From Microstructure to Shade Shift: Confocal and Spectrophotometric Evaluation of Peroxide-Induced Dental Bleaching
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Size
2.2. Specimen Preparation
2.3. Macroscopic Visualization of Dental Samples Used in the Study
2.4. Bleaching Procedure
- Opalescence Quick (Ultradent Products Inc., South Jordan, UT, USA) contains 45% carbamide peroxide (a slower-acting bleaching compound that decomposes into hydrogen peroxide and urea. The gel is white, ready to use, and does not require mixing or light activation, making it suitable for short, in-office procedures. Opalescence Quick (45% PC, Ultradent, USA) was applied directly to the enamel surface in a 1–2 mm layer for 15–30 min per session, without light activation.
- Opalescence Boost (Ultradent Products Inc., South Jordan, UT, USA) is a chemically activated in-office bleaching agent containing 40% hydrogen peroxide (HP). It is supplied in a dual-syringe system that ensures fresh mixing of the peroxide gel immediately prior to application. The product is applied without the need for light activation and has a characteristic red color. Opalescence Boost (40% HP, Ultradent, South Jordan, UT, USA) was applied in a 1–2 mm layer, with one application lasting 20 min, two applications totaling 40 min, and three applications up to 60 min per session (not exceeding three applications per session), without light activation.
- BlancOne Ultra+ (IDS Spa, Savona, Italy) is a professional in-office whitening system based on 35% HP, supplied in powder form within a mixing container. The bleaching agent requires manual preparation by adding and mixing the provided H2O2 solution with the powder. The final product has an orange color and is intended for immediate application after activation. BlancOne Ultra+ (35% HP, IDS, Italy) was applied in a 1–2 mm layer, with light activation for 8–10 min per application, totaling three applications per session.
