Factors Associated with Changes in Skeletal Muscle Mass in Medical Health Checkups
Abstract
1. Introduction
2. Patients and Methods
2.1. Body Composition Analysis and Our Study Subjects
2.2. Type Classification According to the Daily Alcohol Consumed
2.3. Our Study and Ethical Approval
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Comparison of Change Rate in FF Index According to Age
3.3. Comparison of Change Rate in FF Index According to BMI
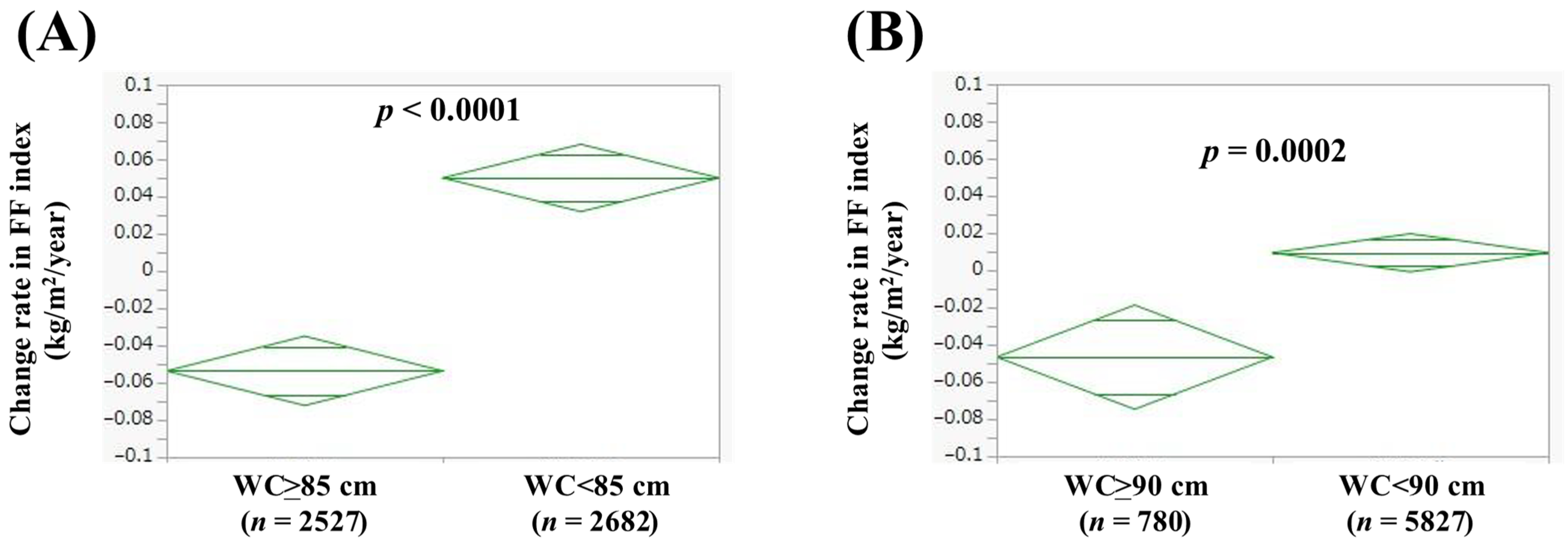
3.4. Comparison of Change Rate in FF Index According to WC
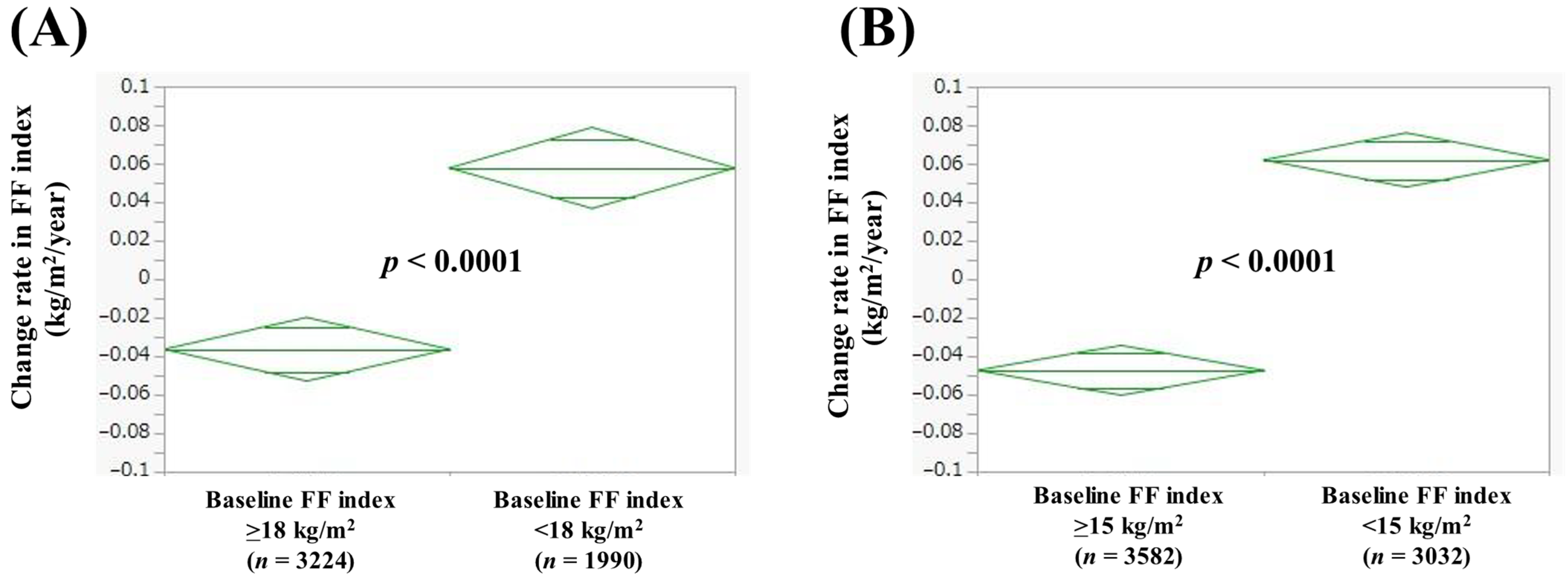
3.5. Comparison of Change Rate in FF Index According to Baseline FF Index
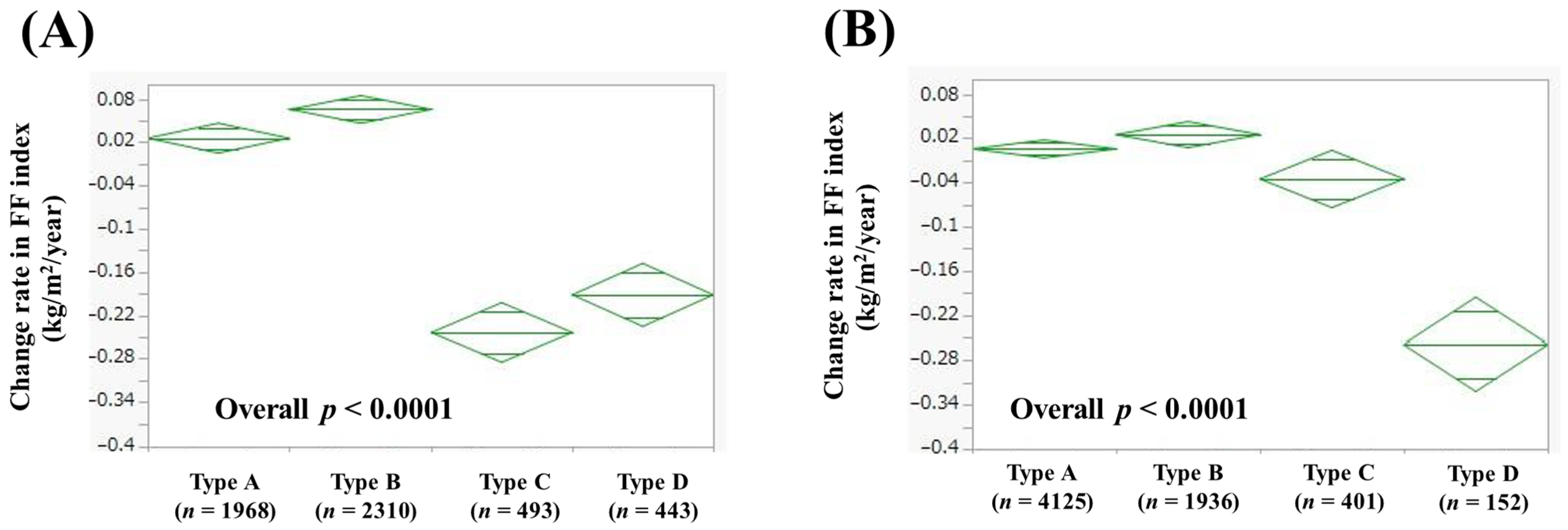
3.6. Comparison of Change Rate in FF Index According to Alcohol Intake
3.7. Univariate and Multivariate Analyses of Factors Linked to Change Rate in FF Index > 0 kg/m2/Year
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef]
- Nishikawa, H.; Fukunishi, S.; Asai, A.; Yokohama, K.; Nishiguchi, S.; Higuchi, K. Pathophysiology and mechanisms of primary sarcopenia (Review). Int. J. Mol. Med. 2021, 48, 156. [Google Scholar] [CrossRef]
- Franco-Romero, A.; Sandri, M.; Schiaffino, S. Autophagy in Skeletal Muscle. Cold Spring Harb. Perspect. Biol. 2025, 17, a041565. [Google Scholar] [CrossRef] [PubMed]
- Kirk, B.; Lombardi, G.; Duque, G. Bone and muscle crosstalk in ageing and disease. Nat. Rev. Endocrinol. 2025, 21, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Lees, M.J.; Prado, C.M.; Wischmeyer, P.E.; Phillips, S.M. Skeletal Muscle: A Critical Organ for Survival and Recovery in Critical Illness. Crit. Care Clin. 2025, 41, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Oliver, C.J.; Climstein, M.; Rosic, N.; Bosy-Westphal, A.; Tinsley, G.; Myers, S. Fat-Free Mass: Friend or Foe to Metabolic Health? J. Cachexia Sarcopenia Muscle 2025, 16, e13714. [Google Scholar] [CrossRef]
