Latest Evidence on Intravascular Imaging: A Literature Review
Abstract
1. Introduction
1.1. Principles of Intravascular Imaging
1.2. Intravascular Imaging Versus Angiography
1.3. IVUS Versus OCT
1.4. QCA- Versus IVUS-Guided PCI
1.5. IVUS- Compared with FFR-Guided PCI in Intermediate-Severity Coronary Lesions
1.6. Intravascular Imaging in Acute Coronary Syndromes
1.7. Meta-Analyses of Intravascular Imaging Guidance Versus Angiographic Guidance
| Study | Year | Enrolled (n) | Modality | Follow-Up (Months) | Primary Endpoint | Outcomes |
|---|---|---|---|---|---|---|
| RENOVATE-COMPLEX PCI [12] | 2023 | 1639 | IVUS vs. Angiography | 25 | TVF: Cardiac death, TV-MI, or TV revascularization |
Stent thrombosis: 0.1% vs. 0.7% |
| OCTOBER [14] | 2023 | 1201 | OCT vs. Angiography | 24 | MACEs: Cardiac death, TL-MI, or TL revascularization |
|
| ILUMIEN IV [15] | 2023 | 2487 | OCT vs. Angiography | 24 | Minimum stent area (MSA) after PCI; Target-vessel failure (TVF) |
|
| OCCUPI [17] | 2024 | 1604 | OCT vs. Angiography | 12 | MACEs: Cardiac death, MI, stent thrombosis, or ischemia-driven TVR |
|
| OCTIVUS [18] | 2023 | 2008 | OCT vs. IVUS | 12 | Cardiac death, TV-MI, or TV revascularization |
|
| GUIDE-DES [19] | 2024 | 1528 | QCA-Angiography vs. IVUS | 12 | Target-lesion failure (TLF) |
|
| FLAVOUR [20] | 2022 | 1682 | FFR vs. IVUS | 24 | Death, MI, or revascularization |
|
| IVUS-ACS [21] | 2024 | 3504 | IVUS vs. Angiography | 12 | TVF |
|
| OPINION ACS [22] | 2024 | 158 | OFDI vs. IVUS | 12 | Minimum stent area |
|
| Study Name | Key Strengths | Key Limitations |
|---|---|---|
| ILUMIEN IV [15] | Large RCT; high-risk lesion focus; imaging and clinical endpoints | Did not meet primary clinical endpoint; COVID-era biases |
| RENOVATE-COMPLEX PCI [12] | Included both IVUS and OCT; multicenter; large sample of complex lesions | Majority IVUS-guided; single-country population (Korea) |
| OCTOBER [14] | Specific to bifurcation and LMCA lesions; clear clinical benefit shown | Benefit mainly in single-stent strategy; limited generalizability |
| ILUMIEN IV [15] | Large, multicenter, randomized trial; direct comparison of OCT-guided vs. angiography-guided PCI; powered for clinical outcomes | No significant difference in primary endpoint; majority stable CAD patients |
| OCCUPI [17] | Strong procedural and optimization data; real-world complexity | Left main and small vessel subgroups underpowered |
| OCTIVUS [18] | Head-to-head comparison; broad lesion types; non-inferiority shown | No clear clinical outcome difference; procedural complication variance |
| GUIDE-DES [19] | First trial using QCA vs. IVUS; novel design | Short follow-up; mostly stable patients |