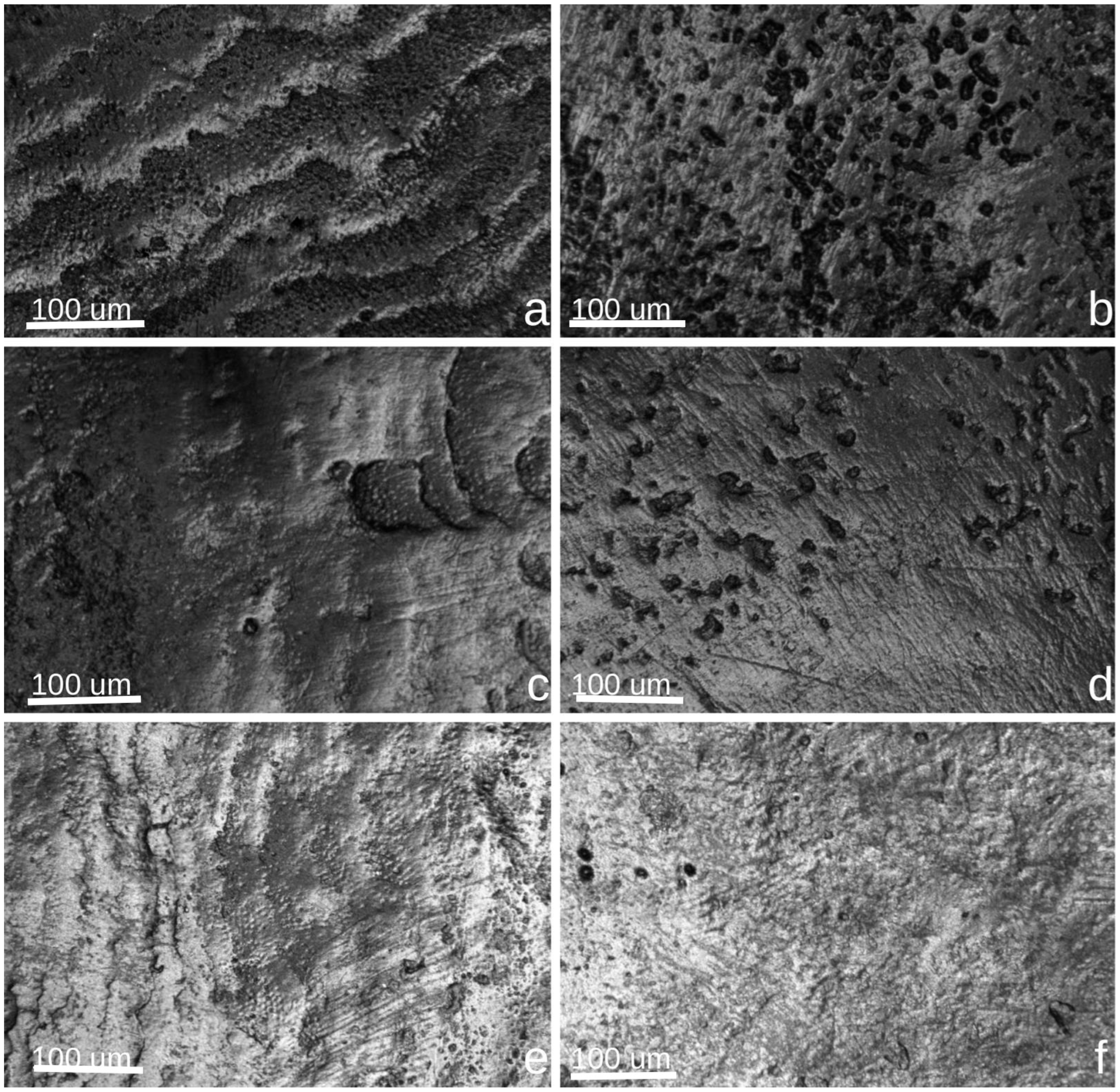
2.5. Confocal Microscopy Evaluation
2.6. Spectrophotometric Shade Evaluation
- -
- ΔE00 < 1: not perceptible;
- -
- 1 ≤ ΔE00 < 2: perceptible by experts only;
- -
- 2 ≤ ΔE00 < 3.3: perceptible by trained observers;
- -
- 3.3 ≤ ΔE00 < 5: clinically visible difference;
- -
- ΔE00 ≥ 5: strong and easily perceptible difference.
2.7. Statistical Analysis
3. Results
3.1. Confocal Microscopy Evaluation
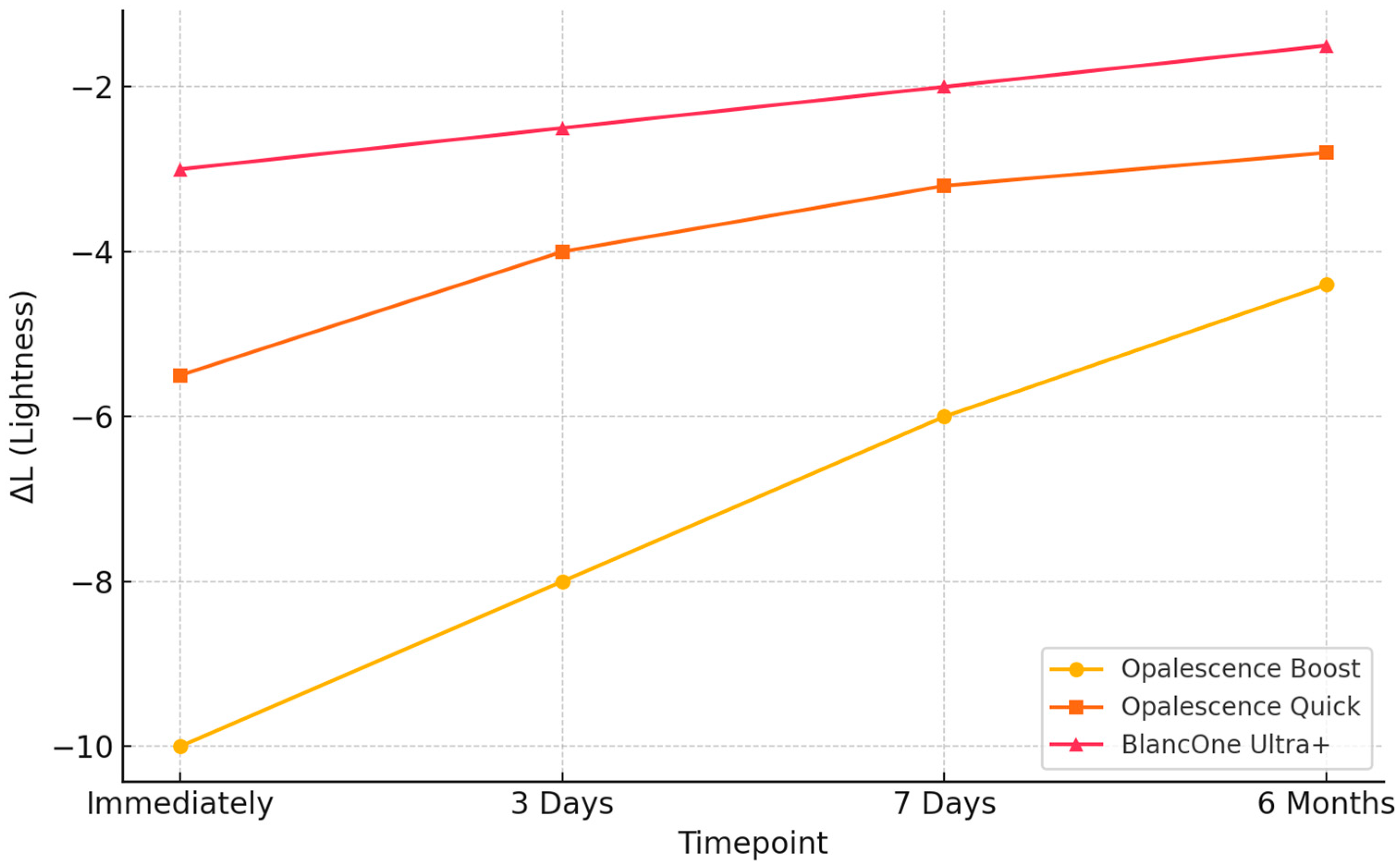
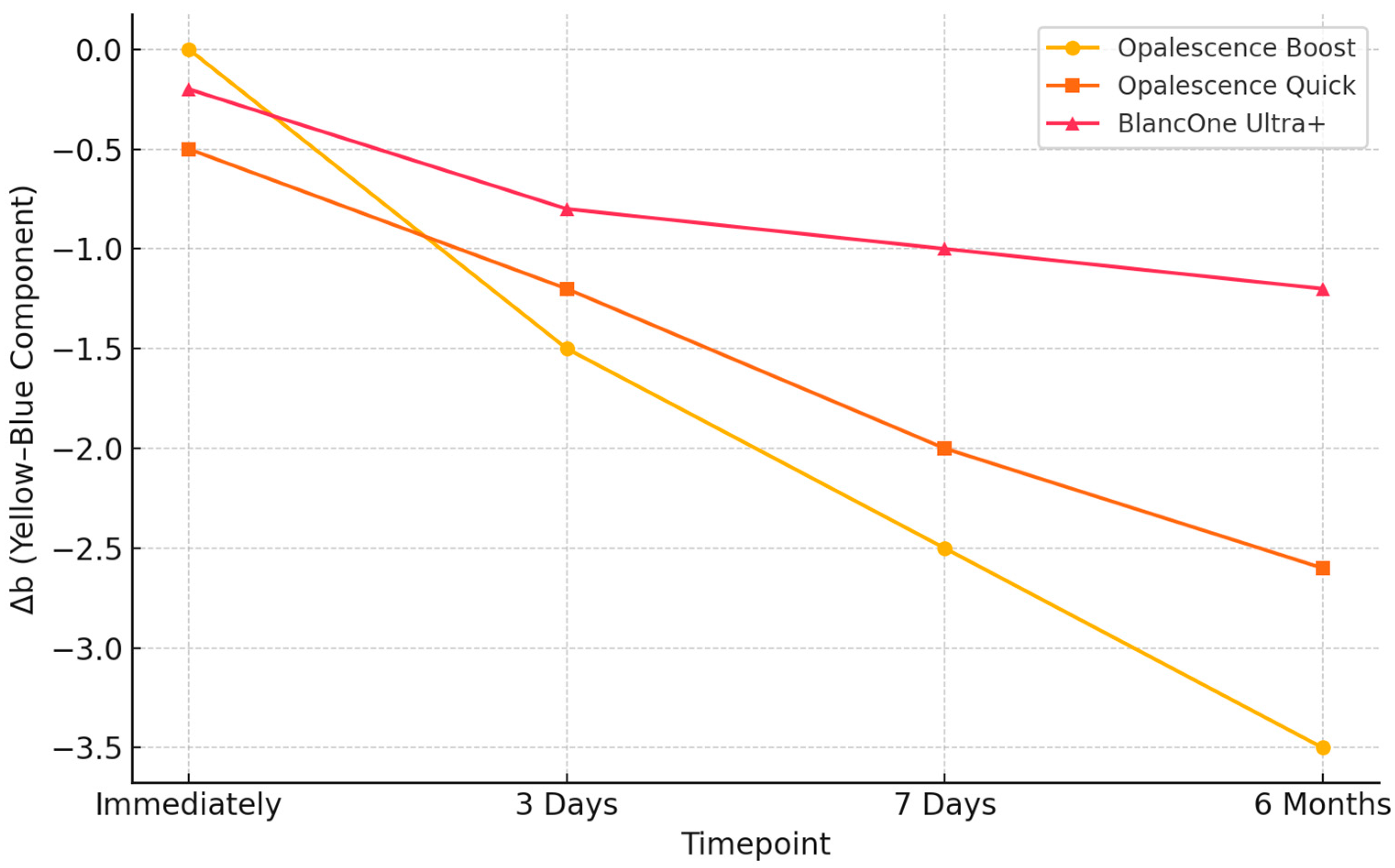
3.2. Evaluation of Color Changes Through Spectrophotometric Shade Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pujari, S.C.; Dey, S.; Pandey, V.; Awasthi, N. In Vitro Comparison of Impact of Different Bleaching Agents on the Microhardness of Enamel. J. Contemp. Dent. Pract. 2016, 17, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Matis, B.; Wang, G.; Matis, J.; Cook, N.; Eckert, G. White Diet: Is It Necessary During Tooth Whitening? Oper. Dent. 2015, 40, 235–240. [Google Scholar] [CrossRef]
- De Rosa, A.; Di Stasio, D.; Lauritano, D.; Santoro, R.; Marotta, A.; Itro, A.; Lucchese, A. Non-Invasive Analysis of Bleaching Effect of Hydrogen Peroxide on Enamel by Reflectance Confocal Microscopy (RCM): Study of Series of Cases. Odontology 2019, 107, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Blatz, M.B.; Chiche, G.; Bahat, O.; Roblee, R.; Coachman, C.; Heymann, H.O. Evolution of Aesthetic Dentistry. J. Dent. Res. 2019, 98, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martínez, J.; Valiente, M.; Sánchez-Martín, M. Tooth Whitening: From the Established Treatments to Novel Approaches to Prevent Side Effects. J. Esthet. Restor. Dent. 2019, 31, 431–440. [Google Scholar] [CrossRef]
