Improving the Compliance of Massive Hemorrhage Protocols Through Education Is Associated with Patient Survival
Abstract
1. Introduction
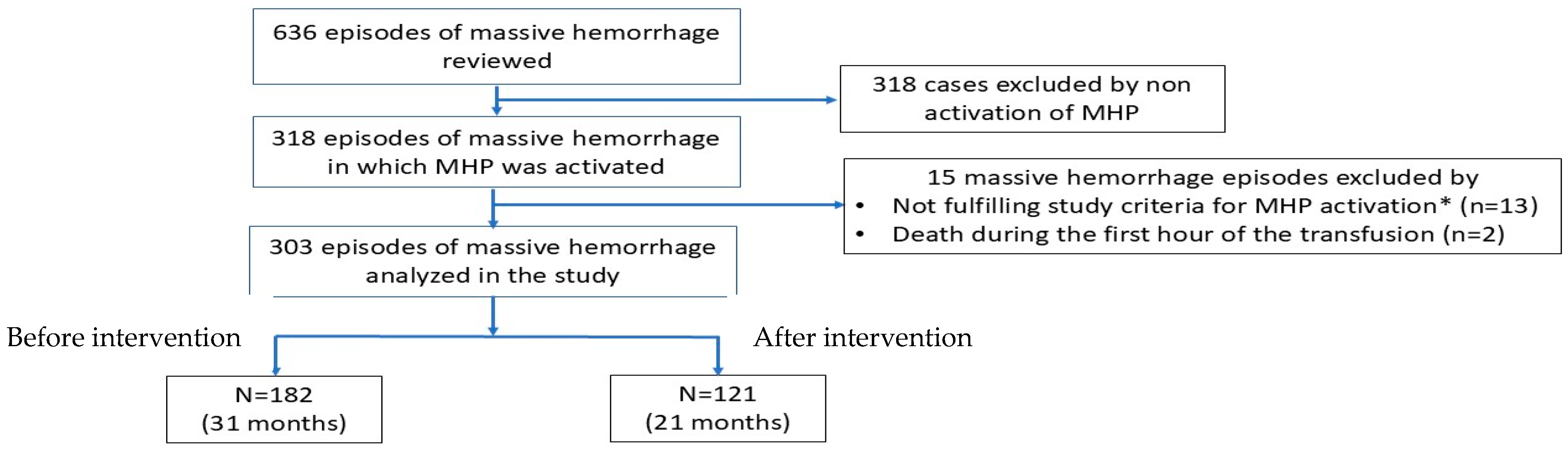
2. Materials and Methods
2.1. Study Design
2.2. Preintervention
2.3. Educational Intervention
2.4. Postintervention
2.5. Data Acquisition
2.6. Study Endpoints
2.7. Ethics
2.8. Statistical Analysis
3. Results
3.1. Clinical and Transfusion Data
3.2. MHP Compliance and Outcomes
3.3. Outcomes and Mortality
3.4. Massive Transfusion
4. Discussion
Limitations and Strengths
5. Conclusions
6. Patients
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Etchill, E.; Sperry, J.; Zuckerbraun, B.; Alarcon, L.; Brown, J.; Schuster, K.; Kaplan, L.; Piper, G.; Peitzman, A.; Neal, M.D. The confusion continues: Results from an American Association for the Surgery of Trauma survey on massive transfusion practices among United States trauma centers. Transfusion 2016, 56, 2478–2486. [Google Scholar] [CrossRef] [PubMed]
- Sihler, K.C.; Napolitano, L.M. Massive transfusion: New insights. Chest 2009, 136, 1654–1667. [Google Scholar] [CrossRef]
- Cotton, B.A.; Au, B.K.; Nunez, T.C.; Gunter, O.L.; Robertson, A.M.; Young, P.P. Predefined Massive Transfusion Protocols are Associated With a Reduction in Organ Failure and Postinjury Complications. J. Trauma Acute Care Surg. 2009, 66, 41–49. [Google Scholar] [CrossRef]
- Dhillon, N.K.; Abumuhor, I.; Hayes, C.; Nammalwar, S.; Ghoulian, J.; Asadi, M.; Ley, E.J. Massive Transfusion Activations in Non-Trauma Patients. Am. Surg. 2023, 89, 1719–1724. [Google Scholar] [CrossRef] [PubMed]
- Holcomb, J.; Caruso, J.; McMullin, N.; Wade, C.E.; Pearse, L.; Oetjen-Gerdes, L.; Champion, H.R.; Lawnick, M.; Farr, W.; Rodriguez, S.; et al. Causes of death in U.S. special operations forces in the global war on terrorism: 2001–2004. Ann. Surg. 2007, 245, 986–991. [Google Scholar] [CrossRef] [PubMed]
- Nunez, T.C.; Young, P.P.; Holcomb, J.B.; Cotton, B.A. Creation, Implementation, and Maturation of a Massive Transfusion Protocol for the Exsanguinating Trauma Patient. J. Trauma Inj. Infect. Crit. Care 2010, 68, 1498–1505. [Google Scholar] [CrossRef]
- Dente, C.J.; Shaz, B.H.; Nicholas, J.M.; Harris, R.S.; Wyrzykowski, A.D.; Patel, S.; Shah, A.; Vercruysse, G.A.; Feliciano, D.V.; Rozycki, G.S.; et al. Improvements in Early Mortality and Coagulopathy are Sustained Better in Patients With Blunt Trauma After Institution of a Massive Transfusion Protocol in a Civilian Level I Trauma Center. J. Trauma Acute Care Surg. 2009, 66, 1616–1624. [Google Scholar] [CrossRef]