- Talani, C.; Astradsson, T.; Farnebo, L.; Mäkitie, A.; Ehrsson, Y.T.; Laurell, G. Pretreatment fat-free mass index correlates with early death in patients with head and neck squamous cell carcinoma. Head Neck 2024, 46, 808–818. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, Z.; Yao, Y.; Wang, S. Variation of All-Cause Mortality with Fat-Free Mass Index (FFMI) and Fat Mass Index (FMI) in Individuals with Asthma: Results from the NHANES Database Retrospective Cohort Study. J. Epidemiol. Glob. Health 2024, 14, 1555–1568. [Google Scholar] [CrossRef]
- Rinaldi, S.; Gilliland, J.; O’Connor, C.; Seabrook, J.A.; Mura, M.; Madill, J. Fat-Free Mass Index Controlled for Age and Sex and Malnutrition Are Predictors of Survival in Interstitial Lung Disease. Respiration 2021, 100, 379–386. [Google Scholar] [CrossRef]
- Yago, A.; Ohkura, Y.; Ueno, M.; Fujisawa, K.; Ogawa, Y.; Shimoyama, H.; Urabe, M.; Haruta, S.; Udagawa, H. Identification of Preoperative Fat-Free Mass Index for the Prognosis of Curatively Resected Esophageal Cancer. World J. Surg. 2022, 46, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Xie, H.; Liu, T.; Zhang, H.; Liu, C.; Li, X.; Bu, Z.; Liu, X.; Lin, S.; Chen, Y.; et al. Prognostic value of the fat-free mass index-based cachexia index in patients with colorectal cancer. Sci. Rep. 2024, 14, 24390. [Google Scholar] [CrossRef] [PubMed]
- Gotsman, I.; Keren, A.; Amir, O.; Zwas, D.R. Increased estimated fat-free mass and fat mass associated with improved clinical outcome in heart failure. Eur. J. Clin. Investig. 2022, 52, e13655. [Google Scholar] [CrossRef]
- Ding, N.S.; Tassone, D.; Al Bakir, I.; Wu, K.; Thompson, A.J.; Connell, W.R.; Malietzis, G.; Lung, P.; Singh, S.; Choi, C.R.; et al. Systematic Review: The Impact and Importance of Body Composition in Inflammatory Bowel Disease. J. Crohns Colitis 2022, 16, 1475–1492. [Google Scholar] [CrossRef]
- Li, Y.L.; Xin, S.Z.; Xiang, H.; Zhao, L.; Lu, L.; Xiu, M.L.; Ye, L.; Jing, C.; Ke, M.Z.; Hong, C.W.; et al. A cross-sectional survey study on the correlation analysis of nutritional status and intestinal flora in patients with esophageal cancer. Front. Nutr. 2024, 11, 1424039. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Shiraki, M.; Hiramatsu, A.; Moriya, K.; Hino, K.; Nishiguchi, S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. 2016, 46, 951–963. [Google Scholar] [CrossRef]