- Kwon, S.R.; Kurti, S.R.; Oyoyo, U.; Li, Y. Effect of Various Tooth Whitening Modalities on Microhardness, Surface Roughness and Surface Morphology of the Enamel. Odontology 2015, 103, 274–279. [Google Scholar] [CrossRef]
- Klaric, E.; Rakic, M.; Sever, I.; Milat, O.; Par, M.; Tarle, Z. Enamel and Dentin Microhardness and Chemical Composition After Experimental Light-Activated Bleaching. Oper. Dent. 2015, 40, E132–E141. [Google Scholar] [CrossRef]
- Soares, A.F.; Bombonatti, J.F.S.; Alencar, M.S.; Consolmagno, E.C.; Honório, H.M.; Mondelli, R.F.L. Influence of pH, Bleaching Agents, and Acid Etching on Surface Wear of Bovine Enamel. J. Appl. Oral Sci. 2016, 24, 24–30. [Google Scholar] [CrossRef]
- Hanna, R.; Miron, I.C.; Benedicenti, S. Feasibility and Safety of Adopting a New Approach in Delivering a 450 Nm Blue Laser with a Flattop Beam Profile in Vital Tooth Whitening. A Clinical Case Series with an 8-Month Follow-Up. J. Clin. Med. 2024, 13, 491. [Google Scholar] [CrossRef]
- Kava, G.G.; Mb, K.; Hegde, S.; Damda, A. Diode Laser Assisted Tooth Bleaching: A Step Towards Beautification. Int. J. Appl. Dent. Sci. 2024, 10, 157–159. [Google Scholar] [CrossRef]
- Petersen, M.; Braun, A.; Franzen, R. Thermal Effects on Dental Pulp During Laser-Assisted Bleaching Procedures with Diode Lasers in a Clinical Study. J. Clin. Med. 2024, 13, 2301. [Google Scholar] [CrossRef]
- Yudaev, P.A.; Chistyakov, E.M. Progress in Dental Materials: Application of Natural Ingredients. Russ. Chem. Rev. 2024, 93, RCR5108. [Google Scholar] [CrossRef]
