Safety and Efficacy of Achieving Very Low LDL Cholesterol Concentrations with PCSK9 Inhibitors
Abstract
1. Introduction
2. Methods
2.1. Data Sources and Search Strategy
2.2. Study Selection
2.3. Outcomes
2.4. Statistical Analysis
3. Results
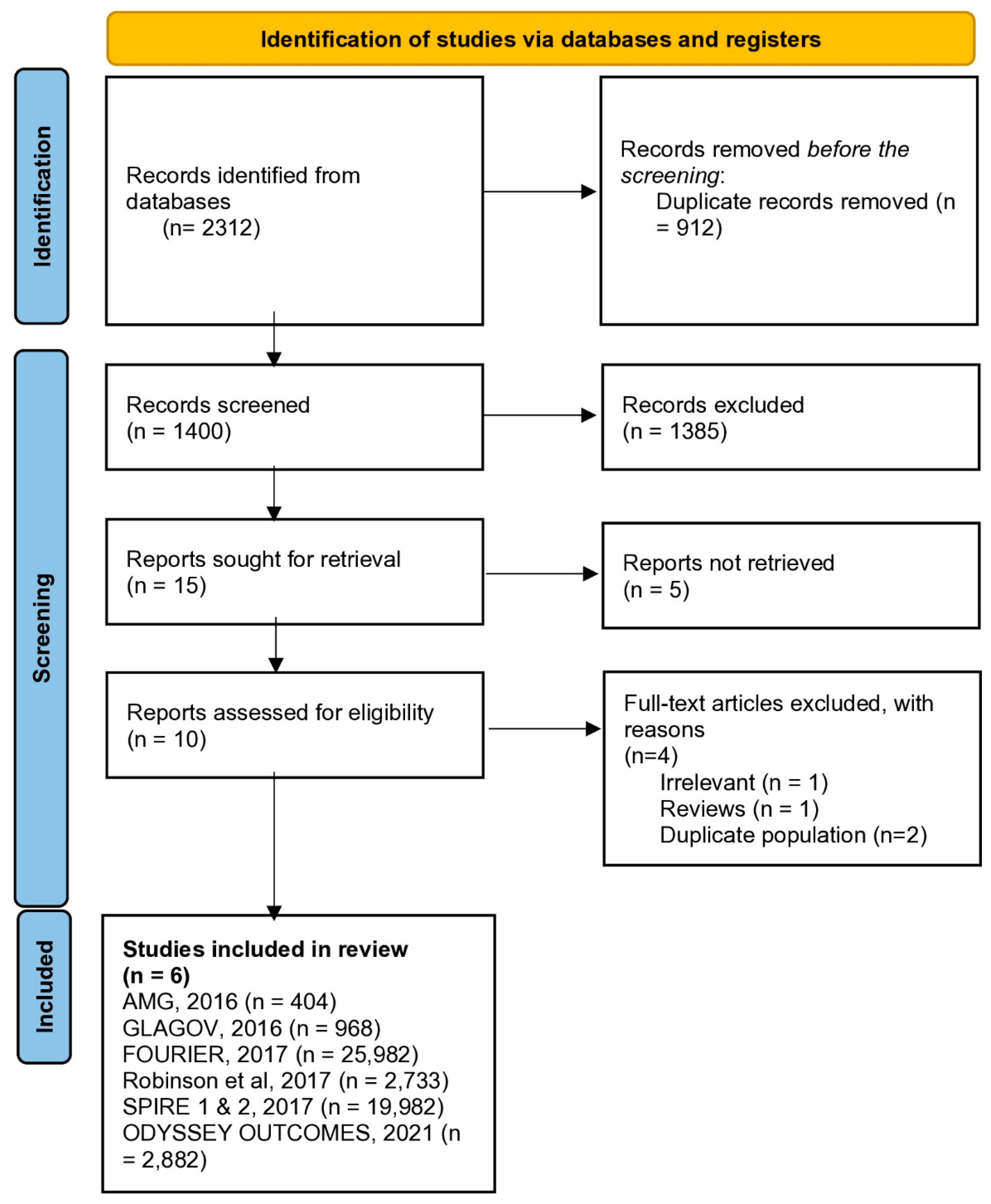
3.1. Search Results
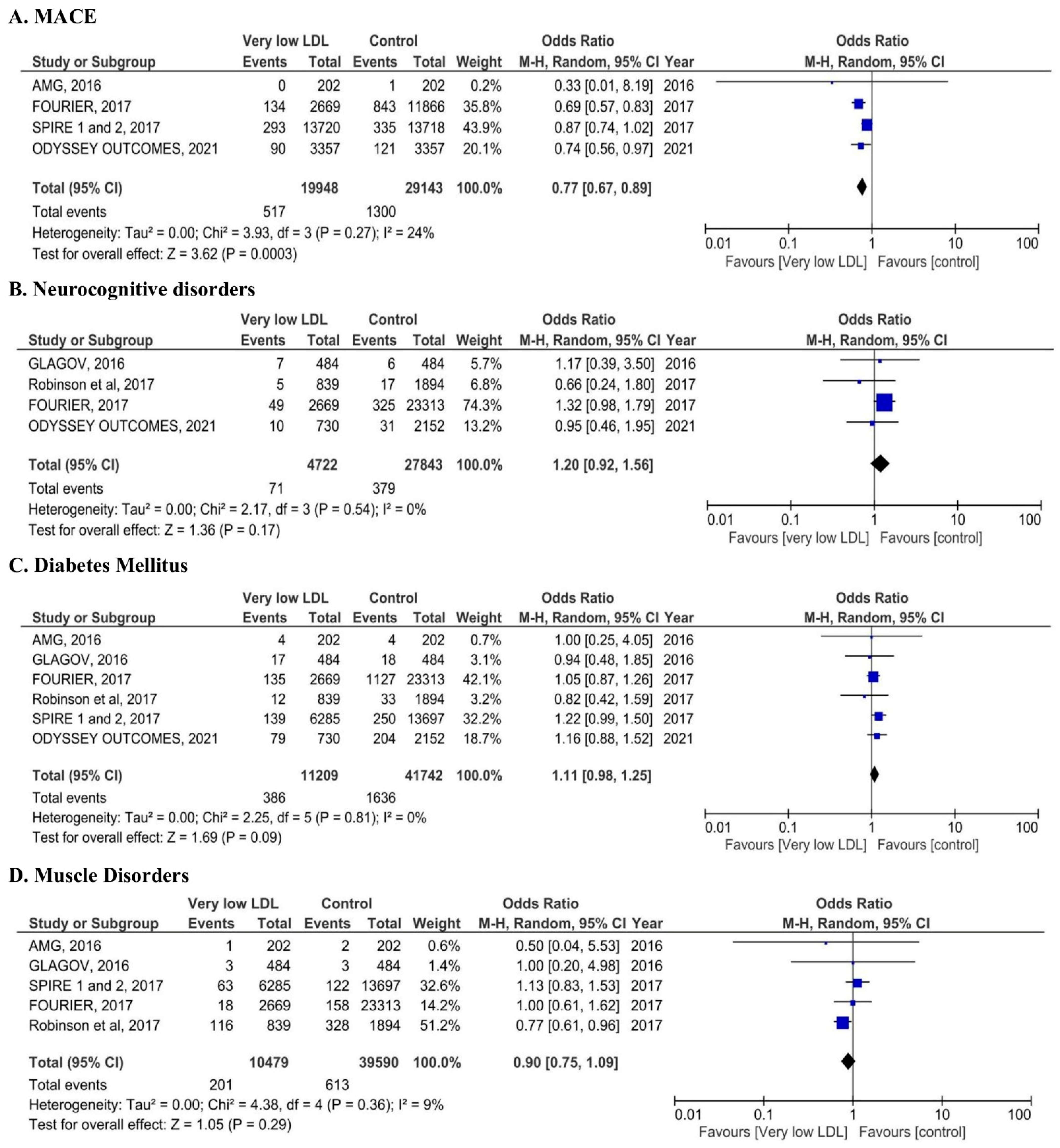
3.2. Safety Outcomes
4. Discussion
4.1. Cardiovascular Benefits of Very Low LDL-C
4.2. Safety Profile of Intensive LDL-C Lowering
4.2.1. Neurocognitive Effects
4.2.2. Diabetes Risk
4.2.3. Myopathy and Cataract
4.2.4. Clinical Standpoint
4.2.5. Mechanistic Considerations
4.2.6. Clinical Implications
4.2.7. Economic Considerations
4.2.8. Alternative Therapies
5. Limitations
6. Future Directions
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CI | Confidence interval |
| LDL-C | Low-density lipoprotein cholesterol |
| MACE | Major adverse cardiovascular event |
| OR | Odds ratio |
| PCSK9 | Proprotein convertase subtilisin/kexin type 9 |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RCT | Randomized controlled trial |
References
- Mhaimeed, O.; Burney, Z.A.; Schott, S.L.; Kohli, P.; Marvel, F.A.; Martin, S.S. The importance of LDL-C lowering in atherosclerotic cardiovascular disease prevention: Lower for longer is better. Am. J. Prev. Cardiol. 2024, 18, 100649. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Borén, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020, 41, 2313–2330. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sniderman, A.D.; Thanassoulis, G.; Glavinovic, T.; Navar, A.M.; Pencina, M.; Catapano, A.; Ference, B.A. Apolipoprotein B Particles and Cardiovascular Disease: A Narrative Review. JAMA Cardiol. 2019, 4, 1287–1295. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cannon, C.P.; Blazing, M.A.; Giugliano, R.P.; McCagg, A.; White, J.A.; Théroux, P.; Darius, H.; Lewis, B.S.; Ophuis, T.O.; Jukema, J.W.; et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N. Engl. J. Med. 2015, 372, 2387–2397. [Google Scholar] [CrossRef] [PubMed]
- Boekholdt, S.M.; Hovingh, G.K.; Mora, S.; Arsenault, B.J.; Amarenco, P.; Pedersen, T.R.; LaRosa, J.C.; Waters, D.D.; DeMicco, D.A.; Simes, R.J.; et al. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: A meta-analysis of statin trials. J. Am. Coll. Cardiol. 2014, 64, 485–494. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ganda, O.P.; Bhatt, D.L.; Mason, R.P.; Miller, M.; Boden, W.E. Unmet Need for Adjunctive Dyslipidemia Therapy in Hypertriglyceridemia Management. J. Am. Coll. Cardiol. 2018, 72, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N. Engl. J. Med. 2018, 379, 2097–2107. [Google Scholar] [CrossRef] [PubMed]
