Chronic Total Occlusions: Current Approaches, Evidence and Outcomes
Abstract
1. Introduction
2. Clinical Presentation
3. CTO Techniques and Strategy Algorithms
4. CTO Procedures Challenges and Complications
5. Evidence from Studies
6. Perspectives
7. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Levine, G.N.; Bates, E.R.; Blankenship, J.C.; Bailey, S.R.; Bittl, J.A.; Cercek, B.; Chambers, C.E.; Ellis, S.G.; Guyton, R.A.; Hollenberg, S.M.; et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011, 124, e574–e651. [Google Scholar] [PubMed]
- Tomasello, S.D.; Boukhris, M.; Giubilato, S.; Marzà, F.; Garbo, R.; Contegiacomo, G.; Marzocchi, A.; Niccoli, G.; Gagnor, A.; Varbella, F.; et al. Management strategies in patients affected by chronic total occlusions: Results from the Italian Registry of Chronic Total Occlusions. Eur. Heart J. 2015, 36, 3189–3198. [Google Scholar] [CrossRef]
- Fefer, P.; Knudtson, M.L.; Cheema, A.N.; Galbraith, P.D.; Osherov, A.B.; Yalonetsky, S.; Gannot, S.; Samuel, M.; Weisbrod, M.; Bierstone, D.; et al. Current Perspectives on Coronary Chronic Total Occlusions. J. Am. Coll. Cardiol. 2012, 59, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Tsuchikane, E.; Katoh, O.; Muramatsu, T.; Muto, M.; Kishi, K.; Hamazaki, Y.; Oikawa, Y.; Kawasaki, T.; Okamura, A. Outcomes of Percutaneous Coronary Interventions for Chronic Total Occlusion Performed by Highly Experienced Japanese Specialists. JACC Cardiovasc. Interv. 2017, 10, 2144–2154. [Google Scholar] [CrossRef]
- Banning, A.P.; Serruys, P.; De Maria, G.L.; Ryan, N.; Walsh, S.; Gonzalo, N.; van Geuns, R.J.; Onuma, Y.; Sabate, M.; Davies, J.; et al. Five-year outcomes after state-of-the-art percutaneous coronary revascularization in patients with de novo three-vessel disease: Final results of the SYNTAX II study. Eur. Heart J. 2022, 43, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Azzalini, L.; Jolicoeur, E.M.; Pighi, M.; Millán, X.; Picard, F.; Tadros, V.-X.; Fortier, A.; L’ALlier, P.L.; Ly, H.Q. Epidemiology, Management Strategies, and Outcomes of Patients With Chronic Total Coronary Occlusion. Am. J. Cardiol. 2016, 118, 1128–1135. [Google Scholar] [CrossRef]
- Werner, G.S.; Surber, R.; Ferrari, M.; Fritzenwanger, M.; Figulla, H.R. The functional reserve of collaterals supplying long-term chronic total coronary occlusions in patients without prior myocardial infarction. Eur. Heart J. 2006, 27, 2406–2412. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D. Writing Group on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction, Third universal definition of myocardial infarction. Nat. Rev. Cardiol. 2012, 9, 620–633. [Google Scholar] [CrossRef]