- Altınışık, H.; Akgül, S.; Nezir, M.; Özcan, S.; Özyurt, E. The Effect of In-Office Bleaching with Different Concentrations of Hydrogen Peroxide on Enamel Color, Roughness, and Color Stability. Materials 2023, 16, 1389. [Google Scholar] [CrossRef] [PubMed]
- De Mendonça, R.; Baliza, J.; Burey, A.; Cavalcante, L.; Loguercio, A.; Calazans, F.; Barceleiro, M. In Vitro Analysis of the pH Stability of Dental Bleaching Gels during In-Office Procedures. J. Clin. Exp. Dent. 2021, 13, e22–e29. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.A.; Strazzi-Sahyon, H.B.; Suzuki, T.Y.U.; Briso, A.L.F.; Dos Santos, P.H. Effect of Dental Bleaching on the Microhardness and Surface Roughness of Sealed Composite Resins. Restor. Dent. Endod. 2020, 45, e12. [Google Scholar] [CrossRef]
- Berger, S.B.; Pavan, S.; Santos, P.H.D.; Giannini, M.; Bedran-Russo, A.K.B. Effect of Bleaching on Sound Enamel and with Early Artificial Caries Lesions Using Confocal Laser Microscopy. Braz. Dent. J. 2012, 23, 110–115. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Epple, M.; Meyer, F.; Enax, J. A Critical Review of Modern Concepts for Teeth Whitening. Dent. J. 2019, 7, 79. [Google Scholar] [CrossRef]
- Carey, C.M. Tooth Whitening: What We Now Know. J. Evid. Based Dent. Pract. 2014, 14, 70–76. [Google Scholar] [CrossRef]
- Alkahtani, R.; Stone, S.; German, M.; Waterhouse, P. A Review on Dental Whitening. J. Dent. 2020, 100, 103423. [Google Scholar] [CrossRef]
- Ghanbarzadeh, M.; Ahrari, F.; Akbari, M.; Hamzei, H. Microhardness of Demineralized Enamel Following Home Bleaching and Laser-Assisted in Office Bleaching. J. Clin. Exp. Dent. 2015, 7, e405–e409. [Google Scholar] [CrossRef]