| FLAVOUR [20] | Head-to-head comparison of physiology vs. imaging-based strategies; long follow-up | Limited to intermediate lesions; not imaging modality vs. angiography |
| IVUS-ACS [21] | Large trial in ACSs; strong evidence for IVUS in urgent settings | Limited OCT comparison; ACS subset only |
| OPINION ACS [22] | Direct comparison of OFDI vs. IVUS; ACS specific | Small sample size; short follow-up |
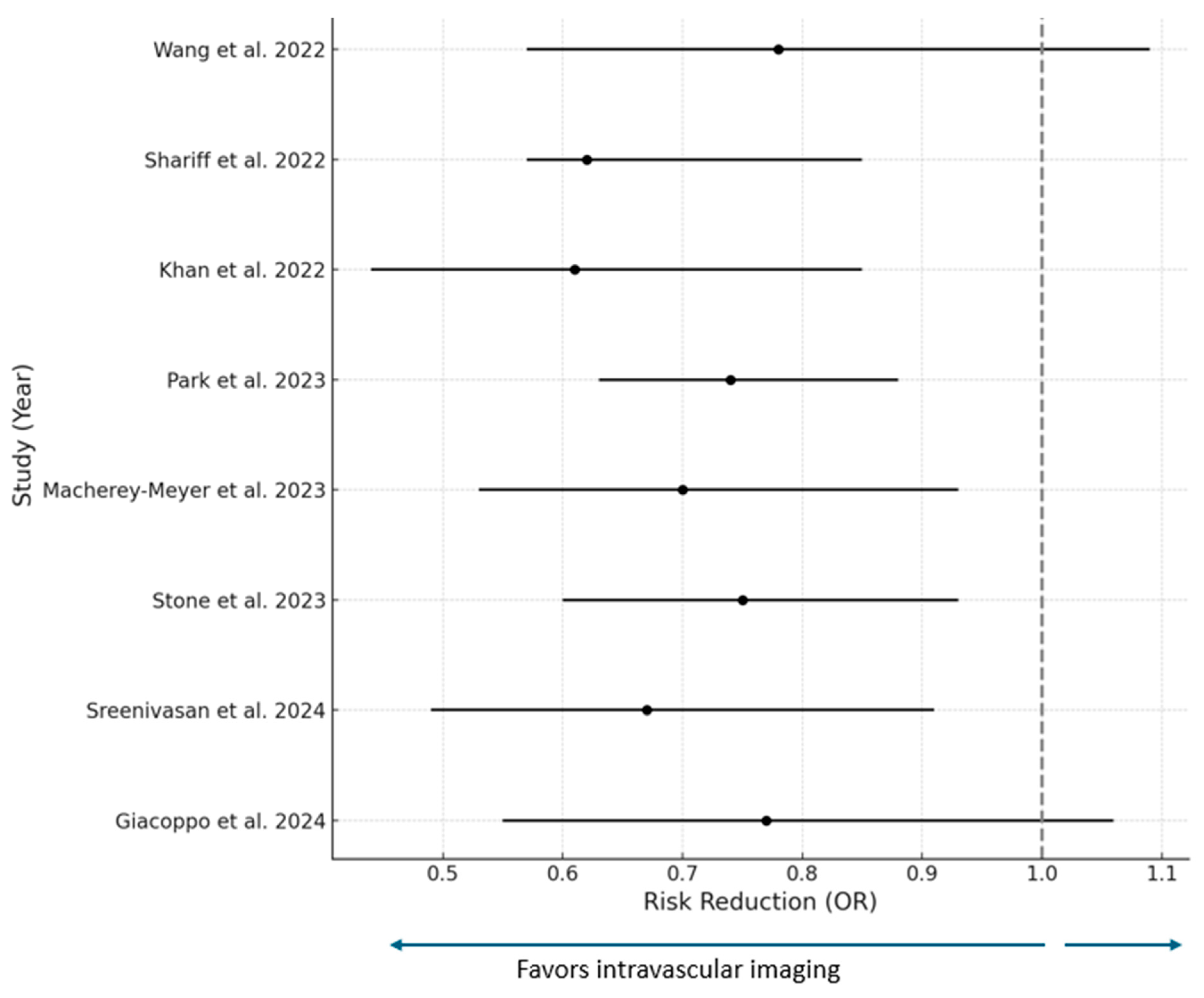
| Study | Study Population | All-Cause Mortality | Cardiovascular Mortality | MACEs | Myocardial Infarction | Stent Thrombosis | TVR | TLR |
|---|---|---|---|---|---|---|---|---|
| Wang et al., 2022 [23] | 5 studies (4 RCTs and 1 observational 3349 patients | OR 0.74 (0.39–1.39) | OR 0.82 (0.55–1.23) | 0.78 (0.57–1.09) | OR 0.41 (0.29–0.58) | OR 0.32 (0.11–0.89) | OR 0.48 (0.36–0.6) | N/R |
| Shariff et al., 2022 [25] | 14 RCTs | OR 0.97 (0.70–0.35) | OR 1.97 (1.25–3.11) | OR 1.62 (1.17–2.24) | OR 1.18 (0.81–1.73) | N/R | OR 1.60 (1.21–2.13) | N/R |
| Khan et al. IVI vs. CA [26] | 20 RCTs 11,698 patients | OR 0.81 (0.64–1.02) | OR 0.53 (0.39–0.72) | N/R | OR 0.81 (0.68–0.97) | OR 0.44 (0.27–0.72) | OR 0.74 (0.61–0.89) | OR 0.71 (0.59–0.86) |
| Park et al., 2023 IVUS vs. CA [27] | 28 studies 12,895 patients | OR 1.15 (0.85–1.56) | OR 0.64 (0.43–0.94) | OR 0.74 (0.63–0.88) | OR 0.82 (0.64–1.04) | OR 0.61 (0.36–1.04) | OR 0.64 (0.5–0.81) | OR 0.68 (0.57–0.8) |
| Macherey-Meyer et al., 2023 OCT vs. ICA [28] | 8 studies 2612 patients | OR (0.51–2.31) | OR 0.49 (0.250.96) | OR 0.7 (0.53–0.93) | OR 0.82 (0.49–1.37) | N/R | OR 0.54 (0.26–1.13) | OR 0.26 (0.07–0.95) |
| Stone et al., 2023 IVI vs. ICA [29] | 22 studies 15,694 patients | OR 0.75 (0.6–0.93) | OR 0.55 (0.41–0.75) | N/R | OR 0.83 (0.71–0.99) | OR 0.52 (0.34–0.81) | OR 0.64 (0.38–1.07) | OR 0.72 (0.60–0.86) |
| Sreenivasan et al., 2024 IVI vs. ICA [30] | 16 studies 7814 patients | OR 0.75 (0.55–1.02) | OR 0.49 (0.34–0.71) | OR 0.67 (0.55–0.82) | N/R | OR 0.63 0.40–0.99 | OR 0.60 (0.45–0.80) | OR 0.67 (0.49–0.91) |
| Giacoppo et al., 2024 IVUS vs. OCT vs. ICA [31] | 24 RCTs 15,489 patients • IVUS vs. ICA • OCT vs. ICA • OCT vs. IVUS | OR 0.77 (0.55–1.06) OR 0.71 (0.51–0.99) OR 0.93 (0.61–1.41) | OR 0.57 (0.37–0.90) OR 0.58 (0.69–0.94) OR 1.01 (0.55–1.84) | OR 0.67 (0.56–0.8) OR 0.77 (0.63–0.94) OR 1.14 (0.9–1.45) | OR 0.88 (0.67–1.17) OR 0.90 (0.70–1.16) OR 0.83 (0.44–1.56) | OR 0.6 (0.35–1.05) OR 0.49 (0.26–0.92) OR 0.81 (0.37–1.79) | OR 0.65 (0.53–0.81) OR 0.87 (0.68–1.11) OR 1.33 (1–1.77) | OR 0.69 (0.54–0.87) OR 0.83 (0.63–1.09) OR 1.2 (0.88–1.66) |
2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACS | Acute Coronary Syndrome |
