Some Levels of Plasma Free Fatty Acids and Amino Acids in the Second Trimester Are Linked to Gestational Diabetes and Are Predictive of Persisting Impaired Glucose Tolerance After Delivery
Abstract
1. Introduction
2. Methods and Materials
2.1. Subjects
2.2. Measurement of Plasma Free Fatty Acids
2.3. Measurement of Plasma Amino Acids
2.4. Statistical Analyses
3. Results
3.1. Comparison of AAs and FFAs in GDM vs. Normal Pregnancy
3.2. Correlation Between AAs and FFAs Levels and Biochemical and Anthropometric Parameters, Respectively
3.3. Association of AAs and FFAs Levels with Maternal and Neonatal Peripartal Data
3.4. Comparison of AAs and FFAs Between GDM Sub-Groups Defined by the Presence or Absence of PGI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1-h PPG | 1-h post-load plasma glucose |
| 2-h PPG | 2-h post-load plasma glucose |
| AAs | amino acids |
| ADA | American Diabetes Association |
| AUCoGGT | area under the oGTT curve |
| BE | base excess |
| BMI | body mass index |
| CI | confidence interval |
| DIP | diabetes in pregnancy |
| DM | diabetes mellitus |
| FFAs | free fatty acids |
| FPG | fasting plasma glucose |
| GDM | gestational diabetes mellitus |
| IADPSG | International Association of Diabetes in Pregnancy Study Group |
| IQR | interquartile range |
| MUFAs | monosaturated fatty acids |
| oGTT | oral glucose tolerance test |
| OR | odds ratio |
| PGI | postpartum glucose intolerance |
| PUFAs | polysaturated fatty acids |
| ROC | Receiver Operating Characteristic |
| SFAs | saturated fatty acids |
| T2DM | type 2 diabetes mellitus |
| VEX | vacuum extractor |
| WHO | World Health Organization |
References
- Bellamy, L.; Casas, J.P.; Hingorani, A.D.; Williams, D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 2009, 373, 1773–1779. [Google Scholar] [CrossRef] [PubMed]
- Standards of Medical Care in Diabetes—2014. Diabetes Care 2014, 37, S14. [CrossRef]
- Barbour, L.A.; McCurdy, C.E.; Hernandez, T.L.; Kirwan, J.P.; Catalano, P.M.; Friedman, J.E. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care 2007, 30 (Suppl. 2), S112–S119. [Google Scholar] [CrossRef] [PubMed]