- Paravina, R.D.; Pérez, M.M.; Ghinea, R. Acceptability and Perceptibility Thresholds in Dentistry: A Comprehensive Review of Clinical and Research Applications. J. Esthet. Restor. Dent. 2019, 31, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.S.; Amin, F.; Fareed, M.A.; Ghabbani, H.; Riaz, S.; Khurshid, Z.; Kumar, N. Biomimetic Aspects of Restorative Dentistry Biomaterials. Biomimetics 2020, 5, 34. [Google Scholar] [CrossRef]
- Pan, Q.; Westland, S. Tooth Color and Whitening—Digital Technologies. J. Dent. 2018, 74, S42–S46. [Google Scholar] [CrossRef] [PubMed]
- Hein, S.; Modrić, D.; Westland, S.; Tomeček, M. Objective Shade Matching, Communication, and Reproduction by Combining Dental Photography and Numeric Shade Quantification. J. Esthet. Restor. Dent. 2021, 33, 107–117. [Google Scholar] [CrossRef]
- Karaman, T.; Altintas, E.; Eser, B.; Talo Yildirim, T.; Oztekin, F.; Bozoglan, A. Spectrophotometric Evaluation of Anterior Maxillary Tooth Color Distribution According to Age and Gender. J. Prosthodont. 2019, 28, e96–e102. [Google Scholar] [CrossRef]
- Mahn, E.; Tortora, S.C.; Olate, B.; Cacciuttolo, F.; Kernitsky, J.; Jorquera, G. Comparison of Visual Analog Shade Matching, a Digital Visual Method with a Cross-Polarized Light Filter, and a Spectrophotometer for Dental Color Matching. J. Prosthet. Dent. 2021, 125, 511–516. [Google Scholar] [CrossRef]
- Romano, A.; Di Spirito, F.; Amato, A.; Ferraro, G.A.; Dipalma, G.; Xhajanka, E.; Serpico, R.; Inchingolo, F.; Contaldo, M. Dental Microstructural Imaging: From Conventional Radiology to In Vivo Confocal Microscopy. Appl. Sci. 2022, 12, 10654. [Google Scholar] [CrossRef]
- ISO 28399:2021; Dentistry—External Tooth Bleaching Products. International Organization for Standardization: Geneva, Switzerland, 2021. Available online: https://www.iso.org/standard/81568.html (accessed on 29 June 2025).
