Sina Score as a New Machine Learning-Derived Online Prediction Model of Mortality for Cirrhotic Patients Awaiting Liver Transplantation: A Prospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Devising a Prediction Scale in Cirrhosis
2.2. The Inclusion and Exclusion Criteria
2.3. Statistical Analysis
3. Results
3.1. Demographic, Anthropometric, and Laboratory Findings
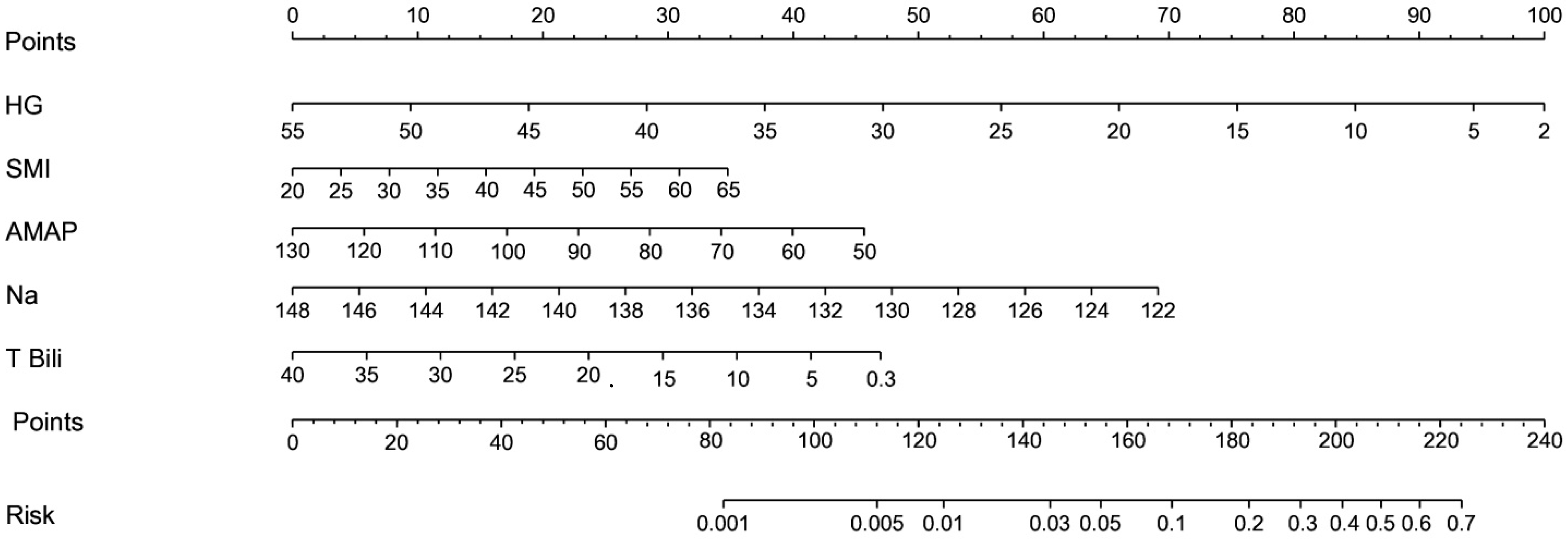
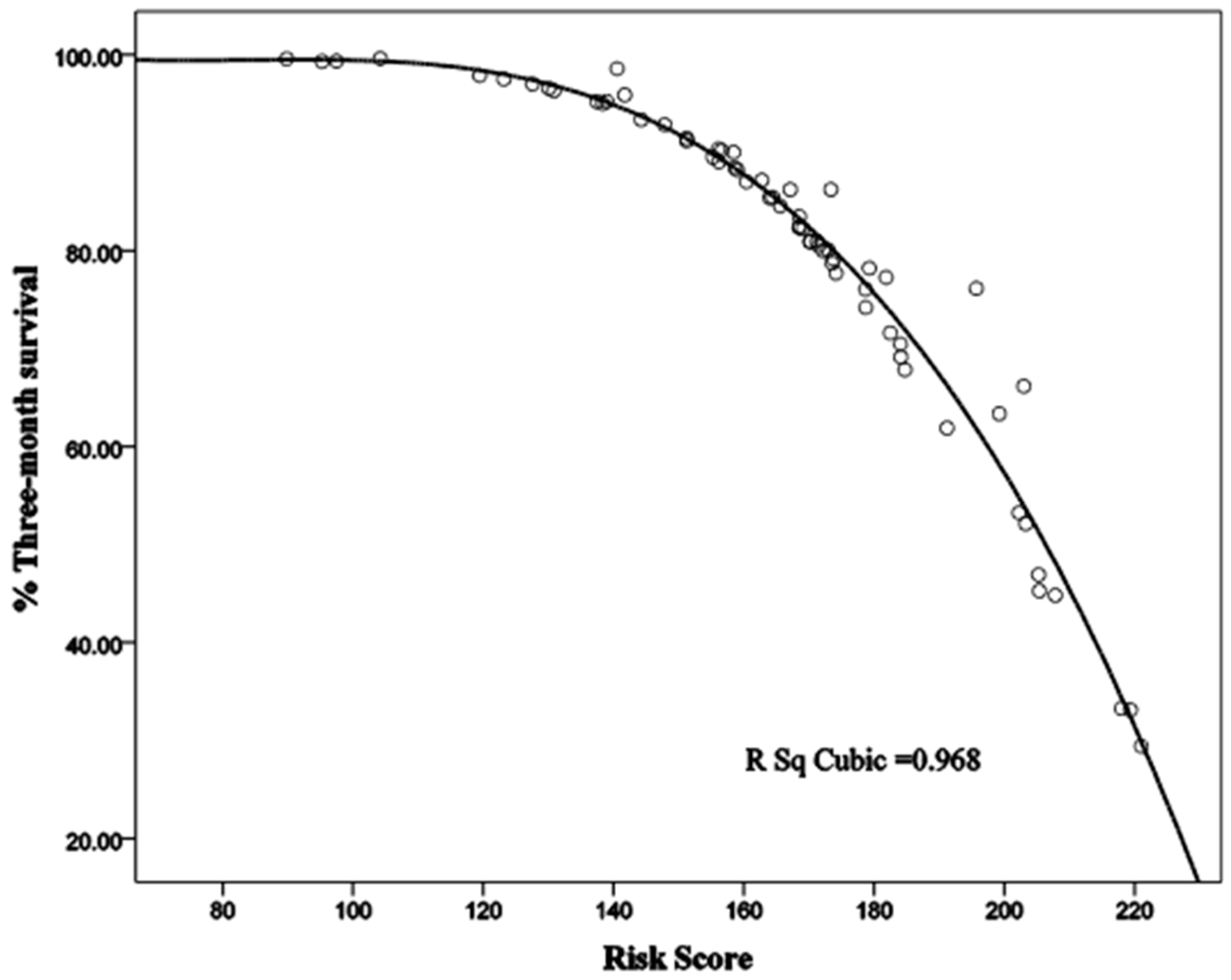
3.2. The Prediction Scale in Cirrhosis
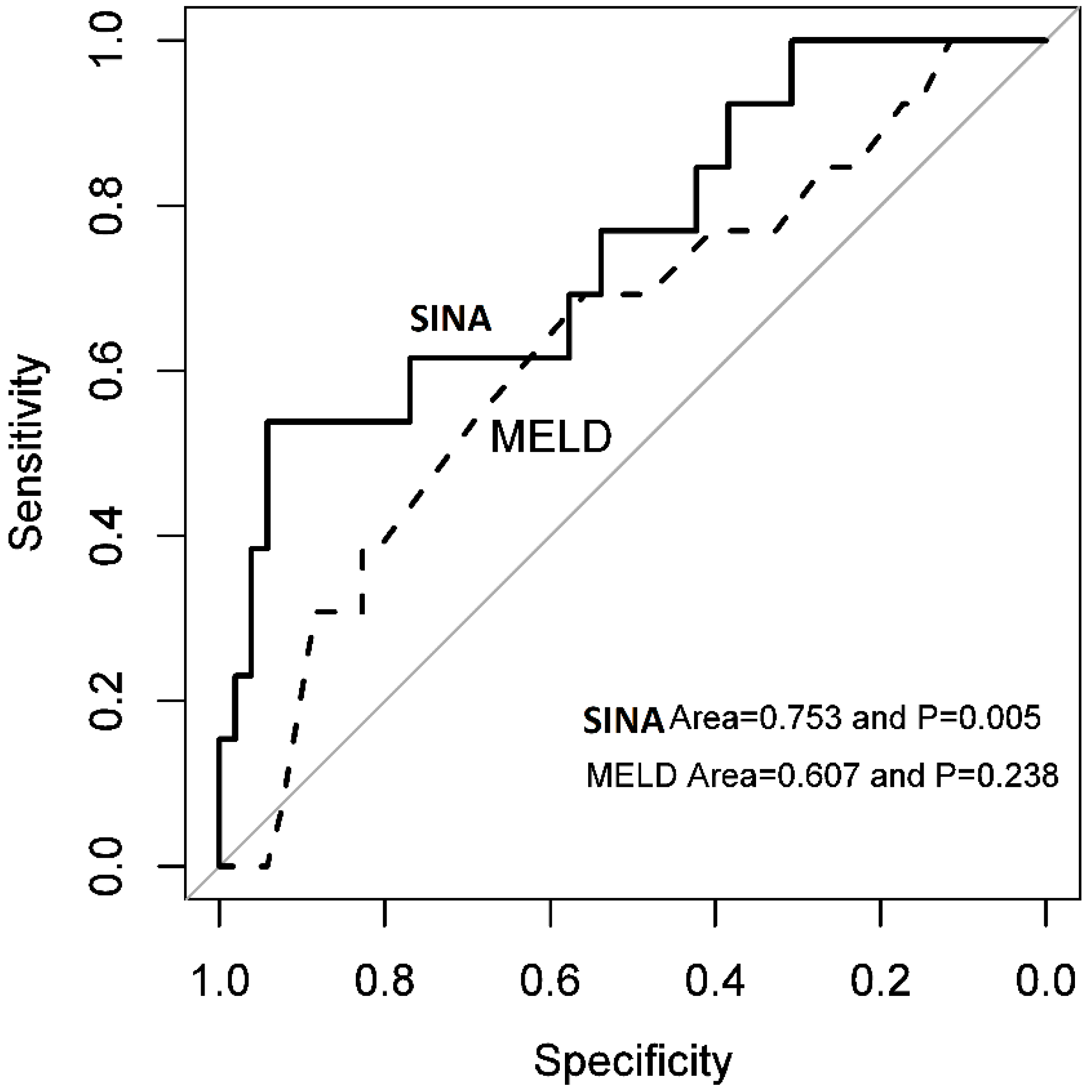
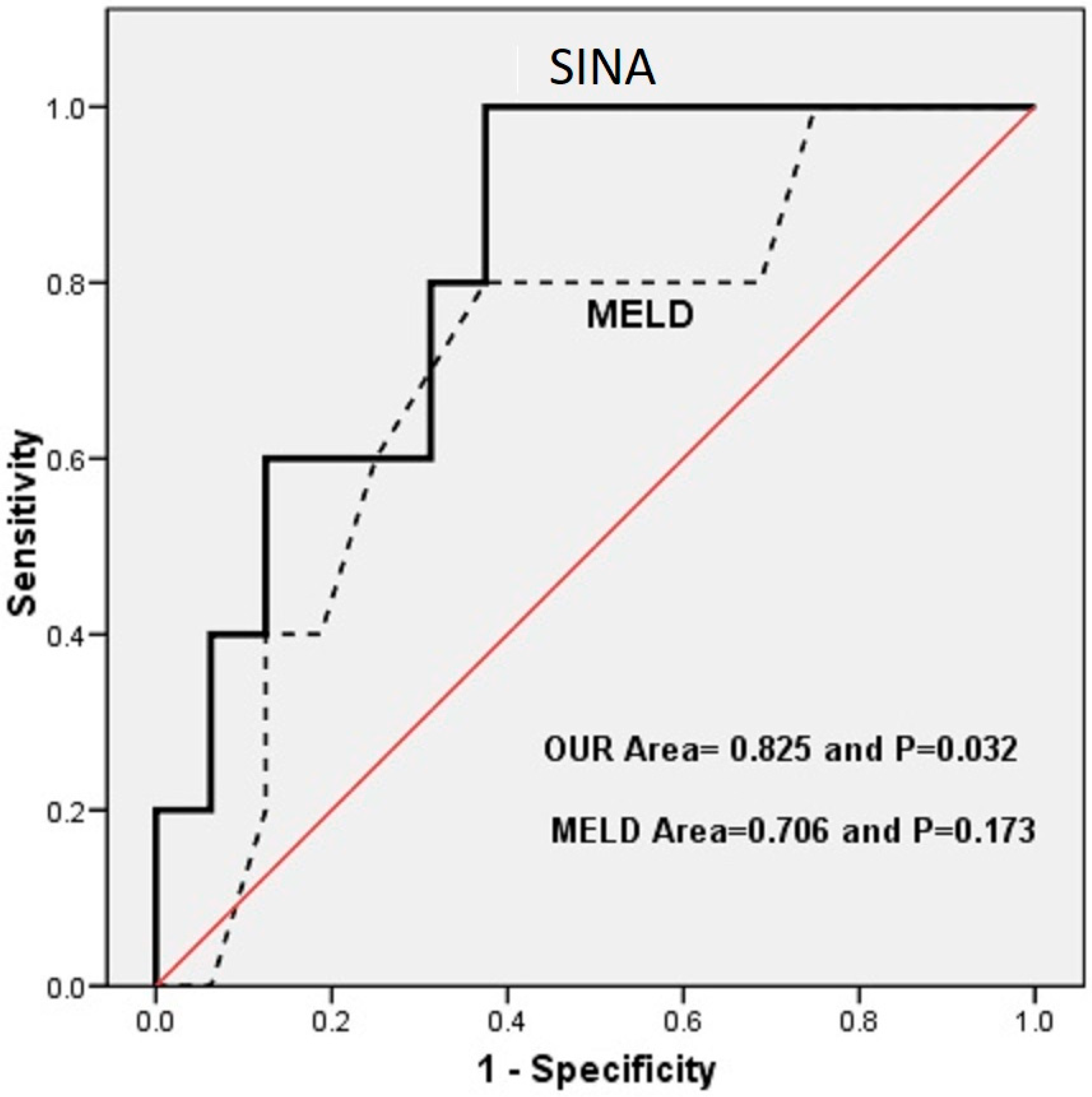
3.3. Sina Scoring System
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 245–266. [Google Scholar] [CrossRef] [PubMed]
- Zhai, M.; Long, J.; Liu, S.; Liu, C.; Li, L.; Yang, L.; Li, Y.; Shu, B. The burden of liver cirrhosis and underlying etiologies: Results from the global burden of disease study 2017. Aging 2021, 13, 279–300. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Hepatitis B Collaborators. Global, regional, and national burden of hepatitis B, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol. Hepatol. 2022, 7, 796–829. [Google Scholar] [CrossRef] [PubMed]