- Bartáková, V.; Chalásová, K.; Pácal, L.; Ťápalová, V.; Máchal, J.; Janků, P.; Kaňková, K. Metabolic Syndrome Prevalence in Women with Gestational Diabetes Mellitus in the Second Trimester of Gravidity. J. Clin. Med. 2024, 13, 1260. [Google Scholar] [CrossRef]
- Alesi, S.; Ghelani, D.; Rassie, K.; Mousa, A. Metabolomic Biomarkers in Gestational Diabetes Mellitus: A Review of the Evidence. Int. J. Mol. Sci. 2021, 22, 5512. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Francis, E.; Hu, G.; Chen, L. Metabolomic profiling of women with gestational diabetes mellitus and their offspring: Review of metabolomics studies. J. Diabetes Complicat. 2018, 32, 512–523. [Google Scholar] [CrossRef]
- Cetin, I.; de Santis, M.S.; Taricco, E.; Radaelli, T.; Teng, C.; Ronzoni, S.; Spada, E.; Milani, S.; Pardi, G. Maternal and fetal amino acid concentrations in normal pregnancies and in pregnancies with gestational diabetes mellitus. Am. J. Obstet. Gynecol. 2005, 192, 610–617. [Google Scholar] [CrossRef]
- Rahimi, N.; Razi, F.; Nasli-Esfahani, E.; Qorbani, M.; Shirzad, N.; Larijani, B. Amino acid profiling in the gestational diabetes mellitus. J. Diabetes Metab. Disord. 2017, 16, 13. [Google Scholar] [CrossRef]
- Bentley-Lewis, R.; Huynh, J.; Xiong, G.; Lee, H.; Wenger, J.; Clish, C.; Nathan, D.; Thadhani, R.; Gerszten, R. Metabolomic profiling in the prediction of gestational diabetes mellitus. Diabetologia 2015, 58, 1329–1332. [Google Scholar] [CrossRef]
- Pappa, K.I.; Vlachos, G.; Theodora, M.; Roubelaki, M.; Angelidou, K.; Antsaklis, A. Intermediate metabolism in association with the amino acid profile during the third trimester of normal pregnancy and diet-controlled gestational diabetes. Am. J. Obstet. Gynecol. 2007, 196, e61–e65. [Google Scholar] [CrossRef]
- Chorell, E.; Hall, U.A.; Gustavsson, C.; Berntorp, K.; Puhkala, J.; Luoto, R.; Olsson, T.; Holmäng, A. Pregnancy to postpartum transition of serum metabolites in women with gestational diabetes. Metabolism 2017, 72, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Bomba-Opon, D.; Wielgos, M.; Szymanska, M.; Bablok, L. Effects of free fatty acids on the course of gestational diabetes mellitus. Neuro Endocrinol. Lett. 2006, 27, 277–280. [Google Scholar]