| FLAVOUR | Fractional Flow Reserve and Intravascular Ultrasound-Guided Intervention Strategy for Clinical Outcomes in Patients with Intermediate Stenosis |
| FFR | Fractional Flow Reserve |
| GUIDE-DES | Quantitative Coronary Angiography-Guidance versus Intravascular Ultrasound-Guidance for Drug-Eluting Stent Implantation |
| ILUMIEN IV | OCT-Guided Coronary Stent Implantation Compared with Angiography: A Multicenter Randomized Trial in PCI, OCT, or Angiography Guidance for PCI in Complex Bifurcation Lesions |
| IVUS | Intravascular Ultrasound |
| IVUS-ACSs | Intravascular Ultrasound-Guided versus Angiography-Guided Percutaneous Coronary Intervention in Acute Coronary Syndromes |
| MACEs | Major Adverse Cardiovascular Events |
| MSA | Minimum Stent Area |
| OCCUPI | Optical Coherence Tomography-Guided Coronary Intervention in Patients With Complex Lesions |
| OCT | Optical Coherence Tomography |
| OCTIVUS | Optical Coherence Tomography versus Intravascular Ultrasound-Guided Percutaneous Coronary Intervention |
| OFDI | Optical Frequency Domain Imaging |
| OPINION ACSs | Optical Frequency Domain Imaging-Guided versus Intravascular Ultrasound-Guided Percutaneous Coronary Intervention for Acute Coronary Syndromes |
| OR | Odds Ratio |
| QFR | Quantitative Flow Ratio |
| RENOVATE-COMPLEX PCI | Randomized Controlled Trial of Intravascular Imaging Guidance versus Angiography-Guidance on Clinical Outcomes After Complex Percutaneous Coronary Intervention |
| TLR | Target-Lesion Revascularization |
| TVR | Target-Vessel Revascularization |
References
- Mintz, G.S.; Guagliumi, G. Intravascular imaging in coronary artery disease. Lancet 2017, 390, 793–809. [Google Scholar] [CrossRef] [PubMed]
- Mintz, G.S.; Popma, J.J.; Pichard, A.D.; Kent, K.M.; Satler, L.F.; Chuang, Y.C.; DeFalco, R.A.; Leon, M.B. Limitations of angiography in the assessment of plaque distribution in coronary artery disease: A systematic study of target lesion eccentricity in 1446 lesions. Circulation 1996, 93, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Mintz, G.S. Clinical utility of intravascular imaging and physiology in coronary artery disease. J. Am. Coll. Cardiol. 2014, 64, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Kobayashi, Y.; Fujii, K.; Sonoda, S.; Tsujita, K.; Hibi, K.; Morino, Y.; Okura, H.; Ikari, Y.; Honye, J. Clinical expert consensus document on intravascular ultrasound from the Japanese Association of Cardiovascular Intervention and Therapeutics (2021). Cardiovasc. Interv. Ther. 2022, 37, 40–51. [Google Scholar] [CrossRef]