- Veracruz, N.; Gish, R.G.; Cheung, R.; Chitnis, A.S.; Wong, R.J. Global trends and the impact of chronic hepatitis B and C on disability-adjusted life years. Liver Int. 2022, 42, 2145–2153. [Google Scholar] [CrossRef]
- Liu, Y.B.; Chen, M.K. Epidemiology of liver cirrhosis and associated complications: Current knowledge and future directions. World J. Gastroenterol. 2022, 28, 5910–5930. [Google Scholar] [CrossRef]
- Ginès, P.; Krag, A.; Abraldes, J.G.; Solà, E.; Fabrellas, N.; Kamath, P.S. Liver cirrhosis. Lancet 2021, 398, 1359–1376. [Google Scholar] [CrossRef]
- O’Leary, J.G.; Lepe, R.; Davis, G.L. Indications for liver transplantation. Gastroenterology 2008, 134, 1764–1776. [Google Scholar] [CrossRef]
- Tenório, A.L.; Macedo, F.I.; Miranda, L.E.; Fernandes, J.L.; da Silva, C.M.; Neto, O.L.; Lacerda, C. Survival on waiting list for liver transplantation before and after introduction of the model for end-stage liver disease score. Transplant. Proc. 2010, 42, 407–411. [Google Scholar] [CrossRef]
- Kim, W.R.; Mannalithara, A.; Heimbach, J.K.; Kamath, P.S.; Asrani, S.K.; Biggins, S.W.; Wood, N.L.; Gentry, S.E.; Kwong, A.J. MELD 3.0: The Model for End-Stage Liver Disease Updated for the Modern Era. Gastroenterology 2021, 161, 1887–1895.e4. [Google Scholar] [CrossRef]