- Meyer, B.; Calvert, D.; Moses, R. Free fatty acids and gestational diabetes mellitus. Aust. N. Z. J. Obstet. Gynaecol. 1996, 36, 255–257. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, T.; Zhu, Y.; Lai, X.; Tao, H.; Xing, Y.; Li, Z. Deciphering the Role of CD36 in Gestational Diabetes Mellitus: Linking Fatty Acid Metabolism and Inflammation in Disease Pathogenesis. J. Inflamm. Res. 2025, 18, 1575–1588. [Google Scholar] [CrossRef] [PubMed]
- Son, N.H.; Basu, D.; Samovski, D.; Pietka, T.A.; Peche, V.S.; Willecke, F.; Fang, X.; Yu, S.Q.; Scerbo, D.; Chang, H.R.; et al. Endothelial cell CD36 optimizes tissue fatty acid uptake. J. Clin. Investig. 2018, 128, 4329–4342. [Google Scholar] [CrossRef]
- Yi, L.Z.; He, J.; Liang, Y.Z.; Yuan, D.L.; Chau, F.T. Plasma fatty acid metabolic profiling and biomarkers of type 2 diabetes mellitus based on GC/MS and PLS-LDA. FEBS Lett. 2006, 580, 6837–6845. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.N.; Altman, D.G.; Campbell, M.J.; Royston, P. Analysis of serial measurements in medical research. BMJ 1990, 300, 230–235. [Google Scholar] [CrossRef]
- Spiller, S.; Blüher, M.; Hoffmann, R. Plasma levels of free fatty acids correlate with type 2 diabetes mellitus. Diabetes Obes. Metab. 2018, 20, 2661–2669. [Google Scholar] [CrossRef]
- Lai, M.; Liu, Y.; Ronnett, G.V.; Wu, A.; Cox, B.J.; Dai, F.F.; Röst, H.L.; Gunderson, E.P.; Wheeler, M.B. Amino acid and lipid metabolism in post-gestational diabetes and progression to type 2 diabetes: A metabolic profiling study. PLoS Med. 2020, 17, e1003112. [Google Scholar] [CrossRef]
- Ortega-Senovilla, H.; Schaefer-Graf, U.; Herrera, E. Pregnant women with gestational diabetes and with well controlled glucose levels have decreased concentrations of individual fatty acids in maternal and cord serum. Diabetologia 2020, 63, 864–874. [Google Scholar] [CrossRef]