- Raal, F.J.; A Stein, E.; Dufour, R.; Turner, T.; Civeira, F.; Burgess, L.; Langslet, G.; Scott, R.; Olsson, A.G.; Sullivan, D.; et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia. Lancet 2015, 385, 331–340. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. ESCScientific Document Group 2021 ESCGuidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, 3168–3209, Erratum in J. Am. Coll. Cardiol. 2019, 73, 3234–3237. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Cannon, C.P.; Morrow, D.A.; Ray, K.K.; Pfeffer, M.A.; Braunwald, E.; PROVE IT-TIMI 22 Investigators. Can low-density lipoprotein be too low? The safety and efficacy of achieving very low low-density lipoprotein with intensive statin therapy: A PROVE IT-TIMI 22 substudy. J. Am. Coll. Cardiol. 2005, 46, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, R.P.; Wiviott, S.D.; Blazing, M.A.; De Ferrari, G.M.; Park, J.G.; Murphy, S.A.; White, J.A.; Tershakovec, A.M.; Cannon, C.P.; Braunwald, E. Long-term Safety and Efficacy of Achieving Very Low Levels of Low-Density Lipoprotein Cholesterol: A Prespecified Analysis of the IMPROVE-IT Trial. JAMA Cardiol. 2017, 2, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for re-porting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Kiyosue, A.; Honarpour, N.; Kurtz, C.; Xue, A.; Wasserman, S.M.; Hirayama, A. A Phase 3 Study of Evolocumab (AMG 145) in Statin-Treated Japanese Patients at High Cardiovascular Risk. Am. J. Cardiol. 2016, 117, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Puri, R.; Anderson, T.; Ballantyne, C.M.; Cho, L.; Kastelein, J.J.; Koenig, W.; Somaratne, R.; Kassahun, H.; Yang, J.; et al. Effect of Evolocumab on Progression of Coronary Disease in Statin-Treated Patients: The GLAGOV Randomized Clinical Trial. JAMA 2016, 316, 2373–2384. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, R.P.; Pedersen, T.R.; Park, J.-G.; De Ferrari, G.M.; A Gaciong, Z.; Ceska, R.; Toth, K.; Gouni-Berthold, I.; Lopez-Miranda, J.; Schiele, F.; et al. Clinical efficacy and safety of achieving very low LDL-cholesterol concentrations with the PCSK9 inhibitor evolocumab: A prespecified secondary analysis of the FOURIER trial. Lancet 2017, 390, 1962–1971. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.G.; Rosenson, R.S.; Farnier, M.; Chaudhari, U.; Sasiela, W.J.; Merlet, L.; Miller, K.; Kastelein, J.J. Safety of Very Low Low-Density Lipoprotein Cholesterol Levels with Alirocumab: Pooled Data from Randomized Trials. J. Am. Coll. Cardiol. 