- Carvalho, L.; Junior, R.M.; Barreira, J.; Schoenfeld, B.J.; Orazem, J.; Barroso, R. Muscle hypertrophy and strength gains after resistance training with different volume-matched loads: A systematic review and meta-analysis. Appl. Physiol. Nutr. Metab. 2022, 47, 357–368. [Google Scholar] [CrossRef]
- Iijima, H.; Ambrosio, F.; Matsui, Y. Network-based systematic dissection of exercise-induced inhibition of myosteatosis in older individuals. J. Physiol. 2025, 603, 45–67. [Google Scholar] [CrossRef]
- Rahmati, M.; Lee, H.; Lee, H.; Park, J.; Vithran, D.T.A.; Li, Y.; Kazemi, A.; Boyer, L.; Fond, G.; Smith, L.; et al. Associations Between Exercise Training, Physical Activity, Sedentary Behaviour and Mortality: An Umbrella Review of Meta-Analyses. J. Cachexia Sarcopenia Muscle 2025, 16, e13772. [Google Scholar] [CrossRef]
- Kawakami, R.; Tanisawa, K.; Ito, T.; Usui, C.; Miyachi, M.; Torii, S.; Midorikawa, T.; Ishii, K.; Muraoka, I.; Suzuki, K.; et al. Fat-Free Mass Index as a Surrogate Marker of Appendicular Skeletal Muscle Mass Index for Low Muscle Mass Screening in Sarcopenia. J. Am. Med. Dir. Assoc. 2022, 23, 1955–1961.e3. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef] [PubMed]
- Matsui, M.; Fukuda, A.; Onishi, S.; Ushiro, K.; Nishikawa, T.; Asai, A.; Kim, S.K.; Nishikawa, H. Impact of Alcohol Intake on Skeletal Muscle: A Large Cross-Sectional Analysis in Japanese Adults. Nutrients 2025, 17, 894. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, K.; Iso, H. The criteria for metabolic syndrome and the national health screening and education system in Japan. Epidemiol. Health 2017, 39, e2017003. [Google Scholar] [CrossRef] [PubMed]
- Strasser, B.; Wolters, M.; Weyh, C.; Krüger, K.; Ticinesi, A. The Effects of Lifestyle and Diet on Gut Microbiota Composition, Inflammation and Muscle Performance in Our Aging Society. Nutrients 2021, 13, 2045. [Google Scholar] [CrossRef]
- Nishikawa, H.; Asai, A.; Fukunishi, S.; Nishiguchi, S.; Higuchi, K. Metabolic Syndrome and Sarcopenia. Nutrients 2021, 13, 3519. [Google Scholar] [CrossRef]