- Layton, J.; Powe, C.; Allard, C.; Battista, M.C.; Doyon, M.; Bouchard, L.; Perron, P.; Wessel, J.; Hivert, M.F. Maternal lipid profile differs by gestational diabetes physiologic subtype. Metabolism 2019, 91, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Stein, T.P.; Steer, R.A.; Scholl, T.O. Individual free fatty acids have unique associations with inflammatory biomarkers, insulin resistance and insulin secretion in healthy and gestational diabetic pregnant women. BMJ Open Diabetes Res. Care 2019, 7, e000632. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Meng, X.; Zhao, A.; Zhao, W.; Pan, J.; Tang, J.; Huang, Y.; Li, H.; Jia, W.; Liu, F. Development of Multimarker Diagnostic Models from Metabolomics Analysis for Gestational Diabetes Mellitus (GDM). Mol. Cell Proteom. 2018, 17, 431–441. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Zhu, S.; Lin, M.; Yang, Q.; Wei, L.; Zhang, J.; Jiang, X.; Zhu, D.; Lu, X.; Chen, Y.Q. Increased GPR120 level is associated with gestational diabetes mellitus. Biochem. Biophys. Res. Commun. 2019, 512, 196–201. [Google Scholar] [CrossRef]
- Zhu, Y.; Tsai, M.Y.; Sun, Q.; Hinkle, S.N.; Rawal, S.; Mendola, P.; Ferrara, A.; Albert, P.S.; Zhang, C. A prospective and longitudinal study of plasma phospholipid saturated fatty acid profile in relation to cardiometabolic biomarkers and the risk of gestational diabetes. Am. J. Clin. Nutr. 2018, 107, 1017–1026. [Google Scholar] [CrossRef]
- Bukowiecka-Matusiak, M.; Burzynska-Pedziwiatr, I.; Sansone, A.; Malachowska, B.; Zurawska-Klis, M.; Ferreri, C.; Chatgilialoglu, C.; Ochedalski, T.; Cypryk, K.; Wozniak, L.A. Lipid profile changes in erythrocyte membranes of women with diagnosed GDM. PLoS ONE 2018, 13, e0203799. [Google Scholar] [CrossRef]
- Burlina, S.; Dalfrà, M.G.; Barison, A.; Marin, R.; Ragazzi, E.; Sartore, G.; Lapolla, A. Plasma phospholipid fatty acid composition and desaturase activity in women with gestational diabetes mellitus before and after delivery. Acta Diabetol. 2017, 54, 45–51. [Google Scholar] [CrossRef]
- Villafan-Bernal, J.R.; Acevedo-Alba, M.; Reyes-Pavon, R.; Diaz-Parra, G.A.; Lip-Sosa, D.L.; Vazquez-Delfin, H.I.; Hernandez-Muñoz, M.; Bravo-Aguirre, D.E.; Figueras, F.; Martinez-Portilla, R.J. Plasma Levels of Free Fatty Acids in Women with Gestational Diabetes and Its Intrinsic and Extrinsic Determinants: Systematic Review and Meta-Analysis. J. Diabetes Res. 2019, 2019, 7098470. [Google Scholar] [CrossRef]