- Riskin, D.J.; Tsai, T.C.; Riskin, L.; Hernandez-Boussard, T.; Purtill, M.; Maggio, P.M.; Spain, D.A.; Brundage, S.I. Massive Transfusion Protocols: The Role of Aggressive Resuscitation Versus Product Ratio in Mortality Reduction. J. Am. Coll. Surg. 2009, 209, 198–205. [Google Scholar] [CrossRef]
- Consunji, R.; Elseed, A.; El-Menyar, A.; Sathian, B.; Rizoli, S.; Al-Thani, H.; Peralta, R. The effect of massive transfusion protocol implementation on the survival of trauma patients: A systematic review and meta-analysis. Blood Transfus. 2020, 18, 434–445. [Google Scholar] [CrossRef]
- Cotton, B.A.; Gunter, O.L.; Isbell, J.; Au, B.K.B.; Robertson, A.M.; Morris, J.A.J.; Jacques, P.S.; Young, P.P. Damage control hematology: The impact of a trauma exsanguination protocol on survival and blood product utilization. J. Trauma Acute Care Surg. 2008, 64, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Malone, D.L.; Hess, J.R.; Fingerhut, A. Massive transfusion practices around the globe and a suggestion for a common massive transfusion protocol. J. Trauma Acute Care Surg. 2006, 60 (Suppl. S6), S91–S96. [Google Scholar] [CrossRef] [PubMed]
- Mellin-Olsen, J.; Staender, S.; Whitaker, D.K.; Smith, A.F. The helsinki declaration on patient safety in anaesthesiology. Curr. Opin. Anaesthesiol. 2014, 27, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Wijaya, R.; Cheng, H.M.G.; Chong, C.K. The use of massive transfusion protocol for trauma and non-trauma patients in a civilian setting: What can be done better? Singapore Med. J. 2016, 57, 238–241. [Google Scholar] [CrossRef]
- Bawazeer, M.; Ahmed, N.; Izadi, H.; McFarlan, A.; Nathens, A.; Pavenski, K. Compliance with a massive transfusion protocol (MTP) impacts patient outcome. Injury 2015, 46, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Cotton, B.A.; Dossett, L.A.; Au, B.K.; Nunez, T.C.; Robertson, A.M.; Young, P.P. Room for (Performance) improvement: Provider-related factors associated with poor outcomes in massive transfusion. J. Trauma Acute Care Surg. 2009, 67, 1004–1011. [Google Scholar] [CrossRef]
- Llau, J.; Acosta, F.; Escolar, G.; Fernández-Mondéjar, E.; Guasch, E.; Marco, P.; Paniagua, P.; Páramo, J.; Quintana, M.; Torrabadella, P. Documento multidisciplinar de consenso sobre el manejo de la hemorragia masiva (documento HEMOMAS). Med. Intensiv. 2015, 39, 483–504. [Google Scholar] [CrossRef]
- Rincón Ferrari, M.D.; Felipe Correoso, M.M.; Candela Toha, Á. Assessment of the degree of knowledge of the massive transfusion protocol in four Spanish hospitals. Med. Clin. 2023, 161, 312–313. [Google Scholar] [CrossRef]