- Izzo, A.; Massimino, E.; Riccardi, G.; Della, P.G. A Narrative Review on Sarcopenia in Type 2 Diabetes Mellitus: Prevalence and Associated Factors. Nutrients 2021, 13, 183. [Google Scholar] [CrossRef]
- Tanaka, M.; Masuda, S.; Yamakage, H.; Inoue, T.; Ohue-Kitano, R.; Yokota, S.; Kusakabe, T.; Wada, H.; Sanada, K.; Ishii, K.; et al. Role of serum myostatin in the association between hyperinsulinemia and muscle atrophy in Japanese obese patients. Diabetes Res. Clin. Pract. 2018, 142, 195–202. [Google Scholar] [CrossRef]
- Yang, M.; Liu, C.; Jiang, N.; Liu, Y.; Luo, S.; Li, C.; Zhao, H.; Han, Y.; Chen, W.; Li, L.; et al. Myostatin: A potential therapeutic target for metabolic syndrome. Front. Endocrinol. 2023, 14, 1181913. [Google Scholar] [CrossRef]
- Prokopidis, K.; Witard, O.C. Understanding the role of smoking and chronic excess alcohol consumption on reduced caloric intake and the development of sarcopenia. Nutr. Res. Rev. 2022, 35, 197–206. [Google Scholar] [CrossRef]
- Tantai, X.; Liu, Y.; Yeo, Y.H.; Praktiknjo, M.; Mauro, E.; Hamaguchi, Y.; Engelmann, C.; Zhang, P.; Jeong, J.Y.; van Vugt, J.L.A.; et al. Effect of sarcopenia on survival in patients with cirrhosis: A meta-analysis. J. Hepatol. 2022, 76, 588–599. [Google Scholar] [CrossRef]
- Hanai, T.; Nishimura, K.; Unome, S.; Miwa, T.; Nakahata, Y.; Imai, K.; Suetsugu, A.; Takai, K.; Shimizu, M. Alcohol-associated liver disease increases the risk of muscle loss and mortality in patients with cirrhosis. J. Gastroenterol. 2024, 59, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Dasarathy, J.; McCullough, A.J.; Dasarathy, S. Sarcopenia in Alcoholic Liver Disease: Clinical and Molecular Advances. Alcohol. Clin. Exp. Res. 2017, 41, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Dolganiuc, A.; Thomes, P.G.; Ding, W.X.; Lemasters, J.J.; Donohue, T.M., Jr. Autophagy in alcohol-induced liver diseases. Alcohol. Clin. Exp. Res. 2012, 36, 1301–1308. [Google Scholar] [CrossRef]
- Ishii, K.; Ogawa, W.; Kimura, Y.; Kusakabe, T.; Miyazaki, R.; Sanada, K.; Satoh-Asahara, N.; Someya, Y.; Tamura, Y.; Ueki, K.; et al. Diagnosis of sarcopenic obesity in Japan: Consensus statement of the Japanese Working Group on Sarcopenic Obesity. Geriatr. Gerontol. Int. 2024, 24, 997–1000. [Google Scholar] [CrossRef]