- Park, T.K.; Lee, S.H.; Choi, K.H.; Lee, J.M.; Yang, J.H.; Bin Song, Y.; Hahn, J.; Choi, J.; Gwon, H.; Lee, S.H.; et al. Late Survival Benefit of Percutaneous Coronary Intervention Compared With Medical Therapy in Patients With Coronary Chronic Total Occlusion: A 10-Year Follow-Up Study. J. Am. Heart Assoc. 2021, 10, e019022. [Google Scholar] [CrossRef]
- Morino, Y.; Abe, M.; Morimoto, T.; Kimura, T.; Hayashi, Y.; Muramatsu, T.; Ochiai, M.; Noguchi, Y.; Kato, K.; Shibata, Y.; et al. Predicting Successful Guidewire Crossing Through Chronic Total Occlusion of Native Coronary Lesions Within 30 Minutes. JACC Cardiovasc. Interv. 2011, 4, 213–221. [Google Scholar] [CrossRef]
- Hong, S.J.; Kim, B.K.; Cho, I.; Kim, H.Y.; Rha, S.W.; Lee, S.H.; Park, S.M.; Kim, Y.H.; Chang, H.J.; Ahn, C.M.; et al. Effect of Coronary CTA on Chronic Total Occlusion Percutaneous Coronary Intervention. JACC Cardiovasc. Imaging 2021, 14, 1993–2004. [Google Scholar] [CrossRef] [PubMed]
- Brilakis, E.S.; Mashayekhi, K.; Tsuchikane, E.; Abi Rafeh, N.; Alaswad, K.; Araya, M.; Avran, A.; Azzalini, L.; Babunashvili, A.M.; Bayani, B.; et al. Guiding Principles for Chronic Total Occlusion Percutaneous Coronary Intervention: A Global Expert Consensus Document. Circulation 2019, 140, 420–433. [Google Scholar] [CrossRef]
- Konstantinidis, N.V.; Werner, G.S.; Deftereos, S.; Di Mario, C.; Galassi, A.R.; Buettner, J.H.; Avran, A.; Reifart, N.; Goktekin, O.; Garbo, R.; et al. Temporal Trends in Chronic Total Occlusion Interventions in Europe: 17 626 Procedures From the European Registry of Chronic Total Occlusion. Circ. Cardiovasc. Interv. 2018, 11, e006229. [Google Scholar] [CrossRef] [PubMed]
- Wu, E.B.; Brilakis, E.S.; Mashayekhi, K.; Tsuchikane, E.; Alaswad, K.; Araya, M.; Avran, A.; Azzalini, L.; Babunashvili, A.M.; Bayani, B.; et al. Global Chronic Total Occlusion Crossing Algorithm. J. Am. Coll. Cardiol. 2021, 78, 840–853. [Google Scholar] [CrossRef]
- Di Mario, C.; Mashayekhi, K.A.; Garbo, R.; Pyxaras, S.A.; Ciardetti, N.; Werner, G.S. Recanalisation of coronary chronic total occlusions. EuroIntervention 2022, 18, 535–561. [Google Scholar] [CrossRef]