- Kalkhoff, R.K. Impact of maternal fuels and nutritional state on fetal growth. Diabetes 1991, 40 (Suppl. 2), 61–65. [Google Scholar] [CrossRef]
- Bartáková, V.; Malúšková, D.; Mužík, J.; Bělobrádková, J.; Kaňková, K. Possibility to predict early postpartum glucose abnormality following gestational diabetes mellitus based on the results of routine mid-gestational screening. Biochem. Med. 2015, 25, 460–468. [Google Scholar] [CrossRef][Green Version]
- Bartáková, V.; Barátová, B.; Pácal, L.; Ťápalová, V.; Šebestová, S.; Janků, P.; Kaňková, K. Development of a New Risk Score for Stratification of Women with Gestational Diabetes Mellitus at High Risk of Persisting Postpartum Glucose Intolerance Using Routinely Assessed Parameters. Life 2021, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Hu, P.; Li, F.; Huang, Y.; Yang, Y.; Sun, F.; Wu, P.; Lai, Y.; Wang, Y.; He, X.; et al. Circulating linoleic acid and its interplay with gut microbiota during pregnancy for gestational diabetes mellitus. BMC Med. 2025, 23, 245. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, S.; Chen, H.; Zhou, L.; Chen, B.; Wang, M.; Zhang, D.; Han, T.L.; Zhang, H. Gut dysbiosis contributes to SCFAs reduction-associated adipose tissue macrophage polarization in gestational diabetes mellitus. Life Sci. 2024, 350, 122744. [Google Scholar] [CrossRef] [PubMed]
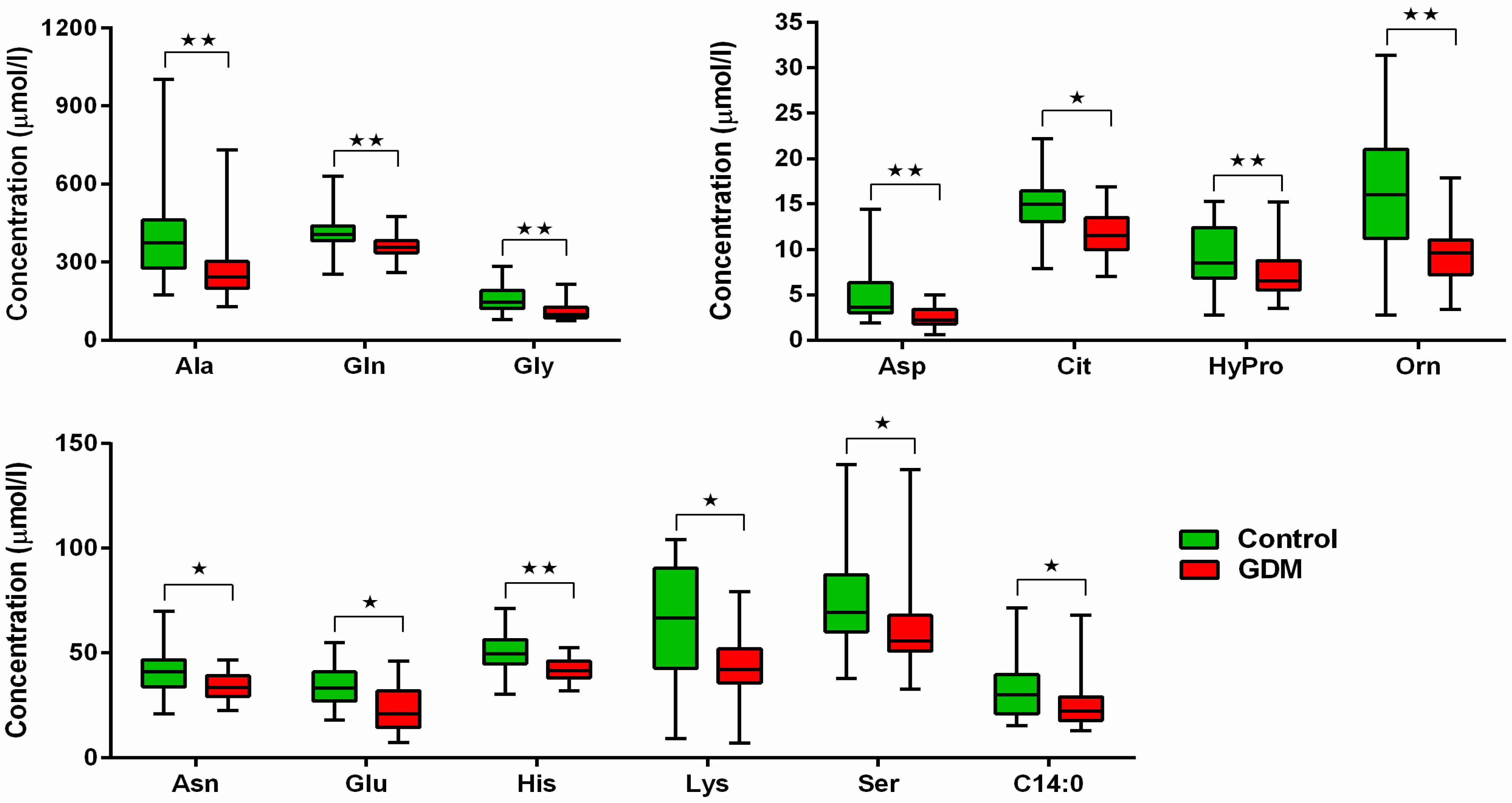
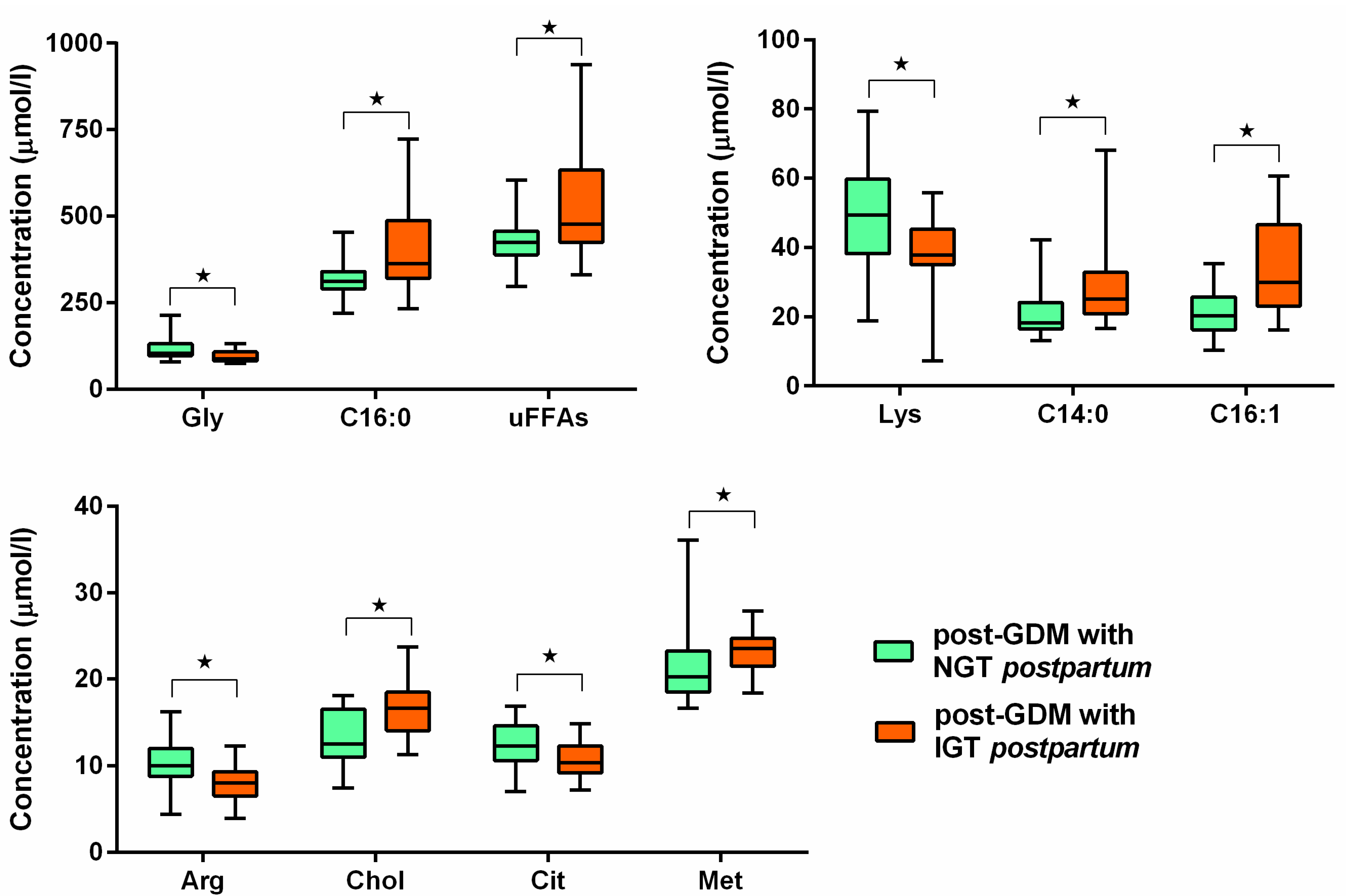
| Parameter | GDM (n = 34) | Controls (n = 20) | p | GDM Normalized After Delivery (n = 20) | GDM with Persisting PGI (n = 14) | p |
|---|---|---|---|---|---|---|
| Age (years) | 33 [30–35] | 32 [29.5–35] | NS | 33 [31–36] | 31.5 [30–34] | NS |
| Pre-gestational BMI (kg/m2) | 25.6 [21.7–28.4] | 21.3 [20.1–23.5] | 0.007 | 26.5 [21.8–27.6] | 24 [20.4–30.6] | NS |
| Obesity (pre-gest. BMI > 30 kg/m2) | 17.6% | 0% | NS | 5.0% | 35.7% | 0.03 |
| Weight gain during pregnancy (kg) | 6 [4–10] | 13 [10–17] | 0.005 | 6 [4–9] | 9 [6–11] | NS |
| Offspring birth weight (g) | 3300 [3050–3550] | 3020 [2900–3450] | NS | 3200 [3020–3500] | 3360 [3060–3630] | NS |