- Llau, J.V.; Aldecoa, C.; Guasch, E.; Marco, P.; Marcos-Neira, P.; Paniagua, P.; Páramo, J.A.; Quintana, M.; Rodríguez-Martorell, F.J.; Serrano, A. Multidisciplinary consensus document on the management of massive haemorrhage. First update 2023 (document HEMOMAS-II). Med. Intensiv. 2023, 47, 454–467. [Google Scholar] [CrossRef]
- Sanderson, B.; Coiera, E.; Asrianti, L.; Field, J.; Estcourt, L.J.; Wood, E.M. How well does your massive transfusion protocol perform? A scoping review of quality indicators. Blood Transfus. 2020, 18, 423–456. [Google Scholar] [CrossRef]
- Margarido, C.; Ferns, J.; Chin, V.; Ribeiro, T.; Nascimento, B.; Barrett, J.; Herer, E.; Halpern, S.; Andrews, L.; Ballatyne, G.; et al. Massive hemorrhage protocol activation in obstetrics: A 5-year quality performance review. Int. J. Obstet. Anesthesia 2019, 38, 37–45. [Google Scholar] [CrossRef]
- McQuilten, Z.K.; Wood, E.M.; Bailey, M.; Cameron, P.A.; Cooper, D.J. Fibrinogen is an independent predictor of mortality in major trauma patients: A five-year statewide cohort study. Injury 2017, 48, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Fominskiy, E.; Nepomniashchikh, V.A.; Lomivorotov, V.V.; Monaco, F.; Vitiello, C.; Zangrillo, A.; Landoni, G. Efficacy and Safety of Fibrinogen Concentrate in Surgical Patients: A Meta-Analysis of Randomized Controlled Trials. J. Cardiothorac. Vasc. Anesth. 2017, 31, e33–e35. [Google Scholar] [CrossRef]
- Rossaint, R.; Afshari, A.; Bouillon, B.; Cerny, V.; Cimpoesu, D.; Curry, N.; Duranteau, J.; Filipescu, D.; Grottke, O.; Grønlykke, L.; et al. The European guideline on management of major bleeding and coagulopathy following trauma: Sixth edition. Crit. Care 2023, 27, 98. [Google Scholar] [CrossRef]
- Holcomb, J.B.; Wade, C.E.; Michalek, J.E.; Chisholm, G.B.; Zarzabal, L.A.; Schreiber, M.A.; Gonzalez, E.A.; Pomper, G.J.; Perkins, J.G.; Spinella, P.C.; et al. Increased Plasma and Platelet to Red Blood Cell Ratios Improves Outcome in 466 Massively Transfused Civilian Trauma Patients. Ann. Surg. 2008, 248, 447–458. [Google Scholar] [CrossRef]
- Holcomb, J.B.; Tilley, B.C.; Baraniuk, S.; Fox, E.E.; Wade, C.E.; Podbielski, J.M.; del Junco, D.J.; Brasel, K.J.; Bulger, E.M.; Callcut, R.A.; et al. Transfusion of Plasma, Platelets, and Red Blood Cells in a 1:1:1 vs a 1:1:2 Ratio and Mortality in Patients With Severe Trauma. JAMA 2015, 313, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Rahouma, M.; Kamel, M.; Jodeh, D.; Kelley, T.; Ohmes, L.B.; de Biasi, A.R.; Abouarab, A.A.; Benedetto, U.; Guy, T.S.; Lau, C.; et al. Does a balanced transfusion ratio of plasma to packed red blood cells improve outcomes in both trauma and surgical patients? A meta-analysis of randomized controlled trials and observational studies. Am. J. Surg. 2018, 216, 342–350. [Google Scholar] [CrossRef]