- Tandon, P.; Montano-Loza, A.J.; Lai, J.C.; Dasarathy, S.; Merli, M. Sarcopenia and frailty in decompensated cirrhosis. J. Hepatol. 2021, 75, S147–S162. [Google Scholar] [CrossRef]
- Pashayee-Khamene, F.; Hajimohammadebrahim-Ketabforoush, M.; Shahrbaf, M.A.; Saadati, S.; Karimi, S.; Hatami, B.; Rashidkhani, B.; Ahmadzadeh, S.; Kord-Varkaneh, H.; Hekmatdoost, A. Malnutrition and its association with the mortality in liver cirrhosis; a prospective nutritional assessment in two referral centers in Iran. Clin. Nutr. ESPEN 2023, 54, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Yang, W.; Wang, S.; Hui, Y.; Cui, B.; Wang, X.; Li, C.; Mao, L.; Fan, X.; Sun, C. Handgrip strength is a substitutive metric to the GLIM criteria-defined malnutrition and predicts long-term mortality among hospitalized patients with cirrhosis. Nutr. Clin. Pract. 2023, 38, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C.P.T.; Dalcumune, L.F.; Fiorese, N.M.; Trindade, L.Z.; Ferreira, F.B.; Pacheco, M.P. Diminished Hand Grip Strength And Cirrhosis: Prevalence And Associated Factors. Arq. Gastroenterol. 2023, 60, 431–437. [Google Scholar] [CrossRef]