- Khand, A.; Patel, B.; Palmer, N.; Jones, J.; Andron, M.; Perry, R.; Mehrotra, S.; Mitsudo, K. Retrograde Wiring of Collateral Channels of the Heart in Chronic Total Occlusions: A Systematic Review and Meta-Analysis of Safety, Feasibility, and Incremental Value in Achieving Revascularization. Angiology 2015, 66, 925–932. [Google Scholar] [CrossRef]
- Matsuno, S.; Tsuchikane, E.; Harding, S.A.; Wu, E.B.; Kao, H.L.; Brilakis, E.S.; Mashayekhi, K.; Werner, G.S. Overview and proposed terminology for the reverse controlled antegrade and retrograde tracking (reverse CART) techniques. EuroIntervention 2018, 14, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Tajti, P.; Karmpaliotis, D.; Alaswad, K.; Jaffer, F.A.; Yeh, R.W.; Patel, M.; Mahmud, E.; Choi, J.W.; Burke, M.N.; Doing, A.H.; et al. The Hybrid Approach to Chronic Total Occlusion Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2018, 11, 1325–1335. [Google Scholar] [CrossRef]
- Zivelonghi, C.; Van Kuijk, J.P.; Suttorp, M.J.; Van der Heyden, J.A.S.; Eefting, F.D.; Rensing, B.J.; Ten Berg, J.M.; Agostoni, P. Implementing a minimally invasive approach (combining radial approach, small guiding catheters and minimization of double access) for coronary chronic total occlusion intervention according to the hybrid algorithm: The Minimalistic Hybrid Algorithm. Int. J. Cardiol. 2019, 283, 84–87. [Google Scholar] [CrossRef]
- Wu, E.B.; Tsuchikane, E.; Ge, L.; Harding, S.A.; Lo, S.; Lim, S.T.; Chen, J.-Y.; Lee, S.-W.; Qian, J.; Kao, H.-L.; et al. Retrograde Versus Antegrade Approach for Coronary Chronic Total Occlusion in an Algorithm-Driven Contemporary Asia-Pacific Multicenter Registry: Comparison of Outcomes. Heart Lung Circ. 2020, 29, 894–903. [Google Scholar] [CrossRef]
- Harding, S.A.; Wu, E.B.; Lo, S.; Lim, S.T.; Ge, L.; Chen, J.-Y.; Quan, J.; Lee, S.-W.; Kao, H.-L.; Tsuchikane, E. A New Algorithm for Crossing Chronic Total Occlusions From the Asia Pacific Chronic Total Occlusion Club. JACC Cardiovasc. Interv. 2017, 10, 2135–2143. [Google Scholar] [CrossRef]
- Patel, V.G.; Brayton, K.M.; Tamayo, A.; Mogabgab, O.; Michael, T.T.; Lo, N.; Alomar, M.; Shorrock, D.; Cipher, D.; Abdullah, S.; et al. Angiographic Success and Procedural Complications in Patients Undergoing Percutaneous Coronary Chronic Total Occlusion Interventions. JACC Cardiovasc. Interv. 2013, 6, 128–136. [Google Scholar] [CrossRef]
- Vadalà, G.; Galassi, A.R.; Werner, G.S.; Sianos, G.; Boudou, N.; Gardo, R.; Maniscalco, L.; Bufe, A.; Avran, A.; Gasparini, G.L.; et al. Contemporary outcomes of chronic total occlusion percutaneous coronary intervention in Europe: The ERCTO registry. EuroIntervention 2024, 20, e185–e197. [Google Scholar] [CrossRef]