- Bezerra, H.G.; Costa, M.A.; Guagliumi, G.; Rollins, A.M.; Simon, D.I. Intracoronary optical coherence tomography: A comprehensive review clinical and research applications. JACC Cardiovasc. Interv. 2009, 2, 1035–1046. [Google Scholar] [CrossRef]
- Ali, Z.A.; Karimi Galougahi, K.; Mintz, G.S.; Maehara, A.; Shlofmitz, R.A.; Mattesini, A. Intracoronary optical coherence tomography: State of the art and future directions. EuroIntervention 2021, 17, e105–e123. [Google Scholar] [CrossRef]
- Hong, S.J.; Mintz, G.S.; Ahn, C.M.; Kim, J.S.; Kim, B.K.; Ko, Y.G.; Kang, T.S.; Kang, W.C.; Kim, Y.H.; Hur, S.H.; et al. Effect of Intravascular Ultrasound-Guided Drug-Eluting Stent Implantation: 5-Year Follow-Up of the IVUS-XPL Randomized Trial. JACC Cardiovasc. Interv. 2020, 13, 62–71. [Google Scholar] [CrossRef]
- Gao, X.F.; Ge, Z.; Kong, X.Q.; Kan, J.; Han, L.; Lu, S.; Tian, N.L.; Lin, S.; Lu, Q.H.; Wang, X.Y.; et al. 3-Year Outcomes of the ULTIMATE Trial Comparing Intravascular Ultrasound Versus Angiography-Guided Drug-Eluting Stent Implantation. JACC Cardiovasc. Interv. 2021, 14, 247–257. [Google Scholar] [CrossRef]
- Antonsen, L.; Thayssen, P.; Maehara, A.; Hansen, H.S.; Junker, A.; Veien, K.T.; Hansen, K.N.; Hougaard, M.; Mintz, G.S.; Jensen, L.O. Optical Coherence Tomography Guided Percutaneous Coronary Intervention With Nobori Stent Implantation in Patients With Non-ST-Segment-Elevation Myocardial Infarction (OCTACS) Trial: Difference in Strut Coverage and Dynamic Malapposition Patterns at 6 Months. Circ. Cardiovasc. Interv. 2015, 8, e002446. [Google Scholar] [CrossRef]
- Meneveau, N.; Souteyrand, G.; Motreff, P.; Caussin, C.; Amabile, N.; Ohlmann, P.; Morel, O.; Lefrancois, Y.; Descotes-Genon, V.; Silvain, J.; et al. Optical Coherence Tomography to Optimize Results of Percutaneous Coronary Intervention in Patients with Non-ST-Elevation Acute Coronary Syndrome: Results of the Multicenter, Randomized DOCTORS Study (Does Optical Coherence Tomography Optimize Results of Stenting). Circulation 2016, 134, 906–917. [Google Scholar] [CrossRef]
- Lee, S.Y.; Ahn, C.M.; Yoon, H.J.; Hur, S.H.; Kim, J.S.; Kim, B.K.; Ko, Y.G.; Choi, D.; Jang, Y.; Hong, M.K. Early Follow-Up Optical Coherence Tomographic Findings of Significant Drug-Eluting Stent Malapposition. Circ. Cardiovasc. Interv. 2018, 11, e007192. [Google Scholar] [CrossRef]
- Lee, J.M.; Choi, K.H.; Song, Y.B.; Lee, J.Y.; Lee, S.J.; Lee, S.Y.; Kim, S.M.; Yun, K.H.; Cho, J.Y.; Kim, C.J.; et al. Intravascular Imaging-Guided or Angiography-Guided Complex PCI. N. Engl. J. Med. 2023, 388, 1668–1679. [Google Scholar] [CrossRef] [PubMed]
