What Is the Link Between Migraine and Hypothyroidism? A Systematic Literature Review
Abstract
1. Introduction
1.1. Background About Migraine
1.2. What Is Hypothyroidism?
1.3. The Purpose of the Review
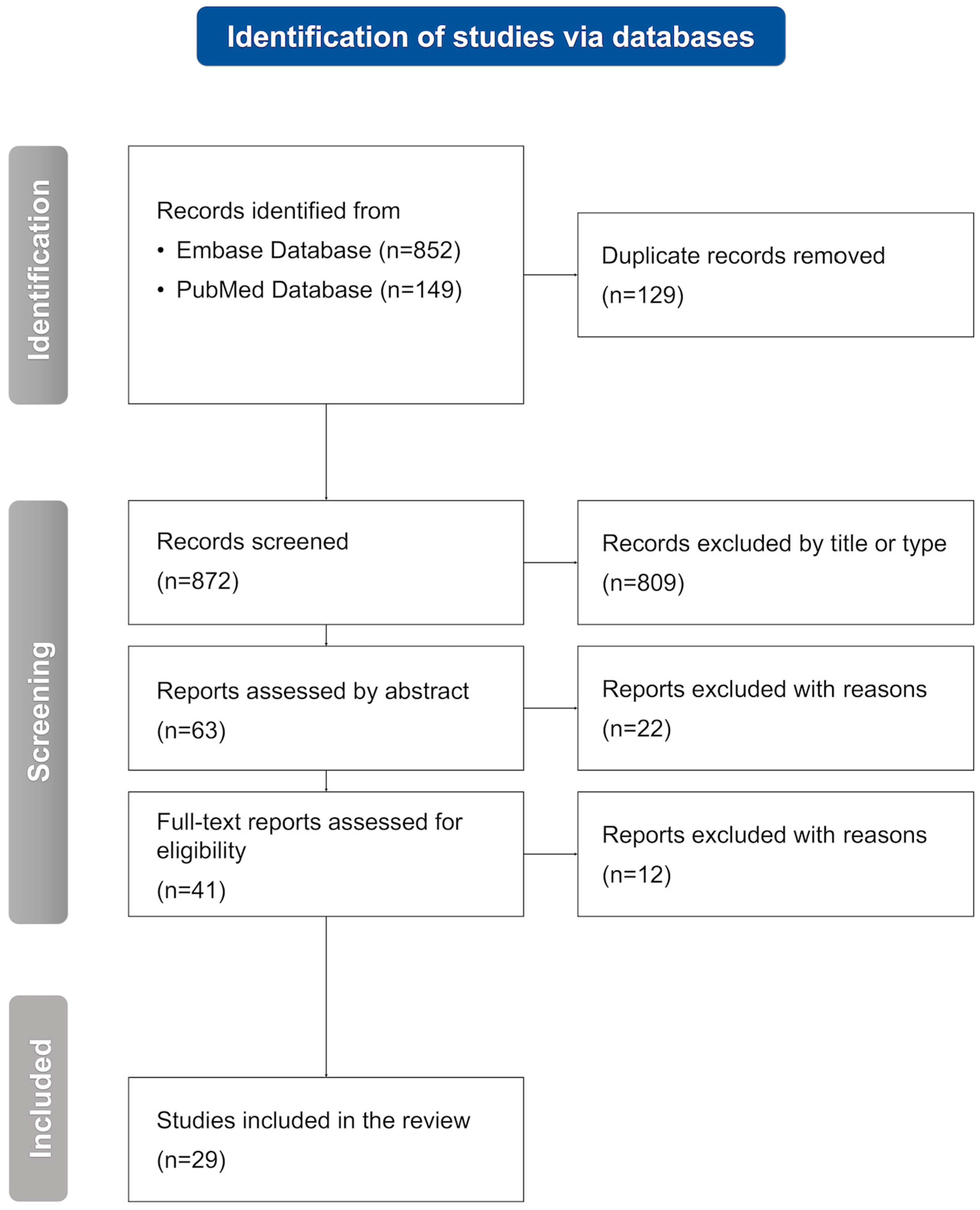
2. Methodology
2.1. Inclusion and Exclusion Criteria
2.2. Selection Process
2.3. Data Extraction and Data Synthesis
3. Findings
3.1. Correlation Between Thyroid Function and Headaches in General
3.2. Migraine and Hypothyroidism Prevalence
3.3. Comparison Between Migraine and Other Headache Types
3.4. Comparison Among Migraine Types
3.5. Levothyroxine Intake and Migraine Course
3.6. Hypothyroidism and Migraine in the Pediatric Population
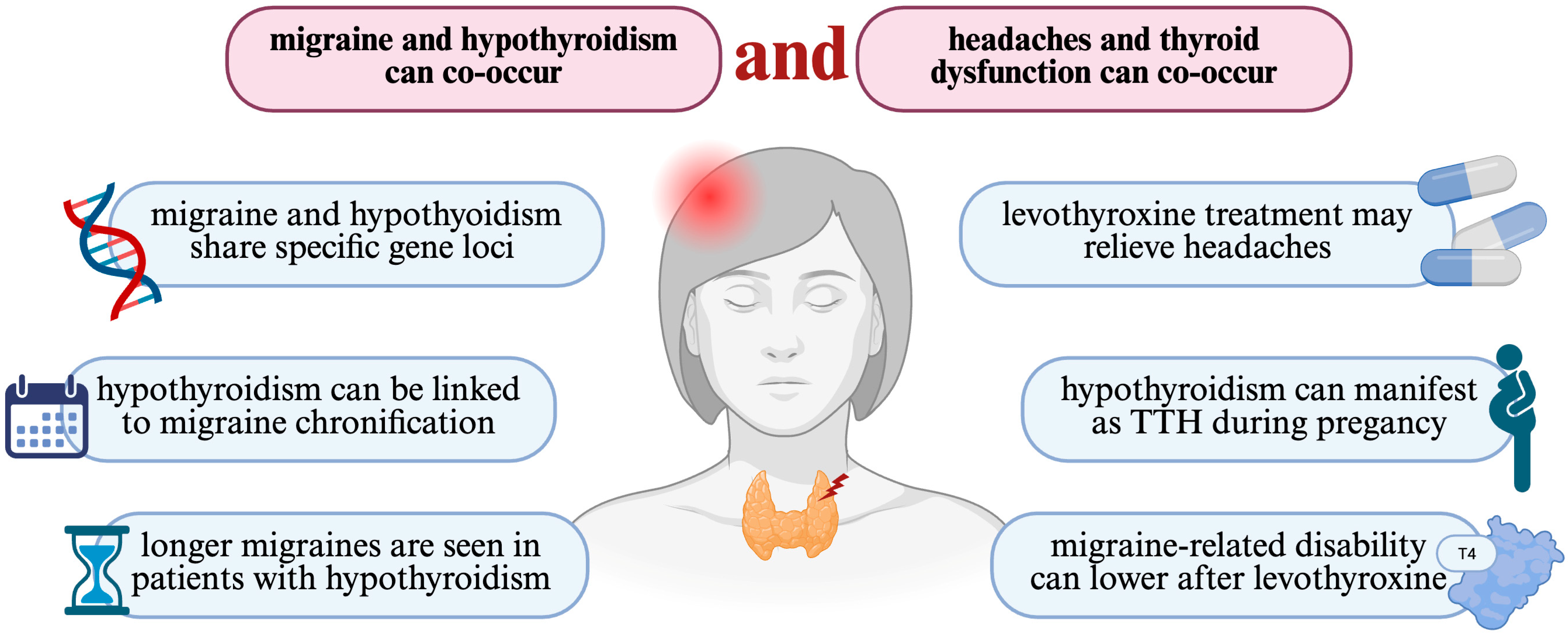
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACTH | adrenocorticotropic hormone |
| BMI | body mass index |
| CGRP | calcitonin gene-related peptide |
| CM | chronic migraine |
| EM | episodic migraine |
| GH | growth hormone |
| HAH | hypothyroidism-associated headaches |
| HD | Hashimoto’s disease |
| ICHD | International Classification of Headache Disorders |
| MIDAS | Migraine Disability Assessment Score |
| NDPH | new daily persistent headaches |
| PRISMA | Preferred Research Items for Systematic Reviews and Meta-Analyses |
| TRH | thyrotropin-releasing hormone |
| TSH | thyroid-stimulating hormone |
| TTH | tension-type headache |
References
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 691–693. [Google Scholar] [CrossRef]
- Kumar, A.; Kadian, R. Migraine Prophylaxis. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Petrušić, I.; Zidverc-Trajković, J. Redefining types of migraine aura. Cephalalgia 2021, 41, 274–275. [Google Scholar] [CrossRef] [PubMed]
- Pescador Ruschel, M.A.; De Jesus, O. Migraine Headache. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Lipton, R.B.; Bigal, M.E.; Diamond, M.; Freitag, F.; Reed, M.L.; Stewart, W.F. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007, 68, 343–349. [Google Scholar] [CrossRef]
- Buse, D.C.; Reed, M.L.; Fanning, K.M.; Bostic, R.C.; Lipton, R.B. Demographics, Headache Features, and Comorbidity Profiles in Relation to Headache Frequency in People With Migraine: Results of the American Migraine Prevalence and Prevention (AMPP) Study. Headache 2020, 60, 2340–2356. [Google Scholar] [CrossRef] [PubMed]
- Sacco, S.; Amin, F.M.; Ashina, M.; Bendtsen, L.; Deligianni, C.I.; Gil-Gouveia, R.; Katsarava, Z.; MaassenVanDenBrink, A.; Martelletti, P.; Mitsikostas, D.D.; et al. European Headache Federation guideline on the use of monoclonal antibodies targeting the calcitonin gene related peptide pathway for migraine prevention—2022 update. J. Headache Pain 2022, 23, 67. [Google Scholar] [CrossRef]