- Guan, C.; Yang, W.; Song, L.; Chen, J.; Qian, J.; Wu, F.; Zou, T.; Shi, Y.; Sun, Z.; Xie, L.; et al. Association of Acute Procedural Results With Long-Term Outcomes After CTO PCI. JACC Cardiovasc. Interv. 2021, 14, 278–288. [Google Scholar] [CrossRef]
- Galassi, A.R.; Tomasello, S.D.; Reifart, N.; Werner, G.S.; Sianos, G.; Bonnier, H.; Sievert, H.; Ehladad, S.; Bufe, A.; Shofer, J.; et al. In-hospital outcomes of percutaneous coronary intervention in patients with chronic total occlusion: Insights from the ERCTO (European Registry of Chronic Total Occlusion) registry. EuroIntervention 2011, 7, 472–479. [Google Scholar] [CrossRef]
- Suero, J.A.; Marso, S.P.; Jones, P.G.; Laster, S.B.; Huber, K.C.; Giorgi, L.V.; Johnson, W.L.; Rutherford, B.D. Procedural outcomes and long-term survival among patients undergoing percutaneous coronary intervention of a chronic total occlusion in native coronary arteries: A 20-year experience. J. Am. Coll. Cardiol. 2001, 38, 409–414. [Google Scholar] [CrossRef]
- Ivanhoe, R.J.; Weintraub, W.S.; Douglas, J.S., Jr.; Lembo, N.J.; Furman, M.; Gershony, G.; Cohen, C.L.; King, S.B., 3rd. Percutaneous transluminal coronary angioplasty of chronic total occlusions. Primary success, restenosis, and long-term clinical follow-up. Circulation 1992, 85, 106–115. [Google Scholar] [CrossRef]
- Finci, L.; Meier, B.; Favre, J.; Righetti, A.; Rutishauser, W. Long-term results of successful and failed angioplasty for chronic total coronary arterial occlusion. Am. J. Cardiol. 1990, 66, 660–662. [Google Scholar] [CrossRef]
- Borgia, F.; Viceconte, N.; Ali, O.; Stuart-Buttle, C.; Saraswathyamma, A.; Parisi, R.; Mirabella, F.; Dimopoulos, K.; Di Mario, C. Improved cardiac survival, freedom from mace and angina-related quality of life after successful percutaneous recanalization of coronary artery chronic total occlusions. Int. J. Cardiol. 2012, 161, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Christakopoulos, G.E.; Carlino, M.; Jeroudi, O.M.; Roesle, M.; Rangan, B.V.; Abdullah, S.; Grodin, J.; Kumbhani, D.J.; Vo, M.; Luna, M.; et al. Meta-Analysis of Clinical Outcomes of Patients Who Underwent Percutaneous Coronary Interventions for Chronic Total Occlusions. Am. J. Cardiol. 2015, 115, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Werner, G.S.; Surber, R.; Kuethe, F.; Emig, U.; Schwarz, G.; Bahrmann, P.; Figulla, H.R. Collaterals and the recovery of left ventricular function after recanalization of a chronic total coronary occlusion. Am. Heart J. 2005, 149, 129–137. [Google Scholar] [CrossRef]