- Kwon, W.; Choi, K.H.; Song, Y.B.; Park, Y.H.; Lee, J.M.; Lee, J.Y.; Lee, S.J.; Lee, S.Y.; Kim, S.M.; Yun, K.H.; et al. Intravascular Imaging in Patients With Complex Coronary Lesions and Chronic Kidney Disease. JAMA Netw. Open 2023, 6, e2345554. [Google Scholar] [CrossRef] [PubMed]
- Holm, N.R.; Andreasen, L.N.; Neghabat, O.; Laanmets, P.; Kumsars, I.; Bennett, J.; Olsen, N.T.; Odenstedt, J.; Hoffmann, P.; Dens, J.; et al. OCT or Angiography Guidance for PCI in Complex Bifurcation Lesions. N. Engl. J. Med. 2023, 389, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.A.; Landmesser, U.; Maehara, A.; Matsumura, M.; Shlofmitz, R.A.; Guagliumi, G.; Price, M.J.; Hill, J.M.; Akasaka, T.; Prati, F.; et al. Optical Coherence Tomography-Guided versus Angiography-Guided PCI. N. Engl. J. Med. 2023, 389, 1466–1476. [Google Scholar] [CrossRef]
- Ali, Z.A.; Landmesser, U.; Maehara, A.; Shin, D.; Sakai, K.; Matsumura, M.; Shlofmitz, R.A.; Leistner, D.; Canova, P.; Alfonso, F.; et al. OCT-Guided vs Angiography-Guided Coronary Stent Implantation in Complex Lesions: An ILUMIEN IV Substudy. J. Am. Coll. Cardiol. 2024, 84, 368–378. [Google Scholar] [CrossRef]
- Hong, S.J.; Lee, S.J.; Lee, S.H.; Lee, J.Y.; Cho, D.K.; Kim, J.W.; Kim, S.M.; Hur, S.H.; Heo, J.H.; Jang, J.Y.; et al. Optical coherence tomography-guided versus angiography-guided percutaneous coronary intervention for patients with complex lesions (OCCUPI): An investigator-initiated, multicentre, randomised, open-label, superiority trial in South Korea. Lancet 2024, 404, 1029–1039. [Google Scholar] [CrossRef]
- Kang, D.Y.; Ahn, J.M.; Yun, S.C.; Hur, S.H.; Cho, Y.K.; Lee, C.H.; Hong, S.J.; Lim, S.; Kim, S.W.; Won, H.; et al. Optical Coherence Tomography-Guided or Intravascular Ultrasound-Guided Percutaneous Coronary Intervention: The OCTIVUS Randomized Clinical Trial. Circulation 2023, 148, 1195–1206. [Google Scholar] [CrossRef]
- Lee, P.H.; Hong, S.J.; Kim, H.S.; Yoon, Y.W.; Lee, J.Y.; Oh, S.J.; Lee, J.S.; Kang, S.J.; Kim, Y.H.; Park, S.W.; et al. Quantitative Coronary Angiography vs Intravascular Ultrasonography to Guide Drug-Eluting Stent Implantation: A Randomized Clinical Trial. JAMA Cardiol. 2024, 9, 428–435. [Google Scholar] [CrossRef]
- Yang, S.; Kang, J.; Hwang, D.; Zhang, J.; Jiang, J.; Hu, X.; Hahn, J.Y.; Nam, C.W.; Doh, J.H.; Lee, B.K.; et al. Physiology- or Imaging-Guided Strategies for Intermediate Coronary Stenosis. JAMA Netw. Open 2024, 7, e2350036. [Google Scholar] [CrossRef]
- Li, X.; Ge, Z.; Kan, J.; Anjum, M.; Xie, P.; Chen, X.; Khan, H.S.; Guo, X.; Saghir, T.; Chen, J.; et al. Intravascular ultrasound-guided versus angiography-guided percutaneous coronary intervention in acute coronary syndromes (IVUS-ACS): A two-stage, multicentre, randomised trial. Lancet 2024, 403, 1855–1865. [Google Scholar] [CrossRef] [PubMed]