2017, 69, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Revkin, J.; Amarenco, P.; Brunell, R.; Curto, M.; Civeira, F.; Flather, M.; Glynn, R.J.; Gregoire, J.; Jukema, J.W.; et al. Cardiovascular Efficacy and Safety of Bococizumab in High-Risk Patients. N. Engl. J. Med. 2017, 376, 1527–1539. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.G.; Steg, P.G.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Goodman, S.G.; Jukema, J.W.; Kim, Y.-U.; Li, Q.H.; Manvelian, G.; et al. Clinical Efficacy and Safety of Alirocumab After Acute Coronary Syndrome According to Achieved Level of Low-Density Lipoprotein Cholesterol: A Propensity Score-Matched Analysis of the ODYSSEY OUTCOMES Trial. Circulation 2021, 143, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Cholesterol Treatment Trialists’ Collaboration. Harmonisation of large-scale, heterogeneous individual participant adverse event data from randomised trials of statin therapy. Clin. Trials 2022, 19, 593–604. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ference, B.A.; Robinson, J.G.; Brook, R.D.; Catapano, A.L.; Chapman, M.J.; Neff, D.R.; Voros, S.; Giugliano, R.P.; Davey Smith, G.; Fazio, S.; et al. Variation in PCSK9 and HMGCR and Risk of Cardiovascular Disease and Diabetes. N. Engl. J. Med. 2016, 375, 2144–2153. [Google Scholar] [CrossRef] [PubMed]
- Iatan, I.; Guan, M.; Humphries, K.H.; Yeoh, E.; Mancini, G.B.J. Atherosclerotic Coronary Plaque Regression and Risk of Adverse Cardiovascular Events: A Systematic Review and Updated Meta-Regression Analysis. JAMA Cardiol. 2023, 8, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Räber, L.; Ueki, Y.; Otsuka, T.; Losdat, S.; Häner, J.D.; Lonborg, J.; Fahrni, G.; Iglesias, J.F.; van Geuns, R.-J.; Ondracek, A.S.; et al. Effect of Alirocumab Added to High-Intensity Statin Therapy on Coronary Atherosclerosis in Patients with Acute Myocardial Infarction: The PACMAN-AMIRandomized Clinical Trial. JAMA 2022, 327, 1771–1781. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Kataoka, Y.; Nissen, S.E.; Prati, F.; Windecker, S.; Puri, R.; Hucko, T.; Aradi, D.; Herrman, J.R.; Hermanides, R.S.; et al. Effect of Evolocumab on Coronary Plaque Phenotype and Burden in Statin-Treated Patients Following Myocardial Infarction. JACC Cardiovasc. Imaging 2022, 15, 1308–1321. [Google Scholar] [CrossRef] [PubMed]