- Sinha, R.; Roxby, D.; Bersten, A. Experience with a massive transfusion protocol in the management of massive haemorrhage. Transfus. Med. 2013, 23, 108–113. [Google Scholar] [CrossRef]
- Holcomb, J.B.; Del Junco, D.J.; Fox, E.E.; Wade, C.E.; Cohen, M.J.; Schreiber, M.A.; Alarcon, L.H.; Bai, Y.; Brasel, K.J.; Bulger, E.M.; et al. The Prospective, Observational, Multicenter, Major Trauma Transfusion (PROMMTT) Study. JAMA Surg. 2013, 148, 127–136. [Google Scholar] [CrossRef]
- Foster, J.C.; Sappenfield, J.W.; Smith, R.S.; Kiley, S.P. Initiation and termination of massive transfusion protocols: Current strategies and future prospects. Anesth. Analg. 2017, 125, 2045–2055. [Google Scholar] [CrossRef]
- Green, L.; Stanworth, S.; McQuilten, Z.; Lin, V.; Tucker, H.; Jackson, B.; Badawi, M.; Hindawi, S.; Chaurasia, R.; Patidar, G.; et al. International Forum on the Management of Major Haemorrhage: Summary. Vox Sang. 2022, 117, 746–753. [Google Scholar] [CrossRef]
- Rijnhout, T.W.H.; Noorman, F.; Bek, A.; Zoodsma, M.; Hoencamp, R. Massive transfusion in The Netherlands. Emerg. Med. J. 2020, 37, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.W.; Booth, G.S. Blood shortages and changes to massive transfusion protocols: Survey of hospital practices during the COVID-19 pandemic. Transfus. Apher. Sci. 2022, 61, 103297. [Google Scholar] [CrossRef]
- Paganini, M.; Abowali, H.; Bosco, G.; Balouch, M.; Enten, G.; Deng, J.; Shander, A.; Ciesla, D.; Wilson, J.; Camporesi, E. Quality Improvement Project of a Massive Transfusion Protocol (MTP) to Reduce Wastage of Blood Components. Int. J. Environ. Res. Public Health 2021, 18, 274. [Google Scholar] [CrossRef]
- Yazer, M.H.; Dunbar, N.M.; Cohn, C.; Dillon, J.; Eldib, H.; Jackson, B.; Kaufman, R.; Murphy, M.F.; O’BRien, K.; Raval, J.S.; et al. Blood product transfusion and wastage rates in obstetric hemorrhage. Transfusion 2018, 58, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, N.M.; Olson, N.J.; Szczepiorkowski, Z.M.; Martin, E.D.; Tysarcyk, R.M.; Triulzi, D.J.; Alarcon, L.H.; Yazer, M.H. Blood component transfusion and wastage rates in the setting of massive transfusion in three regional trauma centers. Transfusion 2017, 57, 45–52. [Google Scholar] [CrossRef]