- Veneri, F.; Cavani, F.; Bolelli, G.; Checchi, V.; Bizzi, A.; Setti, G.; Generali, L. In Vitro Evaluation of the Effectiveness and pH Variation of Dental Bleaching Gels and Their Effect on Enamel Surface Roughness. Dent. J. 2024, 12, 415. [Google Scholar] [CrossRef]
- Pourhoseingholi, M.A.; Vahedi, M.; Rahimzadeh, M. Sample Size Calculation in Medical Studies. Gastroenterol. Hepatol. Bed Bench 2013, 6, 14–17. [Google Scholar]
- Vilhena, K.F.B.; Nogueira, B.C.L.; Fagundes, N.C.F.; Loretto, S.C.; Angelica, R.S.; Lima, R.R.; Silva E Souza, M.H. Dental Enamel Bleached for a Prolonged and Excessive Time: Morphological Changes. PLoS ONE 2019, 14, e0214948. [Google Scholar] [CrossRef]
- De Chaumont, F.; Dallongeville, S.; Chenouard, N.; Hervé, N.; Pop, S.; Provoost, T.; Meas-Yedid, V.; Pankajakshan, P.; Lecomte, T.; Le Montagner, Y.; et al. Icy: An Open Bioimage Informatics Platform for Extended Reproducible Research. Nat. Methods 2012, 9, 690–696. [Google Scholar] [CrossRef] [PubMed]
- VITA Easyshade® V. Available online: https://www.vita-zahnfabrik.com/pdb_GG2G50G200_en-US.html_us (accessed on 23 March 2025).
- Commission Internationale de l’Éclairage (CIE). Colorimetry, 3rd ed.; CIE: Wien, Austria, 2004; ISBN 978-3-901906-33-6. [Google Scholar]
- VITA Zahnfabrik. VITA Easyshade V—Instructions for Use; VITA Zahnfabrik: Bad Säckingen, Germany, 2023; Available online: https://www.google.com/search?q=VITA+Zahnfabrik.+VITA+Easyshade+V%E2%80%94Instructions+for+Use%3B+VITA+Zahnfabrik%3A+Bad+S%C3%A4ckingen%2C+Germany%2C+2023&oq=VITA+Zahnfabrik.+VITA+Easyshade+V%E2%80%94Instructions+for+Use%3B+VITA+Zahnfabrik%3A+Bad+S%C3%A4ckingen%2C+Germany%2C+2023&gs_lcrp=EgZjaHJvbWUyBggAEEUYOdIBCDEyMDZqMGo3qAIHsAIB8QVEUGmDTK0smfEFRFBpg0ytLJk&sourceid=chrome&ie=UTF-8 (accessed on 1 May 2025).
- Sharma, G.; Wu, W.; Dalal, E.N. The CIEDE2000 Color-Difference Formula: Implementation Notes, Supplementary Test Data, and Mathematical Observations. Color Res. Appl. 2005, 30, 21–30. [Google Scholar] [CrossRef]
- Gómez-Polo, C.; Muñoz, M.P.; Lorenzo Luengo, M.C.; Vicente, P.; Galindo, P.; Martín Casado, A.M. Comparison of the CIELab and CIEDE2000 Color Difference Formulas. J. Prosthet. Dent. 2016, 115, 65–70. [Google Scholar] [CrossRef]
- Ghinea, R.; Pérez, M.M.; Herrera, L.J.; Rivas, M.J.; Yebra, A.; Paravina, R.D. Color Difference Thresholds in Dental Ceramics. J. Dent. 2010, 38 (Suppl. 2), e57–e64. [Google Scholar] [CrossRef] [PubMed]
- Elbahary, S.; Gitit, Z.; Flaisher-Salem, N.; Azem, H.; Shemsesh, H.; Rosen, E.; Tsesis, I. Influence of Irrigation Protocol on Peroxide Penetration into Dentinal Tubules Following Internal Bleaching: A Confocal Laser Scanning Microscopy Study. J. Clin. Pediatr. Dent. 2021, 45, 253–258. [Google Scholar] [CrossRef]
- Llena, C.; Esteve, I.; Forner, L. Effects of In-Office Bleaching on Human Enamel and Dentin. Morphological and Mineral Changes. Ann. Anat. Anat. Anz. 2018, 217, 97–102. [Google Scholar] [CrossRef]
- Amer, M. Intracoronal Tooth Bleaching—A Review and Treatment Guidelines. Aust. Dent. J. 2023, 68, S141–S152. [Google Scholar] [CrossRef]
- Mohammadipour, H.S.; Shokrollahi, P.; Gholami, S.; Bagheri, H.; Namdar, F.; Sekandari, S. Do Different Tooth Bleaching–Remineralizing Regimens Affect the Bleaching Effectiveness and Enamel Microhardness In Vitro? Int. J. Dent. 2024, 2024, 6893472. [Google Scholar] [CrossRef]
- Castro, J.; Godinho, J.; Mata, A.; Silveira, J.M.; Pessanha, S. Study of the Effects of Unsupervised Over-the Counter Whitening Products on Dental Enamel Using μ-Raman and μ-EDXRF Spectroscopies. J. Raman Spectrosc. 2016, 47, 444–448. [Google Scholar] [CrossRef]
- Babot-Marquillas, C.; Sánchez-Martín, M.-J.; Amigo, J.M.; Yousef, I.; H.Valido, I.; Boada, R.; Valiente, M. Tooth Whitening, Oxidation or Reduction? Study of Physicochemical Alterations in Bovine Enamel Using Synchrotron Based Micro-FTIR. Dent. Mater. 2022, 38, 670–679. [Google Scholar] [CrossRef]