- Charles, A.C.; Digre, K.B.; Goadsby, P.J.; Robbins, M.S.; Hershey, A. Calcitonin gene-related peptide-targeting therapies are a first-line option for the prevention of migraine: An American Headache Society position statement update. Headache 2024, 64, 333–341. [Google Scholar] [CrossRef]
- Taylor, P.N.; Medici, M.M.; Hubalewska-Dydejczyk, A.; Boelaert, K. Hypothyroidism. Lancet 2024, 404, 1347–1364. [Google Scholar] [CrossRef]
- Vanderpump, M.P. The epidemiology of thyroid disease. Br. Med. Bull. 2011, 99, 39–51. [Google Scholar] [CrossRef]
- Chaker, L.; Razvi, S.; Bensenor, I.M.; Azizi, F.; Pearce, E.N.; Peeters, R.P. Hypothyroidism. Nat. Rev. Dis. Primers 2022, 8, 30. [Google Scholar] [CrossRef]
- Bartalena, L.; Piantanida, E.; Gallo, D.; Ippolito, S.; Tanda, M.L. Management of Graves’ hyperthyroidism: Present and future. Expert Rev. Endocrinol. Metab. 2022, 17, 153–166. [Google Scholar] [CrossRef]
- Basaria, S.; Cooper, D.S. Amiodarone and the thyroid. Am. J. Med. 2005, 118, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Czarnywojtek, A.; Zgorzalewicz-Stachowiak, M.; Czarnocka, B.; Sawicka-Gutaj, N.; Gut, P.; Krela-Kazmierczak, I.; Ruchala, M. Effect of lithium carbonate on the function of the thyroid gland: Mechanism of action and clinical implications. J. Physiol. Pharmacol. 2020, 71, 191–199. [Google Scholar] [CrossRef]
- Garmendia Madariaga, A.; Santos Palacios, S.; Guillén-Grima, F.; Galofré, J.C. The incidence and prevalence of thyroid dysfunction in Europe: A meta-analysis. J. Clin. Endocrinol. Metab. 2014, 99, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Wartofsky, L. Myxedema coma. Endocrinol. Metab. Clin. N. Am. 2006, 35, 687–698. [Google Scholar] [CrossRef]
- Cooper, D.S.; Biondi, B. Subclinical thyroid disease. Lancet 2012, 379, 1142–1154. [Google Scholar] [CrossRef]
- Rodondi, N.; Newman, A.B.; Vittinghoff, E.; de Rekeneire, N.; Satterfield, S.; Harris, T.B.; Bauer, D.C. Subclinical hypothyroidism and the risk of heart failure, other cardiovascular events, and death. Arch. Intern. Med. 2005, 165, 2460–2466. [Google Scholar] [CrossRef]
- Wassner, A.J. Pediatric Hypothyroidism: Diagnosis and Treatment. Paediatr. Drugs 2017, 19, 291–301. [Google Scholar] [CrossRef]
- Duntas, L.H.; Yen, P.M. Diagnosis and treatment of hypothyroidism in the elderly. Endocrine 2019, 66, 63–69. [Google Scholar] [CrossRef]
- Jakubiak, G.K.; Pawlas, N.; Morawiecka-Pietrzak, M.; Zalejska-Fiolka, J.; Stanek, A.; Cieślar, G. Relationship of Thyroid Volume and Function with Ankle-Brachial Index, Toe-Brachial Index, and Toe Pressure in Euthyroid People Aged 18–65. Medicina 2024, 60, 1445. [Google Scholar] [CrossRef]
- Effraimidis, G.; Watt, T.; Feldt-Rasmussen, U. Levothyroxine Therapy in Elderly Patients with Hypothyroidism. Front. Endocrinol. 2021, 12, 641560. [Google Scholar] [CrossRef]