- Baumann Kreuziger, L.M.; Morton, C.T.; Subramanian, A.T.; Anderson, C.P.; Dries, D.J. Not only in trauma patients: Hospital-wide implementation of a massive transfusion protocol. Transfus. Med. 2014, 24, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Casado, M.; Hostigüela-Martín, V.; Raigal-Caño, A.; Labajo, L.; Gómez-Tello, V.; Alonso-Gómez, G.; Aguilera-Cerna, F. Escalas pronósticas en la disfunción multiorgánica: Estudio de cohortes. Med. Intensiv. 2016, 40, 145–153. [Google Scholar] [CrossRef]
- Gunter, O.L.; Au, B.K.; Isbell, J.M.; Mowery, N.T.; Young, P.P.; Cotton, B.A. Optimizing outcomes in damage control resuscitation: Identifying blood product ratios associated with improved survival. J. Trauma Acute Care Surg. 2008, 65, 527–534. [Google Scholar] [CrossRef]
- Paradis, E.; Sutkin, G. Beyond a good story: From Hawthorne Effect to reactivity in health professions education research. Med. Educ. 2017, 51, 31–39. [Google Scholar] [CrossRef]
| Intervention Phase | p Value | ||
|---|---|---|---|
| Before | After | ||
| n = 182 | n = 121 | ||
| Patients by site, n (%) | |||
| Site 1 | 60 (33.0%) | 43 (35.5%) | |
| Site 2 | 91 (50.0%) | 48 (39.7%) | |
| Site 3 | 17 (9.34%) | 20 (16.5%) | |
| Site 4 | 14 (7.69%) | 10 (8.26%) | |
| Male sex, n (%) | 124 (68.1%) | 76 (62.8%) | 0.38 |
| Age, years | 56.0 ± 18.8 | 57.4 ± 16.8 | 0.52 |
| Cause of hemorrhage, n (%) | 0.15 | ||
| Surgery | 80 (43.9%) | 49 (40.5%) | |
| Trauma | 46 (25.3%) | 30 (24.8%) | |
| Gastrointestinal | 23 (12.6%) | 16 (13.2%) | |
| Peripartum | 11 (6.04%) | 2 (1.65%) | |
| Other causes | 22 (12.1%) | 24 (19.8%) | |
| Previous antiaggregant use, n (%) | 38 (20.9%) | 31 (25.6%) | 0.4 |
| Previous anticoagulant use, n (%) | 19 (10.5%) | 15 (12.5%) | 0.58 |
| Systolic blood pressure, n (%) | 155 (85.2%) | 108 (89.2%) | 0.8 |
| mm Hg | 79.9 ± 22.7 | 80.7 ± 25.9 | |
| Diastolic blood pressure, n (%) | 152 (83.5%) | 106 (87.6%) | 0.52 |
| mm Hg | 46.9 ± 13.4 | 48.1 ± 17.3 | |
| Heart rate, n (%) | 149 (81.9%) | 107 (88.4%) | 0.49 |
| bpm, | 101.6 ± 27.7 | 103.9 ± 26.1 | |
| Lactate, n (%) | 45 (24.7%) | 15 (12.4%) | 0.53 |
| mmol/L | 7.26 ± 7.45 | 6.17 ± 5.1 | |
| Hemoglobin, n (%) | 124 (68.1%) | 87 (71.4%) | 0.46 |
| g/L | 86.3± 30.3 | 83.6 ± 23.1 | |
| INR, n (%) | 117 (64.3%) | 84 (69.4%) | 0.86 |
| ratio | 1.56 ± 0.77 | 1.54 ± 0.69 | |
| Partial thromboplastin time, n (%) ratio | 114 (62.6%) | 82 (67.8%) | 0.27 |
| 1.77 ± 2.00 | 2.15 ± 2.61 | ||
| Fibrinogen, n (%) | 97 (53.8%) | 71 (58.7%) | |
| g/L | 2.17 ± 1.42 | 2.01 ± 1.23 | 0.44 |
| Platelets, n (%) | 124 (68.1%) | 87 (71.9%) | 0.96 |
| ×109/L | 1808 ± 99.0 | 179 ±116 | |
| CT-EXTEM, n (%) | 62 (34.1%) | 55 (45.4%) * | 0.61 |
| sec | 94.45 ± 38.6 | 102.45 ± 110.3 | |