- Benn, M.; Nordestgaard, B.G.; Frikke-Schmidt, R.; Tybjærg-Hansen, A. Low LDL cholesterol, PCSK9 and HMGCR genetic variation, and risk of Alzheimer’s disease and Parkinson’s disease: Mendelian randomisation study. BMJ 2017, 357, j1648. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, Y.B.; Kim, M.Y.; Han, K.; Kim, B.; Park, J.; Kim, G.; Hur, K.Y.; Kim, J.H.; Jin, S.M. Association between cholesterol levels and dementia risk according to the presence of diabetes and statin use: A nationwide cohort study. Sci. Rep. 2022, 12, 19383. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giugliano, R.P.; Mach, F.; Zavitz, K.; Kurtz, C.; Im, K.; Kanevsky, E.; Schneider, J.; Wang, H.; Keech, A.; Pedersen, T.R.; et al. Cognitive Function in a Randomized Trial of Evolocumab. N. Engl. J. Med. 2017, 377, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Preiss, D.; Seshasai, S.R.; Welsh, P.; Murphy, S.A.; Ho, J.E.; Waters, D.D.; DeMicco, D.A.; Barter, P.; Cannon, C.P.; Sabatine, M.S.; et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: A meta-analysis. JAMA 2011, 305, 2556–2564. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, D.I.; Preiss, D.; Kuchenbaecker, K.B.; Holmes, M.V.; Engmann, J.E.; Shah, T.; Sofat, R.; Stender, S.; Johnson, P.C.; Scott, R.A.; et al. HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: Evidence from genetic analysis and randomised trials. Lancet 2015, 385, 351–361. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- González-Lleó, A.M.; Sánchez-Hernández, R.M.; Plana, N.; Ibarretxe, D.; Rehues, P.; Ribalta, J.; Llop, D.; Wägner, A.M.; Masana, L.; Boronat, M. Impact of PCSK9 inhibitors in glycaemic control and new-onset diabetes. Cardiovasc. Diabetol. 2024, 23, 4. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Donoghue, M.L.; Giugliano, R.P.; Wiviott, S.D.; Atar, D.; Keech, A.; Kuder, J.F.; Im, K.; Murphy, S.A.; Flores-Arredondo, J.H.; López, J.A.G.; et al. Long-Term Evolocumab in Patients with Established Atherosclerotic Cardiovascular Disease. Circulation 2022, 146, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Everett, B.M.; Mora, S.; Glynn, R.J.; MacFadyen, J.; Ridker, P.M. Safety profile of subjects treated to very low low-density lipoprotein cholesterol levels (<30 mg/dL) with rosuvastatin 20 mg daily (from JUPITER). Am. J. Cardiol. 2014, 114, 1682–1689. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Pradhan, A.; MacFadyen, J.G.; Libby, P.; Glynn, R.J. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: An analysis from the JUPITER trial. Lancet 2012, 380, 565–571. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nissen, S.E.; Stroes, E.; Dent-Acosta, R.E.; Rosenson, R.S.; Lehman, S.J.; Sattar, N.; Preiss, D.; Bruckert, E.; Češka, R.; Lepor, N.; et al. Efficacy Tolerability of Evolocumab vs Ezetimibe in Patients with Muscle-Related Statin Intolerance: The GAUSS-3 Randomized Clinical Trial. JAMA 2016, 315, 1580–1590. [Google Scholar] [CrossRef] [PubMed]
