69 ± 14 | 50.4 | Multicenter retrospective cohort study |
|
|
| Kusayama, 2019 [27] | Japan | 65 | 67 ± 13 | 32.1 | Retrospective chart review study |
|
|
| Pourafkari, 2017 [25] | United States | 200 | 61 ± 17 | 52.5 | Retrospective observational cohort |
|
|
| Çagdas, 2018 [30] | Turkey | 106 | 60 ± 18 | 50.0 | Retrospective observational cohort |
|
|
| Park, 2017 [18] | South Korea | 89 | 68 ± 15 | 59.5% | Retrospective study |
|
|
| Rodger, 2000 [13] | Canada | 49 | N/A | N/A | Retrospective observational case-control |
|
|
| Bahreini, 2024 [9] | Iran | 250 | 54 ± 19 | 60.0 | Prospective cross-sectional study |
|
|
| Thomson, 2019 [33] | Scotland | 189 | 66 (range 20–93) | 43.4 | Retrospective case-control study |
|
|
| Zhan, 2014 [15] | China | 20 | 58 ± 10 | 40 | Retrospective study |
|
|
| Weekes, 2022 [16] | United States | 1676 | 60 ± 17 | 51.8 | Prospective, observational, multicenter cohort study |
|
|
| Richman, 2004 [29] | United States | 49 | 69 (no SD reported) | NI | Retrospective case-control study |
|
|
| Kukla, 2011 [26] | Poland | 292 | 65 ± 15 | 37.3 | Retrospective study |
|
|
| Novicic, 2020 [32] | Serbia | 110 | 65 ± 13 | 45.5 | Retrospective study |
|
|
| Cetin, 2016 [24] | Turkey | 249 | 66 ± 16 | 33.8 | Prospective study |
|
|
| Obradovic, 2016 [28] | Serbia | 144 | 60 ± 16 | 50 | Ambispective study |
|
|
| Ivan, 2017 [8] | United States | 352 | 68 (range 23-96) | 37.5 | Retrospective study |
|
|
| Casazza, 2018 [23] | Italy | 1194 | 70 ± 16 | 42.8 | Prospective multicenter study |
|
|
| Yan, 2024 [20] | China | 383 | 67 ± 13 | 43 | Prospective observational cohort study |
|
|
| Vanni, 2009 [22] | Italy | 386 | 67 ± 16 | 40 | Prospective study |
|
|
| Bolt, 2019 [21] | Switzerland | 390 | 74 (69–81) 1 | 54 | Prospective multicenter cohort study |
|
|
| Geibel, 2005 [7] | Germany | 508 | 63 ± 15 | 42 | Prospective study |
|
|
| Stein, 2013 [31] | United States | 289 | 61 ± 18 | 44 | Review medical records of hospitalized patients |
|
|
| Witting, 2012 [19] | United States | 97 | 50 ± 15 | NI | Retrospective case-control study |
|
|
| Zhang, 2016 [34] | China | 147 | 56 ± 15 | 41.5 | Retrospective observational cohort |
|
|
| ECG Finding | Pathophysiology | ECG Characteristics |
|---|---|---|
| S1Q3T3 pattern (Figure 3) | Acute RV strain → altered conduction and repolarization | S wave in lead I, Q wave, and inverted T wave in lead III |
| Right bundle branch block (Figure 3) | RV dilation → delayed right ventricular conduction | RSR’ in V1, wide QRS > 120 ms, terminal S in I and V6 |
| Right axis deviation | RV pressure overload or hypertrophy | QRS axis > +90°, dominant S in I and R in III |
| Sinus tachycardia | Sympathetic activation due to hypoxia, pain, or RV dysfunction | Regular rhythm, HR >100 bpm, normal P waves |
| Atrial fibrillation/Atrial flutter | Atrial strain or ischemia, particularly of the right atrium | Irregularly irregular rhythm (AF); flutter waves with sawtooth pattern (AFL) |
| Low voltage | Pericardial effusion, obesity, or shock state causing diminished signal | QRS < 5 mm in limb leads or <10 mm in precordial leads |
| T-wave inversion in leads V1–V3 (Figure 3) | Subendocardial ischemia of the RV due to pressure overload | Symmetrical T-wave inversion in V1–V3, sometimes extending to V4–V6 |
| Atrial arrhythmia (other) | Right atrial enlargement or hypoxia-related automaticity | Ectopic P waves, PACs, atrial tachycardia |
| Clockwise rotation | RV enlargement causing altered depolarization vectors | R/S transition delayed beyond V4–V5, persistent S waves in V1–V4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krintratun, S.; Srichuachom, W.; Wongtanasarasin, W. Prevalence of Electrocardiographic Abnormalities in Patients with Acute Pulmonary Embolism: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 4750. https://doi.org/10.3390/jcm14134750
Krintratun S, Srichuachom W, Wongtanasarasin W. Prevalence of Electrocardiographic Abnormalities in Patients with Acute Pulmonary Embolism: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(13):4750. https://doi.org/10.3390/jcm14134750
Chicago/Turabian StyleKrintratun, Sarunsorn, Wuttipong Srichuachom, and Wachira Wongtanasarasin. 2025. "Prevalence of Electrocardiographic Abnormalities in Patients with Acute Pulmonary Embolism: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 13: 4750. https://doi.org/10.3390/jcm14134750
APA StyleKrintratun, S., Srichuachom, W., & Wongtanasarasin, W. (2025). Prevalence of Electrocardiographic Abnormalities in Patients with Acute Pulmonary Embolism: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(13), 4750. https://doi.org/10.3390/jcm14134750