- Spanou, I.; Bougea, A.; Liakakis, G.; Rizonaki, K.; Anagnostou, E.; Duntas, L.; Kararizou, E. Relationship of Migraine and Tension-Type Headache With Hypothyroidism: A Literature Review. Headache 2019, 59, 1174–1186. [Google Scholar] [CrossRef]
- Rubino, E.; Rainero, I.; Garino, F.; Vicentini, C.; Govone, F.; Vacca, A.; Gai, A.; Gentile, S.; Govone, G.; Ragazzoni, F.; et al. Subclinical hypothyroidism is associated with migraine: A case-control study. Cephalalgia 2019, 39, 15–20. [Google Scholar] [CrossRef]
- Tasnim, S.; Nyholt, D.R. Migraine and thyroid dysfunction: Co-occurrence, shared genes and biological mechanisms. Eur. J. Neurol. 2023, 30, 1815–1827. [Google Scholar] [CrossRef] [PubMed]
- Lima Carvalho, M.D.F.; De Medeiros, J.S.; Valença, M.M. Headache in recent onset hypothyroidism: Prevalence, characteristics and outcome after treatment with levothyroxine. Cephalalgia 2017, 37, 938–946. [Google Scholar] [CrossRef]
- Martin, A.T.; Pinney, S.M.; Xie, C.; Herrick, R.L.; Bai, Y.; Buckholz, J.; Martin, V.T. Headache Disorders May Be a Risk Factor for the Development of New Onset Hypothyroidism. Headache 2017, 57, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, B.; Haas, C.S.; Suefke, S.; Kropp, P.; Moenig, H. Headache and Depression in Patients with Hypothalamic-pituitary Disorders-etiology and Risk Factors. Exp. Clin. Endocrinol. Diabetes 2015, 123, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Abou Elmaaty, A.A.; Flifel, M.E.; Belal, T.; Zarad, C.A. Migraine and tension headache comorbidity with hypothyroidism in Egypt. Egypt. J. Neurol. Psychiatry Neurosurg. 2020, 56, 78. [Google Scholar] [CrossRef]
- Khan, H.B.; Shah, P.A.; Bhat, M.H.; Imran, A. Association of hypothyroidism in patients with migraine and tension-type headache disorders in Kashmir, North India. Neurol. Asia 2015, 20, 257–261. [Google Scholar]
- Matias-Guiu, J.; Fernandez, C.; Porta-Etessam, J.; Mateos, V.; Diaz-Insa, S. Factors associated with the differences in migraine prevalence rates between spanish regions. Sci. World J. 2014, 2014, 323084. [Google Scholar] [CrossRef]
- Wouters, H.J.C.M.; Wolffenbuttel, B.H.R.; Kobold, A.C.M.; Links, T.P.; Huls, G.; van der Klauw, M.M. Hypothyroidism, comorbidity and health-related quality of life: A population-based study. Endocr. Connect. 2023, 12, e230266. [Google Scholar] [CrossRef]
- Muddasir, B.; Maqbool, W.; Umer, F.; Bilal, P.; Younis, R. Sociodemographic and comorbidity profiles of migraine patients: An outpatient-based study in a tertiary care hospital. Asian J. Pharm. Clin. Res. 2020, 13, 54–59. [Google Scholar] [CrossRef]
- Panconesi, A.; Bartolozzi, M.L.; Guidi, L. O022. Migraineurs: Seriously ill or basically healthy? J. Headache Pain 2015, 16, 1. [Google Scholar] [CrossRef]
- Tietjen, G.E.; Herial, N.A.; Hardgrove, J.; Utley, C.; White, L. Migraine comorbidity constellations. Headache 2007, 47, 857–865. [Google Scholar] [CrossRef]