- Sirnes, P. Improvement in left ventricular ejection fraction and wall motion after successful recanalization of chronic coronary occlusions. Eur. Heart J. 1998, 19, 273–281. [Google Scholar] [CrossRef]
- Safley, D.M.; Koshy, S.; Grantham, J.A.; Bybee, K.A.; House, J.A.; Kennedy, K.F.; Rutherford, B.D. Changes in myocardial ischemic burden following percutaneous coronary intervention of chronic total occlusions. Catheter. Cardiovasc. Interv. 2011, 78, 337–343. [Google Scholar] [CrossRef]
- Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; Jüni, P.; Kastrati, A.; Koller, A.; Kristensen, S.D.; et al. The Task Force on myocardial revascularization of the European Society of Cardiology (ESC) and European Association for Cardio-Thoracic Surgery (EACTS). Thorac. Surg. 2019, 55, 4–90. [Google Scholar]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization. J. Am. Coll. Cardiol. 2022, 79, e21–e129. [Google Scholar]
- Henriques, J.P.S.; Hoebers, L.P.; Råmunddal, T.; Laanmets, P.; Eriksen, E.; Bax, M.; Ioanes, D.; Suttorp, M.J.; Strauss, B.H.; Barbato, E.; et al. Percutaneous Intervention for Concurrent Chronic Total Occlusions in Patients With STEMI. J. Am. Coll. Cardiol. 2016, 68, 1622–1632. [Google Scholar] [CrossRef]
- Van Veelen, A.; Coerkamp, C.F.; Somsen, Y.B.; Råmunddal, T.; Ioanes, D.; Laanmets, P.; van der Schaaf, R.J.; Eriksen, E.; Bax, M.; Suttorp, M.J.; et al. Ten-Year Outcome of Recanalization or Medical Therapy for Concomitant Chronic Total Occlusion After Myocardial Infarction. J. Am. Heart Assoc. 2024, 13, e033556. [Google Scholar] [CrossRef]
- Mashayekhi, K.; Nührenberg, T.G.; Toma, A.; Gick, M.; Ferenc, M.; Hochholzer, W.; Comberg, T.; Rothe, J.; Valina, C.M.; Löffelhardt, N.; et al. A Randomized Trial to Assess Regional Left Ventricular Function After Stent Implantation in Chronic Total Occlusion. JACC Cardiovasc. Interv. 2018, 11, 1982–1991. [Google Scholar] [CrossRef]
- Obedinskiy, A.A.; Kretov, E.I.; Boukhris, M.; Kurbatov, V.P.; Osiev, A.G.; Ibn Elhadj, Z.; Obedinskaya, N.R.; Kasbaoui, S.; Grazhdankin, I.O.; Prokhorikhin, A.A.; et al. The IMPACTOR-CTO Trial. JACC Cardiovasc. Interv. 2018, 11, 1309–1311. [Google Scholar] [CrossRef]
- Lee, S.W.; Lee, P.H.; Ahn, J.M.; Park, D.W.; Yun, S.C.; Han, S.; Kang, H.; Kang, S.J.; Kim, Y.H.; Lee, C.W.; et al. Randomized Trial Evaluating Percutaneous Coronary Intervention for the Treatment of Chronic Total Occlusion: The DECISION-CTO Trial. Circulation 2019, 139, 1674–1683. [Google Scholar] [CrossRef]