- Braczko, A.; Harasim, G.; Kawecka, A.; Walczak, I.; Kapusta, M.; Narajczyk, M.; Stawarska, K.; Smoleński, R.T.; Kutryb-Zając, B. Blocking cholesterol formation and turnover improves cellular and mitochondria function in murine heart microvascular endothelial cells and cardiomyocytes. Front Physiol. 2023, 14, 1216267. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sever, P.S.; Dahlöf, B.; Poulter, N.R.; Wedel, H.; Beevers, G.; Caulfield, M.; Collins, R.; E Kjeldsen, S.; Kristinsson, A.; McInnes, G.T.; et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): A multicentre randomised controlled trial. Lancet 2003, 361, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Warden, B.A.; Guyton, J.R.; Kovacs, A.C.; Durham, J.A.; Jones, L.K.; Dixon, D.L.; Jacobson, T.A.; Duell, P.B. Assessment and management of statin-associated muscle symptoms (SAMS): A clinical perspective from the National Lipid Association. J. Clin. Lipidol. 2023, 17, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Golder, S.; Klein, A.; O’Connor, K.; Wang, Y.; Gonzalez-Hernandez, G. Social Media Posts on Statins: What Can We Learn About Patient Experiences and Perspectives? J. Am. Heart Assoc. 2024, 13, e033992. [Google Scholar] [CrossRef] [PubMed]
- Lambert, G.; Sjouke, B.; Choque, B.; Kastelein, J.J.; Hovingh, G.K. The PCSK9 decade. J. Lipid Res. 2012, 53, 2515–2524. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Juul, S.E.; Comstock, B.A.; Wadhawan, R.; Mayock, D.E.; Courtney, S.E.; Robinson, T.; Ahmad, K.A.; Bendel-Stenzel, E.; Baserga, M.; LaGamma, E.F.; et al. A Randomized Trial of Erythropoietin for Neuroprotection in Preterm Infants. N. Engl. J. Med. 2020, 382, 233–243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ridker, P.M.; Shih, J.; Cook, T.J.; Clearfield, M.; Downs, J.R.; Pradhan, A.D.; Weis, S.E.; Gotto, A.M., Jr. Plasma homocysteine concentration, statin therapy, and the risk of first acute coronary events. Circulation 2002, 105, 1776–1779. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Catapano, A.L.; Norata, G.D. Biological Consequences of Dysfunctional HDL. Curr. Med. Chem. 2019, 26, 1644–1664. [Google Scholar] [CrossRef] [PubMed]