- Tasnim, S.; Wilson, S.G.; Walsh, J.P.; Nyholt, D.R. Shared genetics and causal relationships between migraine and thyroid function traits. Cephalalgia 2023, 43, 03331024221139253. [Google Scholar] [CrossRef]
- Hautakangas, H.; Winsvold, B.S.; Ruotsalainen, S.E.; Bjornsdottir, G.; Harder, A.V.E.; Kogelman, L.J.A.; Thomas, L.F.; Noordam, R.; Benner, C.; Gormley, P.; et al. Genome-wide analysis of 102,084 migraine cases identifies 123 risk loci and subtype-specific risk alleles. Nat. Genet. 2022, 54, 152–160. [Google Scholar] [CrossRef]
- Teumer, A.; Chaker, L.; Groeneweg, S.; Li, Y.; Di Munno, C.; Barbieri, C.; Schultheiss, U.T.; Traglia, M.; Ahluwalia, T.S.; Akiyama, M.; et al. Genome-wide analyses identify a role for SLC17A4 and AADAT in thyroid hormone regulation. Nat. Commun. 2018, 9, 4455. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, M. Thyroid stimulating hormone level in migraine headache. J. Neurol. Sci. 2021, 429, 119321. [Google Scholar] [CrossRef]
- Fernandez, L.; Marfil, A. Hypothyroidism as comorbidity of chronic headaches. PREMECEF results. Headache 2020, 60, 37–38. [Google Scholar] [CrossRef]
- Gözübatik Çelik, R.G.; Uludüz Ulu, D.; Hatipoğlu, E.; Hacıoğlu, Y.; Alparslan Türk, B.G.; Sungur, M.A.; Göksan, B.; Saip, S.; Siva, A. The frequency and related factors of primary headaches in patients with Hashimoto thyroiditis. Agri J. Turk. Soc. Algol. 2022, 34, 292–297. [Google Scholar] [CrossRef]
- Bigal, M.E.; Sheftell, F.D.; Rapoport, A.M.; Tepper, S.J.; Lipton, R.B. Chronic daily headache: Identification of factors associated with induction and transformation. Headache 2002, 42, 575–581. [Google Scholar] [CrossRef]
- Filipchuk, M.; Gassmann, J.; Castro Zamparella, T.; Tibaldo, M.C.; Carpinella, M.; Sesto Tagliavini, P.; Scarnato, P.; Goicochea, M.T.; Bruera, O.; Conci Magris, D.M.; et al. High rates of (treated) hypothyroidism among chronic migraine patients consulting a specialized headache clinic: Are we missing something? Neurol. Sci. 2022, 43, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Nowaczewska, M.; Straburzyński, M.; Meder, G.; Waliszewska-Prosół, M. The relationship between migraine and Hashimoto’s thyroiditis: A single center experience. Front. Neurol. 2024, 15, 1370530. [Google Scholar] [CrossRef] [PubMed]
- Spierings, E.L.H.; Padamsee, A. Menstrual-Cycle and Menstruation Disorders in Episodic vs Chronic Migraine: An Exploratory Study. Pain Med. 2015, 16, 1426–1432. [Google Scholar] [CrossRef]
- Togha, M.; Karimitafti, M.J.; Ghorbani, Z.; Farham, F.; Naderi-Behdani, F.; Nasergivehchi, S.; Vahabi, Z.; Ariyanfar, S.; Jafari, E. Characteristics and comorbidities of headache in patients over 50 years of age: A cross-sectional study. BMC Geriatr. 2022, 22, 313. [Google Scholar] [CrossRef]