- Hood, D.A.; Memme, J.M.; Oliveira, A.N.; Triolo, M. Maintenance of Skeletal Muscle Mitochondria in Health, Exercise, and Aging. Annu. Rev. Physiol. 2019, 81, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Matthie, J.R. Bioimpedance measurements of human body composition: Critical analysis and outlook. Expert. Rev. Med. Devices 2008, 5, 239–261. [Google Scholar] [CrossRef]


| Male (n = 5214) | Female (n = 6614) | p Value | |
|---|---|---|---|
| Age (years) | 52.4 ± 12.6 | 50.5 ± 11.5 | <0.0001 |
| BMI (kg/m2) | 23.9 ± 3.7 | 21.8 ± 3.7 | <0.0001 |
| Alcohol intake, type A/B/C/D | 1968/2310/493/443 | 4125/1936/401/152 | <0.0001 |
| WC (cm) | 85.5 ± 9.9 | 78.0 ± 10.0 | <0.0001 |
| 30 min or more exercise per day, yes/no/unknown | 1537/3508/169 | 5215/1281/118 | <0.0001 |
| Hemoglobin (g/dL) | 14.9 ± 1.1 | 13.1 ± 1.1 | <0.0001 |
| ALT (IU/L) | 26.9 ± 19.8 | 17.4 ± 11.7 | <0.0001 |
| GGT (IU/L) | 45.5 ± 54.3 | 23.2 ± 23.2 | <0.0001 |
| Serum albumin (g/dL) | 4.4 ± 0.3 | 4.3 ± 0.2 | <0.0001 |
| Triglyceride (mg/dL) | 118.9 ± 86.1 | 79.9 ± 50.6 | <0.0001 |
| FBS (mg/dL) | 95.9 ± 19.6 | 88.6 ± 13.0 | <0.0001 |
| Uric acid (mg/dL) | 6.2 ± 1.3 | 4.6 ± 1.0 | <0.0001 |
| eGFR (ml/min/1.73 m2) | 70.6 ± 13.2 | 73.7 ± 13.2 | <0.0001 |
| sBP (mmHg) | 122.6 ± 16.5 | 115.4 ± 17.2 | <0.0001 |
| dBP (mmHg) | 79.3 ± 12.5 | 72.2 ± 12.0 | <0.0001 |
| Baseline FF index (kg/m2) | 18.5 ± 1.5 | 15.1 ± 1.0 | <0.0001 |
| Change rate in FF index (kg/m2/year) | −0.0005 ± 0.48 | 0.0028 ± 0.40 | 0.2883 |
| Change Rate in FF Index > 0 kg/m2/year (n = 2550) | Change Rate in FF Index ≤ 0 kg/m2/year (n = 2664) | p Value | |
|---|---|---|---|
| Age (years) | 51.4 ± 12.6 | 53.4 ± 12.5 | <0.0001 |
| BMI (kg/m2) | 23.7 ± 3.5 | 24.2 ± 3.8 | <0.0001 |
| Alcohol intake Type A/B/C/D | 1023/1262/70/195 | 945/1048/423/248 | <0.0001 |
| WC (cm) | 84.7 ± 9.6 | 86.2 ± 10.2 | <0.0001 |
| 30 min or more exercise per day, yes/no/unknown | 726/1744/80 | 811/1764/89 | 0.1050 |
| Hemoglobin (g/dL) | 14.9 ± 1.1 | 14.9 ± 1.1 | 0.7808 |
| ALT (IU/L) | 25.6 ± 17.9 | 28.2 ± 21.3 | <0.0001 |
| GGT (IU/L) | 43.6 ± 54.8 | 47.3 ± 53.8 | 0.0119 |