- Writing Committee; Lloyd-Jones, D.M.; Morris, P.B.; Ballantyne, C.M.; Birtcher, K.K.; Covington, A.M.; Wilkins, J.T. 2022 ACC Expert Consensus Decision Pathway on the Role of Nonstatin Therapies for LDL-Cholesterol Lowering in the Management of Atherosclerotic Cardiovascular Disease Risk: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2022, 80, 1366–1418, Erratum in J. Am. Coll. Cardiol. 2023, 81, 104. [Google Scholar] [CrossRef] [PubMed]
- Fonarow, G.C.; Keech, A.C.; Pedersen, T.R.; Giugliano, R.P.; Sever, P.S.; Lindgren, P.; van Hout, B.; Villa, G.; Qian, Y.; Somaratne, R.; et al. Cost-effectiveness of Evolocumab Therapy for Reducing Cardiovascular Events in Patients with Atherosclerotic Cardiovascular Disease. JAMA Cardiol. 2017, 2, 1069–1078. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kazi, D.S.; Penko, J.; Coxson, P.G.; Moran, A.E.; Ollendorf, D.A.; Tice, J.A.; Bibbins-Domingo, K. Updated Cost-effectiveness Analysis of PCSK9 Inhibitors Based on the Results of the FOURIER Trial. JAMA 2017, 318, 748–750. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baum, S.J.; Toth, P.P.; Underberg, J.A.; Jellinger, P.; Ross, J.; Wilemon, K. PCSK9 inhibitor access barriers-issues and recommendations: Improving the access process for patients, clinicians and payers. Clin Cardiol. 2017, 40, 243–254. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bohula, E.A.; Marston, N.A.; Ruzza, A.; Murphy, S.A.; De Ferrari, G.M.; Diaz, R.; Leiter, L.A.; Elliott-Davey, M.; Wang, H.; Bhatia, A.K.; et al. Rationale and design of the effect of evolocumab in patients at high cardiovascular risk without prior myocardial infarction or stroke (VESALIUS-CV) trial. Am. Heart J. 2024, 269, 179–190. [Google Scholar] [CrossRef] [PubMed]

| Trial | Patient Characteristics | n | PCKS9i Tested | LDL-C Values in the Treatment Group | LDL-C Values in the Control Group | Follow up Duration (months) | |
|---|---|---|---|---|---|---|---|
| Very Low LDL-C | Control | ||||||
| AMG 145 [16] | Age: ≥20 and ≤85 years Population: Japanese | 202 | 202 | Evolocumab | Mean 26 mg/dL | Mean 103 mg/dl | 3 |
| GLAGOV [17] | Age: ≥18 years Population: North America | 484 | 484 | Evolocumab | Mean 37 mg/dL | Mean 93 mg/d | 19 |
| FOURIER [18] | Age: ≥40 and ≤85 years Population: Multinational | 2669 | 23,313 | Evolocumab | <19 mg/dL | ≥68 mg/dL | 26.4 |
| Robinson et al. [19] | Age: ≥18 years Population: Multinational | 839 | 1894 | Alirocumab | <25 mg/dL | LDL ≥25 mg/dL | 26 |
| SPIRE 1 and 2 [20] | Age: Men ≥ 50 years *, Women ≥ 60 years * Population: Multinational | 6285 | 13,697 | Bococizumab | ≤25 mg/dL | ≥70 mg/dL | SPIRE 1: 7 SPIRE 2: 12 |