- Spanou, I.; Christidi, F.; Liakakis, G.; Rizonaki, K.; Bougea, A.; Anagnostou, E.; Kararizou, E. Primary headache subtypes and thyroid dysfunction: Is there any association? Arq. Neuro-Psiquiatr. 2020, 78, 695–699. [Google Scholar] [CrossRef]
- Li, R.; Han, J.; Shao, G.; Liu, C.; Li, S.; Wang, M.; Yang, D. Causality between multiple autoimmune disorders and migraine and its subtypes: A two-sample Mendelian randomization study. Front. Neurol. 2024, 15, 1420201. [Google Scholar] [CrossRef] [PubMed]
- Dev, P.; Favas, T.T.; Jaiswal, R.; Cyriac, M.; Mishra, V.N.; Pathak, A. The effect of low dose thyroid replacement therapy in patients with episodic migraine and subclinical hypothyroidism: A randomised placebo-controlled trial. Cephalalgia 2023, 43, 03331024231182684. [Google Scholar] [CrossRef]
- Hepp, Z.; Lage, M.J.; Espaillat, R.; Gossain, V.V. The association between adherence to levothyroxine and economic and clinical outcomes in patients with hypothyroidism in the US. J. Med. Econ. 2018, 21, 912–919. [Google Scholar] [CrossRef]
- Mirouliaei, M.; Fallah, R.; Bashardoost, N.; Partovee, M.; Ordooei, M. Efficacy of Levothyroxine in Migraine Headaches in Children with Subclinical Hypothyroidism. Iran. J. Child. Neurol. 2012, 6, 23–26. [Google Scholar]
- Fallah, R.; Mirouliaei, M.; Bashardoost, N.; Partovee, M. Frequency of subclinical hypothyroidism in 5- to 15-year-old children with migraine headache. J. Pediatr. Endocrinol. Metab. 2012, 25, 859–862. [Google Scholar] [CrossRef]
- Hassan, M.A.E.; El-Gharieb, H.A.; Nasr, M.; Abdelhay, W.M.; Yousef, T.S.M.; El-Zamek, H.M.F.; Zidan, A.M.; Nady, M.; Abdel-Kareem, M.A.; Hasan, A. Potential Association between Subclinical Hypothyroidism and Childhood Migraine. Medicina 2022, 58, 1346. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.S.; Lee, J.; Choi, J.H.; Kwon, H.H.; Kang, J.W. Clinical manifestations of headache in children younger than 7 years. Korean J. Pediatr. 2018, 61, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Ekici, B.; Cebeci, A.N. The debate on the link between subclinical hypothyroidism and childhood migraine: Is initial endocrinological evaluation necessary for children with migraine? Acta Neurol. Belg. 2015, 115, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Biscetti, L.; De Vanna, G.; Cresta, E.; Bellotti, A.; Corbelli, I.; Letizia Cupini, M.; Calabresi, P.; Sarchielli, P. Immunological findings in patients with migraine and other primary headaches: A narrative review. Clin. Exp. Immunol. 2022, 207, 11–26. [Google Scholar] [CrossRef]
- Körtési, T.; Spekker, E.; Vécsei, L. Exploring the Tryptophan Metabolic Pathways in Migraine-Related Mechanisms. Cells 2022, 11, 3795. [Google Scholar] [CrossRef]
- Bauer, M.; Heinz, A.; Whybrow, P.C. Thyroid hormones, serotonin and mood: Of synergy and significance in the adult brain. Mol. Psychiatry 2002, 7, 140–156. [Google Scholar] [CrossRef]