- Ramachandran, G.; Pottakkat, B.; Mohan, P.; Basu, S. Effectiveness of different tools for malnutrition in the assessment of patients with cirrhosis. Am. J. Med. Sci. 2024, 368, 61–67. [Google Scholar] [CrossRef]
- Montano-Loza, A.J.; Meza-Junco, J.; Prado, C.M.; Lieffers, J.R.; Baracos, V.E.; Bain, V.G.; Sawyer, M.B. Muscle wasting is associated with mortality in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 2012, 10, 166–173.e1. [Google Scholar] [CrossRef]
- Dehghani, S.M.; Gholami, S.; Bahador, A.; Haghighat, M.; Imanieh, M.H.; Nikeghbalian, S.; Salahi, H.; Davari, H.; Mehrabani, D.; Malek-Hosseini, S. Comparison of Child-Turcotte-Pugh and pediatric end-stage liver disease scoring systems to predict morbidity and mortality of children awaiting liver transplantation. Transplant. Proc. 2007, 39, 3175–3177. [Google Scholar] [CrossRef]
- Dehghani, S.M.; Gholami, S.; Bahador, A.; Nikeghbalian, S.; Salahi, H.; Imanieh, M.H.; Haghighat, M.; Davari, H.R.; Mehrabani, D.; Malek-Hosseini, S.A. Morbidity and mortality of children with chronic liver diseases who were listed for liver transplantation in Iran. Pediatr. Transplant. 