| History of previous GDM (number of nulliparous) | 23.5% (14) | 5.0% (10) | NS | 15.0% (8) | 42.8% (6) | NS |
| Family history of DM | 76.5% | 40.0% | 0.01 | 70.0% | 92.9% | NS |
| FPG (mmol/L) mid-gestation | 4.9 [4.6–5.3] | 4.0 [3.8–4.3] | <1 × 10−6 | 4.6 [4.4–5.0] | 5.3 [4.9–6.0] | 0.007 |
| 1-h PPG (mmol/L) mid-gestation | 9.2 [8.8–10.1] | 5.8 [5.1–7.6] | 1 × 10−6 | 9.7 [9.1–10.1] | 8.3 [8.2–6.0] | NS |
| 2-h PPG (mmol/L) mid-gestation | 8.0 [7.7–8.6] | 4.8 [4.2–6.6] | <1 × 10−6 | 8.1 [7.6–8.6] | 8.0 [7.8–8.8] | NS |
| AUCoGTT (mmol/L/h) mid-gestation | 13.0 [12.5–13.3] | 8.9 [8.2–10.6] | <1 × 10−6 | 12.8 [12.3–13.2] | 13.1 [12.8–14.6] | NS |
| Insulin treatment | 44.0% | - | - | 35.0% | 50.0% | NS |
| Parameter (n/%) | GDM (n = 34) | Controls (n = 20) | p | GDM Normalized After Delivery (n = 20) | GDM with Persisting PGI (n = 14) | p |
|---|---|---|---|---|---|---|
| Macrosomia (birthweight > 4000 g) | 3/8.8% | 1/5.0% | NS | 1/5.0% | 2/14.2% | NS |
| Pre-term delivery (<38th week of gestation) | 2/5.9% | 2/10.0% | NS | 1/5.0% | 1/7.1% | NS |
| Delivery induction (oxytocin or Prostaglandin E) | 8/23% | 1/5.0% | NS | 7/35% | 1/7.1% | NS |
| Non-physiologic delivery (Caesarean section, VEX, forceps) | 9/26.5% | 4/20% | NS | 3/15% | 6/42.9% | NS |
| Prolonged delivery (>480 min) | 1/2.9% | 0/0% | NS | 0/0% | 1/7.1% | NS |
| Delivery complications (manual extraction of placenta, hypotonia of uterus) | 1/2.9% | 2/10.0% | NS | 0/0% | 1/7.1% | NS |
| Abnormal Apgar score in 5th min (<5) | 0/0% | 0/0% | NS | 0/0% | 0/0% | NS |
| Abnormal cord blood pH (<7.1) | 0/0% | 0/0% | NS | 0/0% | 0/0% | NS |
| Abnormal BE (<−12) | 0/0% | 0/0% | NS | 0/0% | 0/0% | NS |
| Any of the peripartal adverse outcomes (any of those above) | 17/50% | 8/40% | NS | 9/45% | 8/57.1% | NS |
| Any of the offspring’s adverse outcome * | 3/8.8% | 1/5.0% | NS | 1/5.0% | 2/14.2% | NS |
| Predictive Markers for GDM Prediction | Model Coeficients | OR | 95% CI for OR | p-Values |
|---|---|---|---|---|
| BMI | 0.53 | 1.70 | (1.15–2.51) | 0.007 |
| docosahexaenoic acid | 0.11 | 1.12 | (1.03–1.21) | 0.009 |
| cystein | −0.57 | 0.57 | (0.35–0.91) | 0.019 |