| Ref. | Year | Population | Comparison | Findings | HT Diagnosis |
|---|---|---|---|---|---|
| Lima-Carvalho et al. [26] | 2017 | 73 pts with HT of recent onset and HAH | 140 pts with HT of recent onset without HAH | 53% HAH pts had a prior history of MIG compared to 38% non-HAH pts; after 12 months of levothyroxine therapy, 78% of patients with HAH had a reduction in headache frequency or complete remission | assessment of thyroid hormones levels |
| Martin et al. [27] | 2017 | 2021 pts with headache disorders | 6391 pts without headache disorders | 21% increased risk of developing new onset HT in the study group; 41% increased risk of developing HT in pts with possible MIG | assessment of thyroid hormones levels |
| Harbeck et al. [28] | 2015 | 121 HPD pts | 115 CD pts | ACTH deficiency pts had a headache prevalence of 24.5% compared to 43.5% in normal ACTH pts; pts with GH deficiency had a headache prevalence of 22.5% compared to 42% in normal GH pts; pts with TSH deficiency had a headache prevalence of 25% compared to 42.5% in normal TSH pts | assessment of thyroid hormones levels |
| Ref. | Year | Population | Comparison | Findings | HT Diagnosis |
|---|---|---|---|---|---|
| Abou-Elmaaty et al. [29] | 2020 | 94 MIG pts | 100 HC | subclinical or overt HT in 30.8% MIG pts vs. 10% HC; abnormal thyroid ultrasound in 10.6% MIG pts vs. 3% HC | assessment of thyroid hormones and thyroid ultrasound |
| Khan et al. [30] | 2015 | 86 MIG pts | 500 HC | HT in 33.7% MIG pts vs. 11.2% HC with subclinical HT and 1.2% HC with HT | assessment of thyroid hormones |
| Matias-Guiu et al. [31] | 2014 | 31,300 households | negative correlation between MIG pts and hypo/hyperthyroidism | database of the National Statistics Institute of Spain | |
| Wouters et al. [32] | 2023 | 4537 thyroid hormone users among 147,201 adult pts | 142,664 nonusers | MIG in 23.7% thyroid hormone users vs. 17.8% nonusers | questionnaires, physical examination, biochemical measurements, and verified medication use |
| Muddasir et al. [33] | 2020 | 323 MIG pts | - | 9.9% MIG pts with HT | questionnaire |
| Panconesi et al. [34] | 2015 | 1000 MIG pts | - | 6.4% MIG pts with HT | survey |
| Tietjen et al. [35] | 2007 | 223 MIG pts; 55 pts in group 1 (hypertension, hyperlipidemia, DM, and HT) | HT in 27% pts in group 1; group 1 had a higher proportion of males (22%), had an older median age (52 years) and had a later age of headache onset (median years: 22) | questionnaire | |
| Tasnim et al. [36] | 2023 | a positive genetic correlation between MIG and HT, MIG and secondary HT, and MIG and fT4 levels | the GWAS summary statistics from the latest migraine GWAS [37]; statistics for HT, and secondary HT from PANUK Biobank; statistics for TSH and fT4 from GWAS data [38] |