| MCF-EXTEM, n (%) | 61 (33.5%) | 54 (44.6%) * | 0.76 |
| mm | 51.7 ± 12.3 | 51.0 ± 11.5 | |
| MCF-FIBTEM, n (%) | 58 (31.9%) | 54 (44.6%) * | 0.89 |
| mm | 11.8 ± 10.9 | 11.5 ± 6.94 | |
| TASH, n (%) | 39 (21.4%) | 20 (16.5%) | 0.52 |
| score | 18.3 ± 5.89 | 17.25 ± 6.08 | |
| ABC, n (%) | 39 (21.4%) | 20 (16.5%) | 0.24 |
| score | 2.31 ± 1.00 | 2.00 ± 0.79 | |
| ISS, n (%) | 45 (24.7%) | 28 (23.1%) | 0.53 |
| score | 39.4 ± 18.3 | 36.5 ± 19.2 | |
| GCS score | 9.13 ± 5.43 | 10.6 ± 4.97 | 0.38 |
| Trauma patients with TBI (%) | 50% | 36.70% | |
| ACS NSQIP **, n (%) | 134 (73.6%) | 83 (68.6%) | |
| Serious complications (%) | 21.9 ± 12.3 | 21.6 ± 12.3 | 0.84 |
| Mortality (%) | 6.3 ± 8.8 | 6.6 ± 9.5 | 0.79 |
| ASA **, n (%) | 155 (86.2%) | 93 (76.8%) | 0.06 |
| Median (IQR) | 4 (2–4) | 4 (3–4) | |
| Compliance Criteria | Intervention | p Value | Comments | |
|---|---|---|---|---|
| Before n = 182 | After n = 121 | |||
| 1 MHP activation based on the pre-specified indications | 182/193 (94.3%) | 121/123 (98.3%) | 0.049 | Thirteen patients (5.6%) did not meet the prespecified criteria. They were not shocked, nor did they require ≥6 pRBC/24 h. |
| 2 Timely communication with blood bank (<30 min from arrival at ED or from fulfillment of criteria to activation) | (72.8%) | (67.3%) | 0.345 | Time of 30 min was agreed instead of 15 min to better match MH episodes both in traumatic and non-traumatic patients. |
| 3 Group and screen sent | (84.5%) | (87.0%) | 0.615 | |
| 4 Hemorrhage panel sent to lab (ABG with lactate, CBC; INR, Fibrinogen or Fibtem and electrolytes) | (39.0%) | (50.4%) | 0.05 | Clauss fibrinogen and FITBEM are closely correlated, thus compliance was considered present if either of these was measured. |
| 5 Recording of initial hemodynamic variables | (81.3%) | (86.8%) | 0.269 | |
| 6 Administration of blood products to achieve ≥ 1:2 plasma to red blood cell ratio | (50.0%) | (62.0%) | 0.05 | Ratios were calculated at the end of the 24 h massive transfusion period. |
| 7 Measures to correct or prevent hypothermia | (31.3%) | (43.8%) | 0.029 | Recorded how we warm patients or avoid heat loss. |
| 8 Correction of acidosis | (79.1%) | (81.0%) | 0.770 | |
| 9 Correction of hyperpotassemia | (77.9%) | (74.8%) | 0.578 | |
| 10 Correction of hypocalcemia | (67.6%) | (75.2%) | 0.159 | |
| 11 Viscoelastic tests used | (60.5%) | (70.6%) | 0.083 | |
| 12 Prevent blood product wastage. | (83.5%) | (84.0%) | 1.000 | |
| 13 Timely MHP deactivation | (93.2%) | (90.5%) | 0.506 | Within 1 h of last blood product given. |