- Cavalli, V.; Rosa, D.A.D.; Silva, D.P.D.; Kury, M.; Liporoni, P.C.S.; Soares, L.E.S.; Martins, A.A. Effects of Experimental Bleaching Agents on the Mineral Content of Sound and Demineralized Enamels. J. Appl. Oral Sci. 2018, 26, e20170589. [Google Scholar] [CrossRef] [PubMed]
- Safitri, D.; Pamungkasari, E.P.; Murti, B. Meta-Analysis the Effect of a Potassium Nitrate Desensitizing Agent and Casein Phosphopeptide Amorphous Calcium Phosphate on Tooth Sensitivity after Office Bleaching Treatment. Indones. J. Med. 2023, 8, 423–434. [Google Scholar] [CrossRef]
- Tarique, N.; Awan, R.; Saleh, M.I. Vital Tooth Bleaching and Management of Post Operative Sensitivity: A Clinical Trial Evaluating the Efficacy of Different Desensitizing Materials. Pak. J. Med. Health Sci. 2017, 11, 1564–1567. [Google Scholar]
- Oldoini, G.; Bruno, A.; Genovesi, A.; Parisi, L. Effects of Amorphous Calcium Phosphate Administration on Dental Sensitivity during In-Office and At-Home Interventions. Dent. J. 2018, 6, 52. [Google Scholar] [CrossRef]
- Rashid, S.; ElSalhy, M. Efficacy of MI Paste® on Bleaching-Related Sensitivity: Randomized Clinical Trial. Int. J. Dent. 2021, 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Markovic, L.; Jordan, R.A.; Lakota, N.; Gaengler, P. Micromorphology of Enamel Surface After Vital Tooth Bleaching. J. Endod. 2007, 33, 607–610. [Google Scholar] [CrossRef]
- Chang, H.-H.; Cheng, C.-L.; Huang, P.-J.; Lin, S.-Y. Application of Scanning Electron Microscopy and X-Ray Microanalysis: FE-SEM, ESEM-EDS, and EDS Mapping for Studying the Characteristics of Topographical Microstructure and Elemental Mapping of Human Cardiac Calcified Deposition. Anal. Bioanal. Chem. 2014, 406, 359–366. [Google Scholar] [CrossRef]
- Paepegaey, A.-M.; Barker, M.L.; Bartlett, D.W.; Mistry, M.; West, N.X.; Hellin, N.; Brown, L.J.; Bellamy, P.G. Measuring Enamel Erosion: A Comparative Study of Contact Profilometry, Non-Contact Profilometry and Confocal Laser Scanning Microscopy. Dent. Mater. 2013, 29, 1265–1272. [Google Scholar] [CrossRef]
- Naim, S.; Spagnuolo, G.; Osman, E.; Mahdi, S.S.; Battineni, G.; Qasim, S.S.B.; Cernera, M.; Rifai, H.; Jaafar, N.; Maalouf, E.; et al. Quantitative Measurements of the Depth of Enamel Demineralization Before and After Bleach: An In Vitro Study. BioMed Res. Int. 2022, 2022, 2805343. [Google Scholar] [CrossRef]
- Baia, J.C.P.; Oliveira, R.P.; Ribeiro, M.E.S.; Lima, R.R.; Loretto, S.C.; Silva E Souza Junior, M.H. Influence of Prolonged Dental Bleaching on the Adhesive Bond Strength to Enamel Surfaces. Int. J. Dent. 2020, 2020, 2609359. [Google Scholar] [CrossRef]
- De Carvalho, A.; De Souza, T.; Liporoni, P.; Pizi, E.; Matuda, L.; Catelan, A. Effect of Bleaching Agents on Hardness, Surface Roughness and Color Parameters of Dental Enamel. J. Clin. Exp. Dent. 2020, 12, e670–e675. [Google Scholar] [CrossRef] [PubMed]
- Mondelli, R.F.L.; Gabriel, T.R.C.G.; Rizzante, F.A.P.; Magalhães, A.C.; Bombonatti, J.F.S.; Ishikiriama, S.K. Do Different Bleaching Protocols Affect the Enamel Microhardness? Eur. J. Dent. 2015, 9, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Seto, T.; Grymak, A.; Mudliar, V.; Choi, J. Effect of Enamel Bleaching on the Bond Strength of Ceramic—A Systematic Review. Oral 2022, 2, 182–197. [Google Scholar] [CrossRef]
- Ozdemir, Z.M.; Surmelioglu, D. Effects of Different Bleaching Application Time on Tooth Color and Mineral Alteration. Ann. Anat. Anat. Anz. 2021, 233, 151590. [Google Scholar] [CrossRef]
- Mazur, M.; Corridore, D.; Ndokaj, A.; Ardan, R.; Vozza, I.; Babajko, S.; Jedeon, K. MIH and Dental Caries in Children: A Systematic Review and Meta-Analysis. Healthcare 2023, 11, 1795. [Google Scholar] [CrossRef]
- ISO 4287:1997; Geometrical Product Specifications (GPS)—Surface Texture: Profile Method—Terms, Definitions and Surface Texture Parameters. International Organization for Standardization: Geneva, Switzerland, 1997.