| Serum albumin (g/dL) | 4.4 ± 0.3 | 4.4 ± 0.3 | 0.0974 |
| Triglyceride (mg/dL) | 116.1 ± 84.2 | 121.7 ± 87.9 | 0.0184 |
| FBS (mg/dL) | 94.3 ± 15.7 | 97.5 ± 22.7 | <0.0001 |
| Uric acid (mg/dL) | 6.2 ± 1.2 | 6.2 ± 1.3 | 0.5697 |
| eGFR (mL/min/1.73 m2) | 70.9 ± 13.4 | 70.4 ± 13.0 | 0.2186 |
| sBP (mmHg) | 121.8 ± 16.5 | 123.3 ± 16.5 | 0.0007 |
| dBP (mmHg) | 78.9 ± 12.3 | 79.7 ± 12.5 | 0.0176 |
| Baseline FF index (kg/m2) | 18.3 ± 1.5 | 18.6 ± 1.5 | <0.0001 |
| HR | 95% CI | p Value | |
|---|---|---|---|
| Age (per one years) | 0.989 | 0.984–0.994 | <0.0001 |
| BMI (per one kg/m2) | 0.864 | 0.811–0.921 | <0.0001 |
| Alcohol intake | |||
| Type A | 1.000 (reference) | ||
| Type B | 1.100 | 0.971–1.246 | 0.1345 |
| Type C | 0.153 | 0.117–0.201 | <0.0001 |
| Type D | 0.713 | 0.571–0.890 | 0.0027 |
| WC (per one cm) | 0.984 | 0.969–0.999 | 0.0365 |
| ALT (per one IU/L) | 0.993 | 0.989–0.996 | <0.0001 |
| GGT (per one IU/L) | 1.001 | 0.999–1.002 | 0.1850 |
| Triglyceride (per one mg/dL) | 1.000 | 0.999–1.001 | 0.4706 |
| FBS (per one mg/dL) | 0.995 | 0.991–0.998 | 0.0012 |
| sBP (per one mmHg) | 0.998 | 0.992–1.004 | 0.4740 |
| dBP (per one mmHg) | 1.006 | 0.998–1.014 | 0.1238 |
| Baseline FF index (per one kg/m2) | 0.706 | 0.637–0.781 | <0.0001 |
| Change Rate in FF Index > 0 kg/m2/year (n = 3258) | Change Rate in FF Index ≤ 0 kg/m2/year (n = 3356) | p Value | |
|---|---|---|---|
| Age (years) | 50.0 ± 11.4 | 51.1 ± 11.6 | <0.0001 |
| BMI (kg/m2) | 21.6 ± 3.6 | 22.0 ± 3.8 | 0.0001 |
| Alcohol intake Type A/B/C/D | 2048/995/196/19 | 2077/941/205/133 | <0.0001 |
| WC (cm) | 77.5 ± 9.7 | 78.5 ± 10.3 | 0.0002 |
| 30 min or more exercise per day, yes/no/unknown | 622/2588/48 | 659/2627/70 | 0.4929 |
| Hemoglobin (g/dL) | 13.1 ± 1.1 | 13.0 ± 1.2 | 0.0014 |
| ALT (IU/L) | 17.1 ± 10.9 | 17.8 ± 12.5 | 0.0517 |
| GGT (IU/L) | 23.1 ± 23.6 | 23.4 ± 22.7 | 0.5574 |
| Serum albumin (g/dL) | 4.3 ± 0.2 | 4.3 ± 0.2 | 0.0369 |
| Triglyceride (mg/dL) | 78.4 ± 48.7 | 81.5 ± 52.3 | 0.0390 |
| FBS (mg/dL) | 88.0 ± 11.1 | 89.3 ± 14.5 | 0.0085 |
| Uric acid (mg/dL) | 4.6 ± 1.0 | 4.6 ± 1.0 | 0.8223 |
| eGFR (mL/min/1.73 m2) | 73.7 ± 13.3 | 73.7 ± 13.2 | 0.9824 |