| histidine | −0.24 | 0.78 | (0.67–0.92) | 0.003 |
| Predictive Markers for PGI Prediction | Model Coeficients | OR | 95% CI for OR | p-Values |
|---|---|---|---|---|
| choline | 0.78 | 2.19 | (1.01–4.76) | 0.048 |
| tetradecanoic acid | 0.20 | 1.23 | (0.97–1.54) | 0.085 |
| glutamate | −0.26 | 0.77 | (0.60–0.99) | 0.041 |
| citruline | −0.43 | 0.65 | (0.36–1.19) | 0.165 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartáková, V.; Pleskačová, A.; Pácal, L.; Langmajerová, M.S.; Smutná, J.; Chalásová, K.; Eclerová, V.; Glatz, Z.; Kaňková, K.; Tomandl, J. Some Levels of Plasma Free Fatty Acids and Amino Acids in the Second Trimester Are Linked to Gestational Diabetes and Are Predictive of Persisting Impaired Glucose Tolerance After Delivery. J. Clin. Med. 2025, 14, 4744. https://doi.org/10.3390/jcm14134744
Bartáková V, Pleskačová A, Pácal L, Langmajerová MS, Smutná J, Chalásová K, Eclerová V, Glatz Z, Kaňková K, Tomandl J. Some Levels of Plasma Free Fatty Acids and Amino Acids in the Second Trimester Are Linked to Gestational Diabetes and Are Predictive of Persisting Impaired Glucose Tolerance After Delivery. Journal of Clinical Medicine. 2025; 14(13):4744. https://doi.org/10.3390/jcm14134744
Chicago/Turabian StyleBartáková, Vendula, Anna Pleskačová, Lukáš Pácal, Monika Skrutková Langmajerová, Jindra Smutná, Katarína Chalásová, Veronika Eclerová, Zdeněk Glatz, Kateřina Kaňková, and Josef Tomandl. 2025. "Some Levels of Plasma Free Fatty Acids and Amino Acids in the Second Trimester Are Linked to Gestational Diabetes and Are Predictive of Persisting Impaired Glucose Tolerance After Delivery" Journal of Clinical Medicine 14, no. 13: 4744. https://doi.org/10.3390/jcm14134744
APA StyleBartáková, V., Pleskačová, A., Pácal, L., Langmajerová, M. S., Smutná, J., Chalásová, K., Eclerová, V., Glatz, Z., Kaňková, K., & Tomandl, J. (2025). Some Levels of Plasma Free Fatty Acids and Amino Acids in the Second Trimester Are Linked to Gestational Diabetes and Are Predictive of Persisting Impaired Glucose Tolerance After Delivery. Journal of Clinical Medicine, 14(13), 4744. https://doi.org/10.3390/jcm14134744