| Product Name | Composition | Manufacturer | Lot Number |
|---|---|---|---|
| Opalescence Boost | 40% HP pH = 7 | Ultradent Products Inc., South Jordan, UT, USA | ULT4754 |
| Opalescence Quick | 45% CP pH = 5.6–7.2 | Ultradent Products Inc., South Jordan, UT, USA | BWNV9 |
| BlancOne Ultra+ | 35% HP pH = 6–7.2 | IDS Spa, Savona, Italy | 00422 |
| Bleaching Agent | Mean ΔE00 (±SD) Immediately | Mean ΔE00 (±SD) 3 Days | Mean ΔE00 (±SD) 7 Days | Mean ΔE00 (±SD) 6 Months |
|---|---|---|---|---|
| Opalescence Boost 40% HP | 13.85 ± 4.65 | 13.94 ± 4.49 | 11.18 ± 3.70 | 13.08 ± 2.41 |
| Opalescence Quick 35% CP | 5.85 ± 4.18 | 5.85 ± 4.18 | 8.56 ± 4.43 | 8.55 ± 2.23 |
| BlancOne Ultra+ 35% HP | 4.03 ± 1.36 | 4.02 ± 1.28 | 3.18 ± 4.07 | 4.67 ± 1.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buzatu, B.L.R.; Luca, M.M.; Galuscan, A.; Vaduva, A.O.; Fratila, A.D.; Dumitrescu, R.; Sava-Rosianu, R.; Balean, O.; Buzatu, R.; Jumanca, D. From Microstructure to Shade Shift: Confocal and Spectrophotometric Evaluation of Peroxide-Induced Dental Bleaching. J. Clin. Med. 2025, 14, 4642. https://doi.org/10.3390/jcm14134642
Buzatu BLR, Luca MM, Galuscan A, Vaduva AO, Fratila AD, Dumitrescu R, Sava-Rosianu R, Balean O, Buzatu R, Jumanca D. From Microstructure to Shade Shift: Confocal and Spectrophotometric Evaluation of Peroxide-Induced Dental Bleaching. Journal of Clinical Medicine. 2025; 14(13):4642. https://doi.org/10.3390/jcm14134642
Chicago/Turabian StyleBuzatu, Berivan Laura Rebeca, Magda Mihaela Luca, Atena Galuscan, Adrian Ovidiu Vaduva, Aurora Doris Fratila, Ramona Dumitrescu, Ruxandra Sava-Rosianu, Octavia Balean, Roxana Buzatu, and Daniela Jumanca. 2025. "From Microstructure to Shade Shift: Confocal and Spectrophotometric Evaluation of Peroxide-Induced Dental Bleaching" Journal of Clinical Medicine 14, no. 13: 4642. https://doi.org/10.3390/jcm14134642
APA StyleBuzatu, B. L. R., Luca, M. M., Galuscan, A., Vaduva, A. O., Fratila, A. D., Dumitrescu, R., Sava-Rosianu, R., Balean, O., Buzatu, R., & Jumanca, D. (2025). From Microstructure to Shade Shift: Confocal and Spectrophotometric Evaluation of Peroxide-Induced Dental Bleaching. Journal of Clinical Medicine, 14(13), 4642. https://doi.org/10.3390/jcm14134642