| Ref. | Year | Population | Comparison | Findings | HT Diagnosis |
|---|---|---|---|---|---|
| Bhattacharjee et al. [39] | 2021 | 50 MIG pts | 50 non-MIG pts | high TSH level higher in MIG pts compared to HC; subclinical HT more prevalent in MIG pts than in HC | survey, family history |
| L.E. Fernández-Garza et al. [40] | 2020 | 792 pts with 1 headache diagnosis | 73 pts with 2 headache diagnoses; 4 pts with 3 diagnoses | 35 pts with confirmed HT diagnosis | clinical interview |
| Gozubatik Celik et al. [41] | 2022 | 95 HD pts with headaches (38 HRH pts, 57 PH pts) (21 MIG pts, 17 TTH pts, 20 NDPH pts) | 60 HD pts without headache disorders | subclinical HT in 22% with PH; overt HT in 7.2% with PH | clinical observation and interview |
| Bigal et al. [42] | 2002 | 791 pts | 399 CM pts had analgesic overuse, 158 CM pts without analgesic overuse, 69 NDPH pts, 100 EM pts, 65 CPTH pts | the transformation of episodic headaches into CDH, or the development of NDPH, was associated with factors beyond just medication overuse | HT diagnosed in people with CM, NDPH |
| Ref. | Year | Population | Comparison | Findings | HT Diagnosis |
|---|---|---|---|---|---|
| Filipchuk et al. [43] | 2022 | 67 EM pts | 44 CM pts | HT in 8.96% EM pts vs. 29.55% CM pts | the medical records of MIG patients |
| Nowaczewska et al. [44] | 2024 | 592 EM pts | 336 CM pts; | CM in 47.2% pts with HD vs. 34.8% pts without HD; HD in 9.46% EM pts vs. 14.88% CM pts | the medical records of MIG patients |
| Bigal et al. [42] | 2002 | 100 EM pts, 399 CM with MOH, 158 CM without MOH | 69 de novo development of NDPH, 65 chronic posttraumatic headache | strong correlations between EM and CM pts and HT (odds ratios of 8.4) | the medical records of MIG patients |
| Spierings et al. [45] | 2015 | 45 EM pts | 51 CM pts | HT in 11.1% EM pts vs. 11.8% CM pts | questionnaire |
| Togha et al. [46] | 2022 | 198 EM pts | 61 CM pts | HT in 25.3% EM pts vs. 24.6% CM pts | questionnaire |
| Spanou et al. [47] | 2020 | 253 MO pts, 49 MA pts | 53 TTH pts, 29 MOH pts, 23 mixed-type headache pts (MA/MO and TTH), 9 CH pts, and 11 pts with other primary headaches | no significant association between headache subtypes and thyroid dysfunction; HT in 4% MIG pts | the medical records of MIG patients |
| Li et al. [48] | 2024 | 3541 MA pts | 3215 MO pts | a positive causal association between HT and MO | statistics from Europe’s largest genome-wide association study |
| Rubino et al. [24] | 2018 | 151 HT pts | - | MO in 37.75% and MA in 8.61% HT pts | interview and biochemical parameters |
| Ref. | Year | Population | Comparison | Findings | HT Diagnosis |
|---|---|---|---|---|---|