- Werner, G.S.; Martin-Yuste, V.; Hildick-Smith, D.; Boudou, N.; Sianos, G.; Gelev, V.; Rumoroso, J.R.; Erglis, A.; Christiansen, E.H.; Escaned, J.; et al. A randomized multicentre trial to compare revascularization with optimal medical therapy for the treatment of chronic total coronary occlusions. Eur. Heart J. 2018, 39, 2484–2493. [Google Scholar] [CrossRef]
- Werner, G.S.; Hildick-Smith, D.; Yuste, V.M.; Boudou, N.; Sianos, G.; Gelev, V.; Rumoroso, J.R.; Erglis, A.; Christiansen, E.H.; Escaned, J.; et al. Three-year outcomes of A Randomized Multicentre Trial Comparing Revascularization and Optimal Medical Therapy for Chronic Total Coronary Occlusions (EuroCTO). EuroIntervention 2023, 19, 571–579. [Google Scholar] [CrossRef]
- Juricic, S.A.; Tesic, M.B.; Galassi, A.R.; Petrovic, O.N.; Dobric, M.R.; Orlic, D.N.; Vukcevic, V.D.; Stankovic, G.R.; Aleksandric, S.B.; Tomasevic, M.V.; et al. Randomized Controlled Comparison of Optimal Medical Therapy with Percutaneous Recanalization of Chronic Total Occlusion (COMET-CTO). Int. Heart J. 2021, 62, 16–22. [Google Scholar] [CrossRef]
- Juricic, S.A.; Stojkovic, S.M.; Galassi, A.R.; Stankovic, G.R.; Orlic, D.N.; Vukcevic, V.D.; Milasinovic, D.G.; Aleksandric, S.B.; Tomasevic, M.V.; Dobric, M.R.; et al. Long-term follow-up of patients with chronic total coronary artery occlusion previously randomized to treatment with optimal drug therapy or percutaneous revascularization of chronic total occlusion (COMET-CTO). Front. Cardiovasc. Med. 2023, 9, 1014664. [Google Scholar] [CrossRef]
- Strauss, B.H.; Knudtson, M.L.; Cheema, A.N.; Galbraith, P.D.; Elbaz-Greener, G.; Abuzeid, W.; Henning, K.A.; Qiu, F.; Wijeysundera, H.C. Canadian Multicenter Chronic Total Occlusion Registry: Ten-Year Follow-Up Results of Chronic Total Occlusion Revascularization. Circ. Cardiovasc. Interv. 2021, 14, e010546. [Google Scholar] [CrossRef]
- Arnold, R.; Gervasoni, R.; Ledermann, B.; Moulis, L.; Soltani, S.; Lattuca, B.; Robert, P.; Leclercq, F. Long-Term Follow-up After Percutaneous Coronary Interventions for Chronic Total Occlusions; NCT06544174; University Hospital of Montpellier: Montpellier, France, 2025. [Google Scholar]