| sBP (mmHg) | 114.9 ± 16.7 | 116.0 ± 17.6 | 0.0362 |
| dBP (mmHg) | 71.9 ± 11.9 | 72.4 ± 12.1 | 0.0722 |
| Baseline FF index (kg/m2) | 15.0 ± 1.0 | 15.3 ± 1.0 | <0.0001 |
| HR | 95% CI | p Value | |
|---|---|---|---|
| Age (per one years) | 0.997 | 0.989–1.006 | 0.5546 |
| BMI (per one kg/m2) | 0.830 | 0.771–0.894 | <0.0001 |
| Alcohol intake | |||
| Type A | 1.000 (reference) | ||
| Type B | 1.049 | 0.881–1.249 | 0.5888 |
| Type C | 1.015 | 0.733–1.405 | 0.9286 |
| Type D | 0.096 | 0.038–0.242 | <0.0001 |
| WC (per one cm) | 0.983 | 0.964–1.003 | 0.0922 |
| Hemoglobin (per one g/dL) | 1.047 | 0.971–1.128 | 0.2362 |
| Serum albumin (per one g/dL) | 1.164 | 0.812–1.670 | 0.4081 |
| Triglyceride (per one mg/dL) | 0.997 | 0.995–0.999 | 0.0031 |
| FBS (per one mg/dL) | 0.990 | 0.983–0.997 | 0.0064 |
| sBP (per one mmHg) | 1.002 | 0.997–1.008 | 0.3744 |
| Baseline FF index (per one kg/m2) | 0.515 | 0.432–0.612 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onishi, S.; Fukuda, A.; Matsui, M.; Ushiro, K.; Nishikawa, T.; Asai, A.; Kim, S.K.; Yoshio, S.; Nishikawa, H. Factors Associated with Changes in Skeletal Muscle Mass in Medical Health Checkups. J. Clin. Med. 2025, 14, 4683. https://doi.org/10.3390/jcm14134683
Onishi S, Fukuda A, Matsui M, Ushiro K, Nishikawa T, Asai A, Kim SK, Yoshio S, Nishikawa H. Factors Associated with Changes in Skeletal Muscle Mass in Medical Health Checkups. Journal of Clinical Medicine. 2025; 14(13):4683. https://doi.org/10.3390/jcm14134683
Chicago/Turabian StyleOnishi, Saori, Akira Fukuda, Masahiro Matsui, Kosuke Ushiro, Tomohiro Nishikawa, Akira Asai, Soo Ki Kim, Sachiyo Yoshio, and Hiroki Nishikawa. 2025. "Factors Associated with Changes in Skeletal Muscle Mass in Medical Health Checkups" Journal of Clinical Medicine 14, no. 13: 4683. https://doi.org/10.3390/jcm14134683
APA StyleOnishi, S., Fukuda, A., Matsui, M., Ushiro, K., Nishikawa, T., Asai, A., Kim, S. K., Yoshio, S., & Nishikawa, H. (2025). Factors Associated with Changes in Skeletal Muscle Mass in Medical Health Checkups. Journal of Clinical Medicine, 14(13), 4683. https://doi.org/10.3390/jcm14134683