| Dev et al. [49] | 2023 | 43 MIG and subclinical HT pts treated with LT4 | 44 MIG and subclinical HT pts treated with placebo | ↓ headache frequency and severity, ↓ MIDAS score, ↓ MIDAS grade in LT4 pts compared to placebo pts | assessment of thyroid hormones |
| Hepp et al. [50] | 2018 | 159,314 HT pts nonadherent to LT4 treatment | 159,314 HT pts adherent to LT4 treatment | study group more likely to suffer from MIG compared to control group | not given |
| Mirouliaei et al. [51] | 2012 | 25 MIG and subclinical HT pts | before and after LT4 treatment | ↓ headache frequency and severity after LT4 treatment compared to before | assessment of thyroid hormones |
| Ref. | Year | Population | Comparison | Findings | HT Diagnosis |
|---|---|---|---|---|---|
| Ekici et al. [55] | 2015 | 98 pediatric MIG pts | - | only 5 pts with subclinical HT | assessment of thyroid hormones |
| Fallah et al. [52] | 2012 | 104 pediatric MIG pts | - | 25 pts with subclinical HT; ↑ headache frequency and duration in subclinical HT pts vs. those without | assessment of thyroid hormones |
| Hassan et al. [53] | 2022 | 100 pediatric MIG pts | 100 non-MIG pts | subclinical HT in 17 MIG pts vs. 2 non-MIG pts; overt HT in 2 MIG pts vs. 0 non-MIG pts; obesity and overweight more frequent in MIG pts vs. non-MIG pts subclinical HT in 77% obese/overweight MIG pts vs. 8% normal body weight pts; overt HT in 8% obese/overweight pts vs. 0% normal body weight pts | assessment of thyroid hormones |
| Kang et al. [54] | 2018 | 146 pediatric headache pts | 39 primary headache pts (16 MIG pts, 18 probable MIG pts, 5 TTH pts); 18 secondary headache pts; 89 unclassified headache pts | mean symptom duration: 5.8 ± 7.9 months, attack frequency: 15.1 ± 10.6 times per month mean pain severity score: 5.1 ± 2.2 in VAS | assessment of thyroid hormones |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalik, M.; Łapicka, J.; Sota, M.; Zawieska, J.; Grodzka, O.; Kępczyńska, K. What Is the Link Between Migraine and Hypothyroidism? A Systematic Literature Review. J. Clin. Med. 2025, 14, 4645. https://doi.org/10.3390/jcm14134645
Michalik M, Łapicka J, Sota M, Zawieska J, Grodzka O, Kępczyńska K. What Is the Link Between Migraine and Hypothyroidism? A Systematic Literature Review. Journal of Clinical Medicine. 2025; 14(13):4645. https://doi.org/10.3390/jcm14134645
Chicago/Turabian StyleMichalik, Martyna, Justyna Łapicka, Marcin Sota, Julia Zawieska, Olga Grodzka, and Katarzyna Kępczyńska. 2025. "What Is the Link Between Migraine and Hypothyroidism? A Systematic Literature Review" Journal of Clinical Medicine 14, no. 13: 4645. https://doi.org/10.3390/jcm14134645
APA StyleMichalik, M., Łapicka, J., Sota, M., Zawieska, J., Grodzka, O., & Kępczyńska, K. (2025). What Is the Link Between Migraine and Hypothyroidism? A Systematic Literature Review. Journal of Clinical Medicine, 14(13), 4645. https://doi.org/10.3390/jcm14134645