- Wu, E.B.; Brilakis, E.S.; Lo, S.; Kalyanasundaram, A.; Mashayekhi, K.; Kao, H.; Lim, S.; Ge, L.; Chen, J.; Qian, J.; et al. Advances in CrossBoss/Stingray use in antegrade dissection reentry from the Asia Pacific Chronic Total Occlusion Club. Catheter. Cardiovasc. Interv. 2020, 96, 1423–1433. [Google Scholar] [CrossRef]
- Xenogiannis, I.; Jaffer, F.A.; Shah, A.R.; Omer, M.; Megaly, M.; Vemmou, E.; Nikolakopoulos, I.; Rangan, B.; Garcia, S.; Lesser, J.R.; et al. Computed tomography angiography co-registration with real-time fluoroscopy in percutaneous coronary intervention for chronic total occlusions. EuroIntervention 2021, 17, e433–e435. [Google Scholar] [CrossRef]
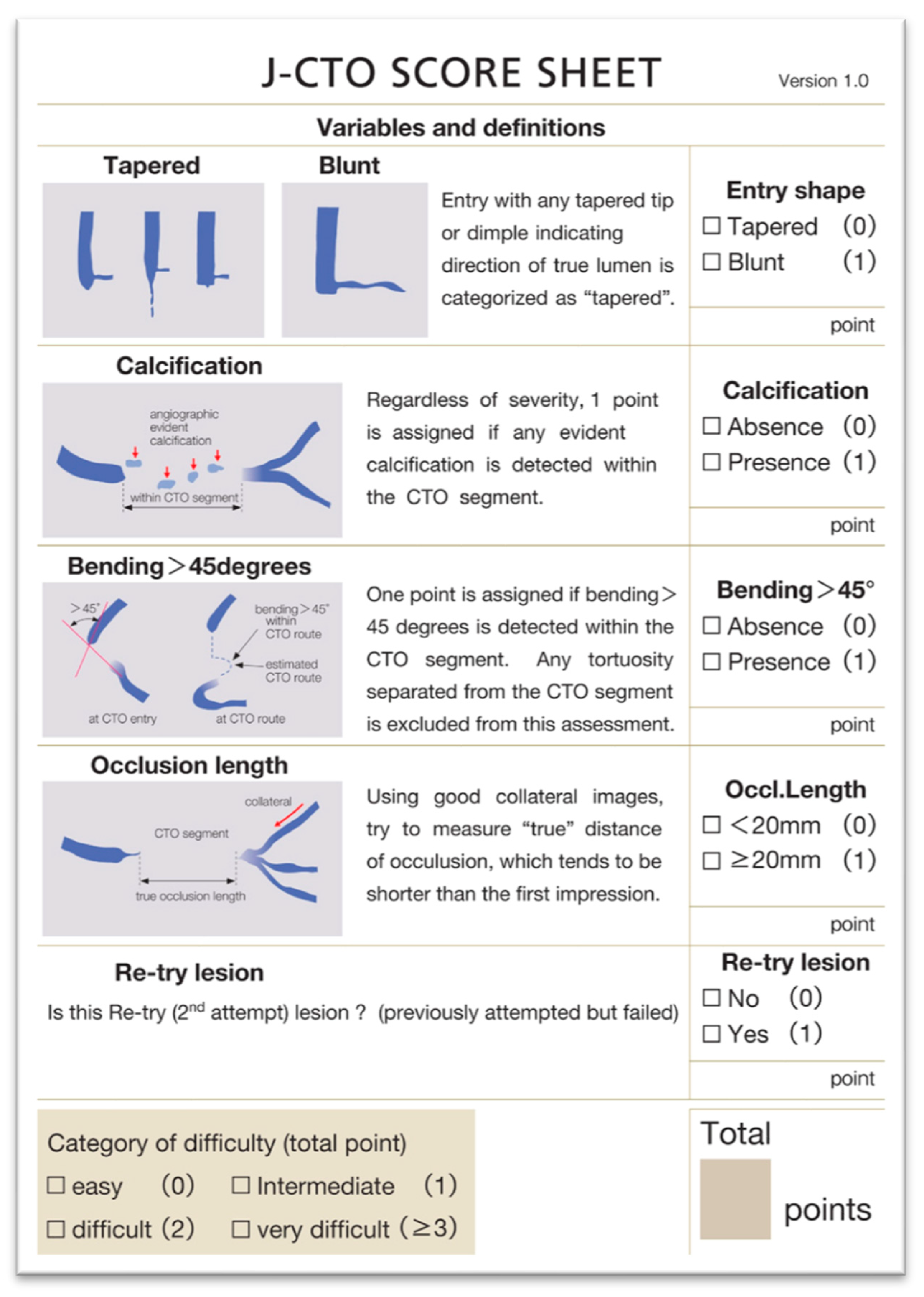
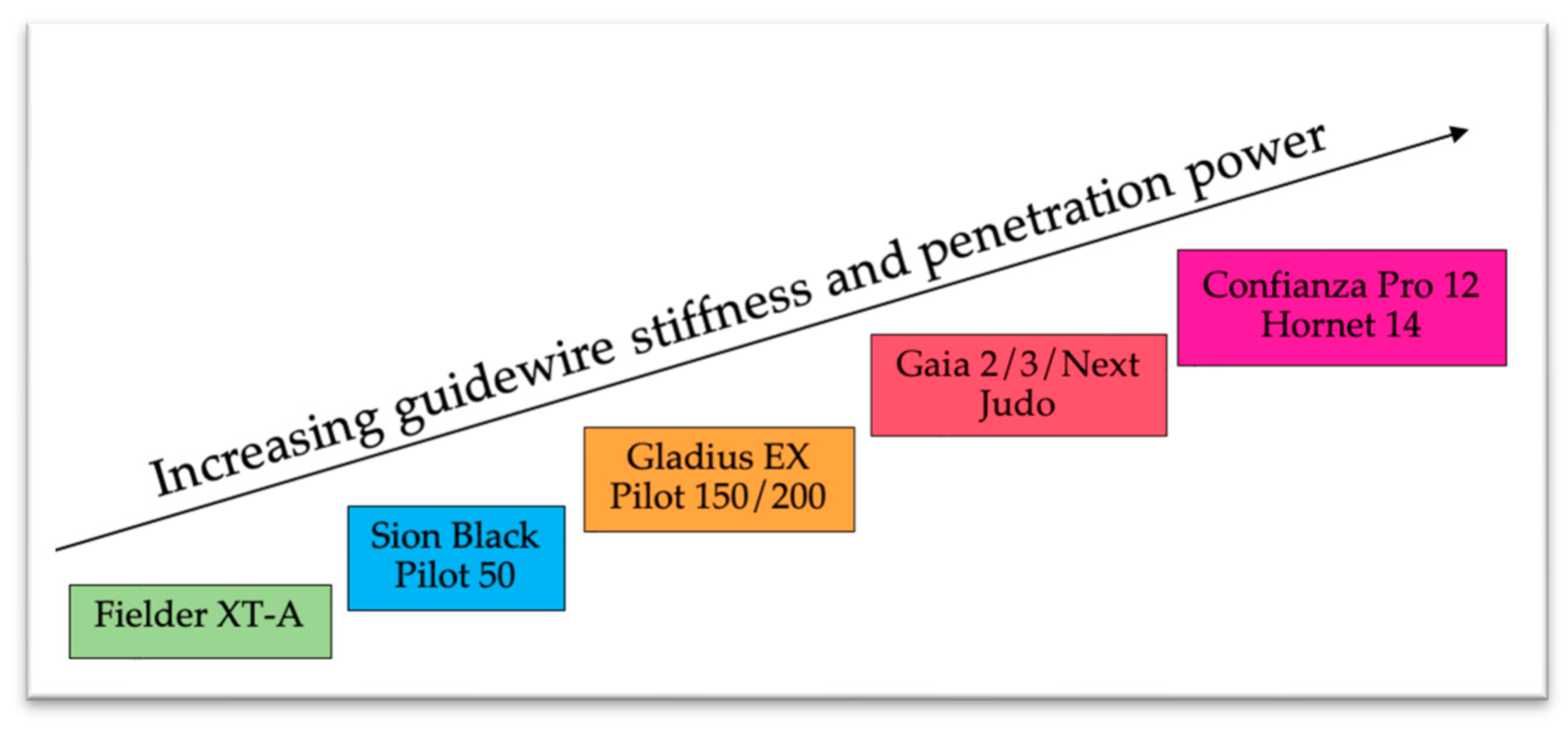
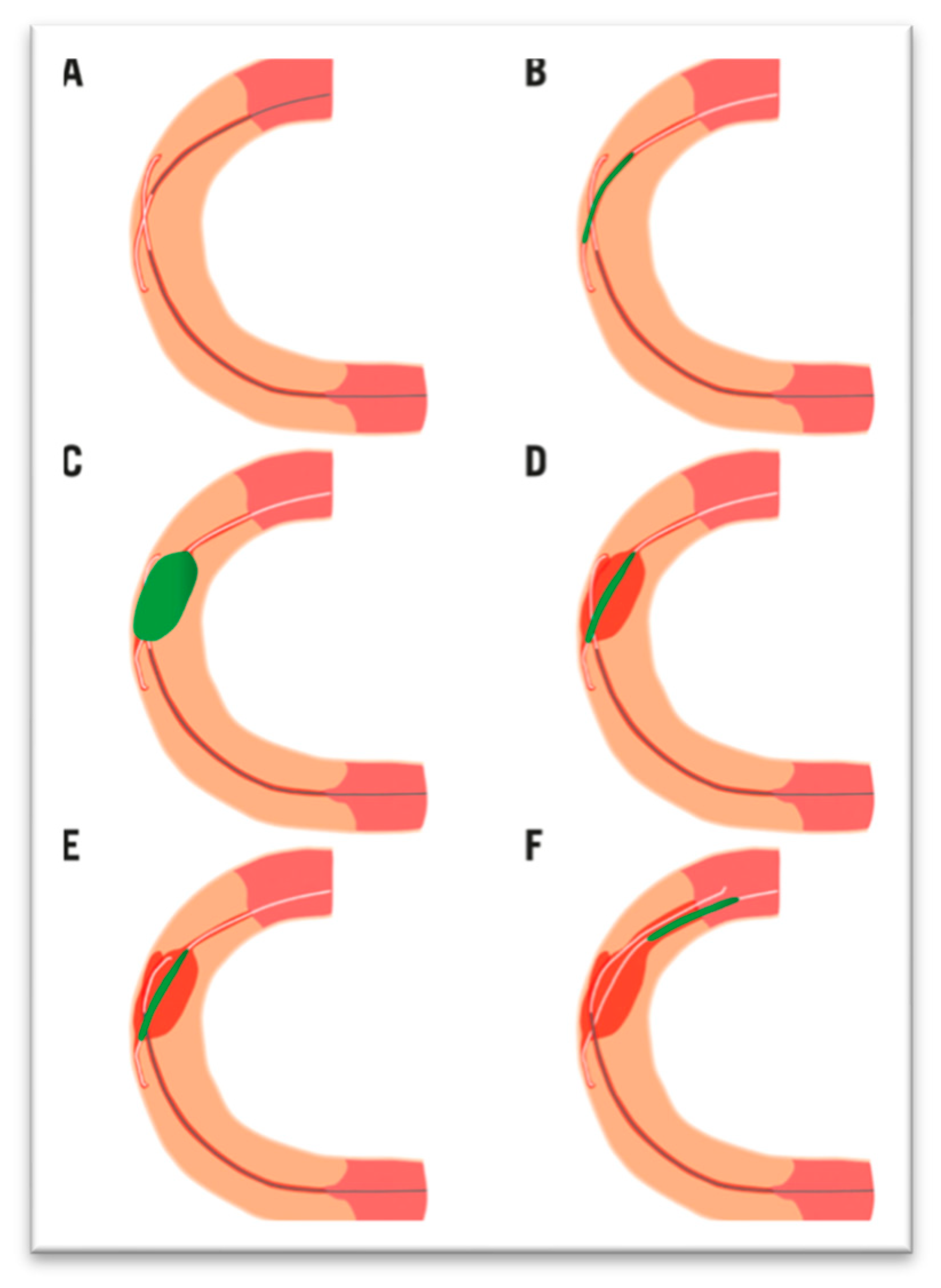
| Trials | Study Period | Population | Patients Included | Groups | Primary Endpoints Results | Secondary Endpoints Results |
|---|---|---|---|---|---|---|
| EXPLORE [36,37] | 2007–2015 | Patients with STEMI and concurrent CTO. | 304 | CTO-PCI vs. OMT | LVEF and LVEDV on cardiac MRI at 4 months: no difference between groups. In the ten-year outcomes EXPLORE trial, there was no difference in MACE (cardiac death, myocardial infarction, or CABG) at 10 years. | Infarct size and regional myocardial function: no difference between groups. Higher LVEF in LAD CTO-PCI group. No difference in MACE (cardiac death, myocardial infarction, or CABG) at 4 months. No difference in cardiac death at 4 months. In the ten-year outcomes EXPLORE trial, dyspnea relief was more frequent in the CTO-PCI group. No difference in all-cause death at 10 years. |
| REVASC [38] | 2007–2015 | Patients with symptoms and/or proof of ischemia. | 205 | CTO-PCI vs. OMT | Change in segmental wall thickening in the CTO territory at 6 months: no difference between groups. | Improvement of regional wall motion and changes in LV volumes and ejection fraction: no difference between groups. No difference in all-cause death at 12 months. Lower MACE (all-cause death, myocardial infarction, or any clinically driven repeat revascularization) at 12 months in the CTO-PCI group. |
| IMPACTOR-CTO [39] | 2010–2014 | Patients with stable angina and isolated RCA CTO. | 94 | CTO-PCI + OMT vs. OMT | Change in myocardial ischemia burden assessed with adenosine stress cardiac MRI at 12 months: lower in the CTO-PCI group. | Change in 6-min walk distance and QoL: improvement in the CTO-PCI group only. No difference in MACE (all-cause death, myocardial infarction, or unplanned revascularization) at 12 months. |