- Otake, H.; Kubo, T.; Hibi, K.; Natsumeda, M.; Ishida, M.; Kataoka, T.; Takaya, T.; Iwasaki, M.; Sonoda, S.; Shinke, T.; et al. Optical frequency domain imaging-guided versus intravascular ultrasound-guided percutaneous coronary intervention for acute coronary syndromes: The OPINION ACS randomised trial. EuroIntervention 2024, 20, e1086–e1097. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liang, C.; Wang, Y.; Sun, S.; Wang, Y.; Suo, M.; Ye, M.; Li, X.; Liu, X.; Zhang, M.; et al. The long-term clinical outcomes of intravascular ultrasound-guided versus angiography-guided coronary drug eluting stent implantation in long de novo coronary lesions: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2022, 9, 944143. [Google Scholar] [CrossRef]
- Zhang, Y.; Farooq, V.; Garcia-Garcia, H.M.; Bourantas, C.V.; Tian, N.; Dong, S.; Li, M.; Yang, S.; Serruys, P.W.; Chen, S.L. Comparison of intravascular ultrasound versus angiography-guided drug-eluting stent implantation: A meta-analysis of one randomised trial and ten observational studies involving 19,619 patients. EuroIntervention 2012, 8, 855–865. [Google Scholar] [CrossRef]
- Shariff, M.; Kumar, A.; Kansara, T.; Majmundar, M.; Doshi, R.; Stulak, J.M.; Kapadia, S.R.; Reed, G.W.; Puri, R.; Kalra, A. Network Meta-analysis of Trials Comparing Intravascular Ultrasound, Optical Coherence Tomography, and Angiography-Guided Technique for Drug-Eluting Stent Implantation. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100507. [Google Scholar] [CrossRef]
- Khan, S.U.; Agarwal, S.; Arshad, H.B.; Akbar, U.A.; Mamas, M.A.; Arora, S.; Baber, U.; Goel, S.S.; Kleiman, N.S.; Shah, A.R. Intravascular imaging guided versus coronary angiography guided percutaneous coronary intervention: Systematic review and meta-analysis. BMJ 2023, 383, e077848. [Google Scholar] [CrossRef]
- Park, D.Y.; An, S.; Jolly, N.; Attanasio, S.; Yadav, N.; Gutierrez, J.A.; Nanna, M.G.; Rao, S.V.; Vij, A. Comparison of intravascular ultrasound, optical coherence tomography, and conventional angiography-guided percutaneous coronary interventions: A systematic review, network meta-analysis, and meta-regression. Catheter. Cardiovasc. Interv. 2023, 102, 440–450. [Google Scholar] [CrossRef]
- Macherey-Meyer, S.; Meertens, M.M.; Heyne, S.; Braumann, S.; Tichelbacker, T.; Wienemann, H.; Mauri, V.; Baldus, S.; Adler, C.; Lee, S. Optical coherence tomography-guided versus angiography-guided percutaneous coronary intervention in acute coronary syndrome: A meta-analysis. Clin. Res. Cardiol. 2024, 113, 967–976. [Google Scholar] [CrossRef]