| ODYSSEY OUTCOMES [21] | Age: ≥18 years Population: Multinational | 730 | 2152 | Alirocumab | <15 mg/dL for safety Analyses <25 mg/dL for efficacy analyses | ≥50 mg/dL | 36 |
| AMG 145 [16] | GLAGOV [17] | FOURIER [18] | Robinson et al. [19] | SPIRE 1 and 2 [20] | ODYSSEY OUTCOMES [21] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Very Low LDL-C | Control | Very Low LDL-C | Control | Very Low LDL-C | Control | Very Low LDL-C | Control | Very Low LDL-C | Control | Very Low LDL-C | Control | |
| % | 50 | 50 | 50 | 50 | 17 | 83 | 25 | 75 | 50 | 50 | 50 | 50 |
| Age (years) | 61 | 62 | 60 | 60 | 63 | 61 | 62 | 59 | 63 | 63 | 58 | 58 |
| Female Gender | 39 | 40 | 28 | 28 | 28 | 22 | 25 | 43 | 29 | 30 | 20 | 33 |
| CAD | 11 | 15 | - | - | - | - | 77 | 61 | - | - | - | - |
| PAD | 14 | 12 | - | - | 14 | 14 | - | - | 3 | 5 | ||
| DM | 51 | 47 | 20 | 22 | 34 | 38 | 42 | 28 | 48 | 47 | 29 | 28 |
| HTN | 72 | 75 | 82 | 84 | 81 | 80 | - | - | 81 | 80 | - | - |
| Current smokers | 26 | 23 | 26 | 23 | 32 | 28 | 22 | 26 | 25 | 24 | 25 | 26 |
| Prior MI | - | - | 35 | 35 | 81 | 81 | - | - | - | - | - | - |
| Prior PCI | - | - | 39 | 39 | - | - | - | - | - | - | 15 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machanahalli Balakrishna, A.; Kaushik, S.; Tandalam Palanivelu, S.; Monther, N.; Ponamgi, S.P.; Alla, V.M.; Patil, S.M. Safety and Efficacy of Achieving Very Low LDL Cholesterol Concentrations with PCSK9 Inhibitors. J. Clin. Med. 2025, 14, 4562. https://doi.org/10.3390/jcm14134562
Machanahalli Balakrishna A, Kaushik S, Tandalam Palanivelu S, Monther N, Ponamgi SP, Alla VM, Patil SM. Safety and Efficacy of Achieving Very Low LDL Cholesterol Concentrations with PCSK9 Inhibitors. Journal of Clinical Medicine. 2025; 14(13):4562. https://doi.org/10.3390/jcm14134562
Chicago/Turabian StyleMachanahalli Balakrishna, Akshay, Sharanya Kaushik, Sangeetha Tandalam Palanivelu, Noorhan Monther, Shiva P. Ponamgi, Venkata Mahesh Alla, and Shantanu M. Patil. 2025. "Safety and Efficacy of Achieving Very Low LDL Cholesterol Concentrations with PCSK9 Inhibitors" Journal of Clinical Medicine 14, no. 13: 4562. https://doi.org/10.3390/jcm14134562
APA StyleMachanahalli Balakrishna, A., Kaushik, S., Tandalam Palanivelu, S., Monther, N., Ponamgi, S. P., Alla, V. M., & Patil, S. M. (2025). Safety and Efficacy of Achieving Very Low LDL Cholesterol Concentrations with PCSK9 Inhibitors. Journal of Clinical Medicine, 14(13), 4562. https://doi.org/10.3390/jcm14134562