| Average criterion degree of compliance (CDC) | 68.5% | 72.9% | 0.05 | |
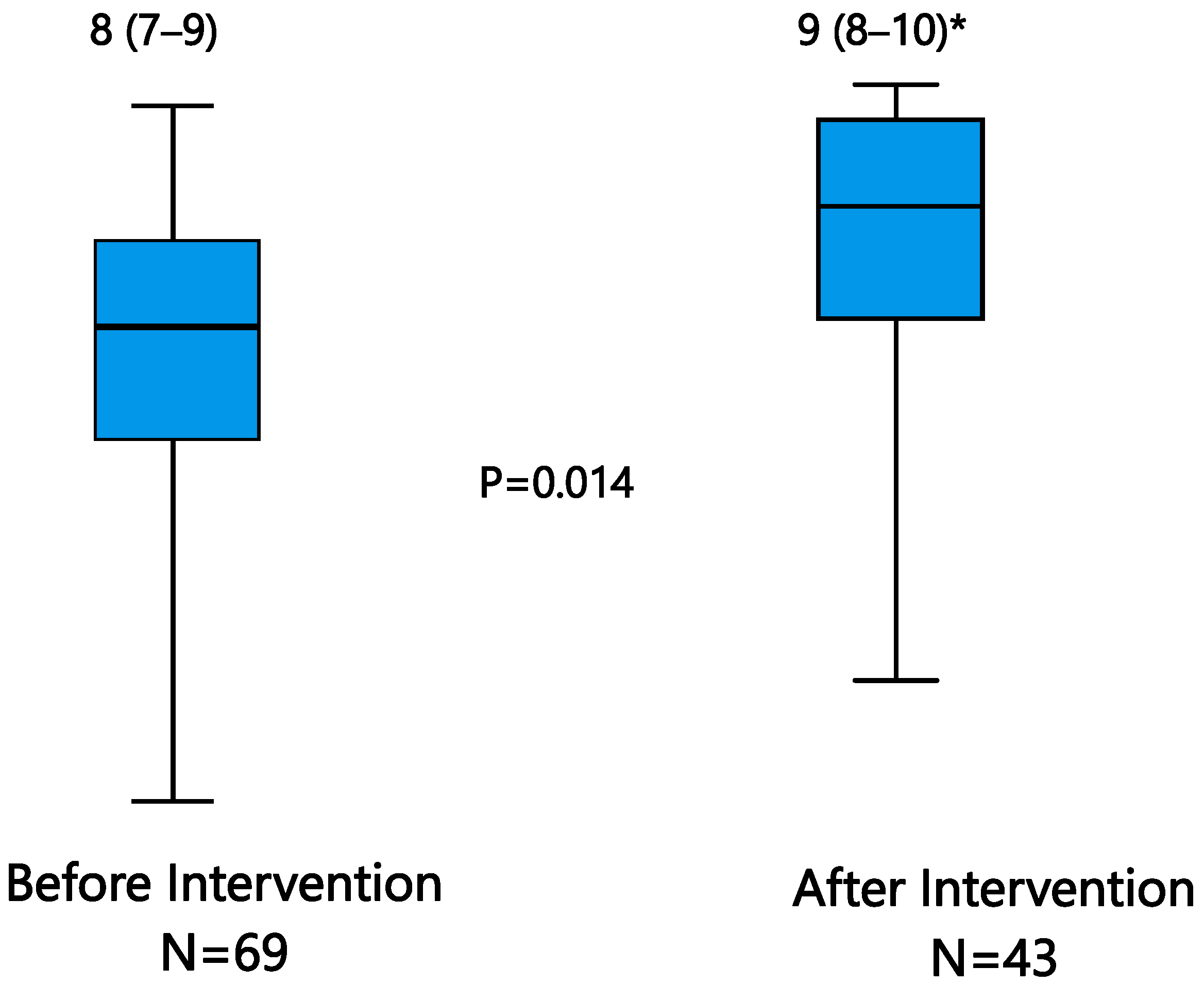
| Overall episode degree of compliance (EDC), median (IQR) | 8 (6–9) | 8 (7–9) | 0.053 | |
| Outcomes | Intervention Phase | p | |
|---|---|---|---|
| Before | After | Value | |
| SAPS II | 52.25 ±22.3 | 47.1 ±19.4 | 0.049 |
| 24 h-mortality | 43 (23.6%) | 34 (28.1%) | 0.42 |
| In-hospital mortality | 77 (42.3%) | 51 (42.5%) | 0.97 |
| Mechanical ventilation, days | 2 (1–8.5) | 2 (1–4) | 0.21 |
| ICU stay, days | 6 (3–13) | 5 (2–11) | 0.13 |
| Length of hospital stay, days | 11 (1–28) | 9 (1–28) | 0.45 |
| 24 h | In-Hospital | Mortality by Hemorrhage | |||
|---|---|---|---|---|---|
| Mortality | Mortality | ||||
| Dead | Alive | Dead | Alive | Dead | Alive |
| 7 (6–9) | 8 (7–9) | 8 (6–9) | 8 (7–9) | 7 (6–8) | 8 (7–9) |
| p = 0.003 | p = 0.049 | p = 0.012 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paniagua-Iglesias, P.; Rincón-Ferrari, M.D.; Candela-Toha, A.; Marcos-Jubilar, M.; Barquero-Lopez, M.; Gich-Saladich, I.; Medina-Marrero, L.; Bosch-Llobet, A.; Garrido-Fleischmann, D.; Ordoñez-Llanos, J.; et al. Improving the Compliance of Massive Hemorrhage Protocols Through Education Is Associated with Patient Survival. J. Clin. Med. 2025, 14, 4632. https://doi.org/10.3390/jcm14134632
Paniagua-Iglesias P, Rincón-Ferrari MD, Candela-Toha A, Marcos-Jubilar M, Barquero-Lopez M, Gich-Saladich I, Medina-Marrero L, Bosch-Llobet A, Garrido-Fleischmann D, Ordoñez-Llanos J, et al. Improving the Compliance of Massive Hemorrhage Protocols Through Education Is Associated with Patient Survival. Journal of Clinical Medicine. 2025; 14(13):4632. https://doi.org/10.3390/jcm14134632
Chicago/Turabian StylePaniagua-Iglesias, Pilar, Maria Dolores Rincón-Ferrari, Angel Candela-Toha, Maria Marcos-Jubilar, Marta Barquero-Lopez, Ignasi Gich-Saladich, Laura Medina-Marrero, Alba Bosch-Llobet, Daniela Garrido-Fleischmann, Jordi Ordoñez-Llanos, and et al. 2025. "Improving the Compliance of Massive Hemorrhage Protocols Through Education Is Associated with Patient Survival" Journal of Clinical Medicine 14, no. 13: 4632. https://doi.org/10.3390/jcm14134632
APA StylePaniagua-Iglesias, P., Rincón-Ferrari, M. D., Candela-Toha, A., Marcos-Jubilar, M., Barquero-Lopez, M., Gich-Saladich, I., Medina-Marrero, L., Bosch-Llobet, A., Garrido-Fleischmann, D., Ordoñez-Llanos, J., & Urrutia-Cuchí, G. (2025). Improving the Compliance of Massive Hemorrhage Protocols Through Education Is Associated with Patient Survival. Journal of Clinical Medicine, 14(13), 4632. https://doi.org/10.3390/jcm14134632