- Stone, G.W.; Christiansen, E.H.; Ali, Z.A.; Andreasen, L.N.; Maehara, A.; Ahmad, Y.; Landmesser, U.; Holm, N.R. Intravascular imaging-guided coronary drug-eluting stent implantation: An updated network meta-analysis. Lancet 2024, 403, 824–837. [Google Scholar] [CrossRef]
- Sreenivasan, J.; Reddy, R.K.; Jamil, Y.; Malik, A.; Chamie, D.; Howard, J.P.; Nanna, M.G.; Mintz, G.S.; Maehara, A.; Ali, Z.A.; et al. Intravascular Imaging-Guided Versus Angiography-Guided Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis of Randomized Trials. J. Am. Heart Assoc. 2024, 13, e031111. [Google Scholar] [CrossRef]
- Giacoppo, D.; Laudani, C.; Occhipinti, G.; Spagnolo, M.; Greco, A.; Rochira, C.; Agnello, F.; Landolina, D.; Mauro, M.S.; Finocchiaro, S.; et al. Coronary Angiography, Intravascular Ultrasound, and Optical Coherence Tomography for Guiding of Percutaneous Coronary Intervention: A Systematic Review and Network Meta-Analysis. Circulation 2024, 149, 1065–1086. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. EuroIntervention 2019, 14, 1435–1534. [Google Scholar] [CrossRef] [PubMed]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e4–e17. [Google Scholar] [CrossRef] [PubMed]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. G. Ital. Cardiol. 2024, 25 (Suppl. S1), e1–e132. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koros, R.; Karanasos, A.; Papafaklis, M.I.; Xygka, G.; Vasilagkos, G.; Apostolos, A.; Kallinikos, F.; Papageorgiou, M.; Tampaki, N.-M.; Fotopoulou, C.-M.; et al. Latest Evidence on Intravascular Imaging: A Literature Review. J. Clin. Med. 2025, 14, 4714. https://doi.org/10.3390/jcm14134714
Koros R, Karanasos A, Papafaklis MI, Xygka G, Vasilagkos G, Apostolos A, Kallinikos F, Papageorgiou M, Tampaki N-M, Fotopoulou C-M, et al. Latest Evidence on Intravascular Imaging: A Literature Review. Journal of Clinical Medicine. 2025; 14(13):4714. https://doi.org/10.3390/jcm14134714
Chicago/Turabian StyleKoros, Rafail, Antonios Karanasos, Michail I. Papafaklis, Georgia Xygka, Georgios Vasilagkos, Anastasios Apostolos, Fotios Kallinikos, Maria Papageorgiou, Nikoletta-Maria Tampaki, Charikleia-Maria Fotopoulou, and et al. 2025. "Latest Evidence on Intravascular Imaging: A Literature Review" Journal of Clinical Medicine 14, no. 13: 4714. https://doi.org/10.3390/jcm14134714
APA StyleKoros, R., Karanasos, A., Papafaklis, M. I., Xygka, G., Vasilagkos, G., Apostolos, A., Kallinikos, F., Papageorgiou, M., Tampaki, N.-M., Fotopoulou, C.-M., Lolou, E., Gkioni, G., Davlouros, P., & Tsigkas, G. (2025). Latest Evidence on Intravascular Imaging: A Literature Review. Journal of Clinical Medicine, 14(13), 4714. https://doi.org/10.3390/jcm14134714