| DECISION-CTO [40] | 2010–2016 | Patients presenting with stable angina, silent ischemia, or ACS. | 834 | CTO-PCI + OMT vs. OMT | MACE (death, myocardial infarction, stroke, or any revascularization) at 4 years: no difference between groups. | No difference in QoL improvement in both groups (EQ-5D, SAQ). No difference in all-cause death at 4 years. |
| EURO-CTO [41,42] | 2012–2015 | Patients with symptoms and proof of myocardial viability. | 396 | CTO-PCI + OMT vs. OMT | Change in health status subscales as assessed by SAQ at 12 months: higher improvements in QoL, angina frequency, and freedom from angina subscales in the CTO-PCI group. In the three-year outcomes of the EURO-CTO trial, MACE was significantly higher in the OMT group (largely due to ischemia-driven revascularizations). | Better EQ-5D improvement in the CTO-PCI group Better CCS classification improvement in the CTO-PCI group. No difference in MACE (cardiac death, non-fatal myocardial infarction, and ischemia-driven target lesion revascularization) at 12 months. No difference in cardiac death at 3 years |
| COMET-CTO [43,44] | 2015–2017 | Patients with stable angina and/or proof of myocardial ischemia and/or proof of myocardial viability. | 100 | CTO-PCI + OMT vs. OMT | Change in QoL assessed with SAQ at 6 months: better improvements in all QoL subscales in the CTO-PCI group. In long-term, follow-up COMET-CTO trial, there was no difference in MACE (non-fatal myocardial infarction and recurrent revascularization) at 4.7 years. | No difference in LVEF improvement. No difference in MACE (non-fatal myocardial infarction and recurrent revascularization) at 275 days. No difference in cardiac death at 4.7 years. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arnold, R.; Gervasoni, R.; Leclercq, F. Chronic Total Occlusions: Current Approaches, Evidence and Outcomes. J. Clin. Med. 2025, 14, 4695. https://doi.org/10.3390/jcm14134695
Arnold R, Gervasoni R, Leclercq F. Chronic Total Occlusions: Current Approaches, Evidence and Outcomes. Journal of Clinical Medicine. 2025; 14(13):4695. https://doi.org/10.3390/jcm14134695
Chicago/Turabian StyleArnold, Remi, Richard Gervasoni, and Florence Leclercq. 2025. "Chronic Total Occlusions: Current Approaches, Evidence and Outcomes" Journal of Clinical Medicine 14, no. 13: 4695. https://doi.org/10.3390/jcm14134695
APA StyleArnold, R., Gervasoni, R., & Leclercq, F. (2025). Chronic Total Occlusions: Current Approaches, Evidence and Outcomes. Journal of Clinical Medicine, 14(13), 4695. https://doi.org/10.3390/jcm14134695