2007, 11, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, S.M.; Bahador, A.; Gholami, S.; Nikeghbalian, S.; Salahi, H.; Imanieh, M.H.; Haghighat, M.; Davari, H.R.; Serati, Z.; Mehrabani, D.; et al. Pediatric liver transplantation in Iran: Evaluation of the first 50 cases. Pediatr. Transplant. 2007, 11, 256–260. [Google Scholar] [CrossRef]
- Buchard, B.; Boirie, Y.; Cassagnes, L.; Lamblin, G.; Coilly, A.; Abergel, A. Assessment of Malnutrition, Sarcopenia and Frailty in Patients with Cirrhosis: Which Tools Should We Use in Clinical Practice? Nutrients 2020, 12, 186. [Google Scholar] [CrossRef]
- Beaudart, C.; McCloskey, E.; Bruyère, O.; Cesari, M.; Rolland, Y.; Rizzoli, R.; Araujo De Carvalho, I.; Amuthavalli Thiyagarajan, J.; Bautmans, I.; Bertière, M.-C.; et al. Sarcopenia in daily practice: Assessment and management. BMC Geriatr. 2016, 16, 170. [Google Scholar] [CrossRef]
- Ahmed, I. Sarcopenia: Hand grip dynamometers, the latest addition to the doctor’s bag. Br. J. Gen. Pract. 2020, 70, 279–280. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.J.; Lal, S.; Strauss, B.J.; Todd, C.; Pilling, M.; Burden, S.T. Measurement of Muscle Mass and Sarcopenia Using Anthropometry, Bioelectrical Impedance, and Computed Tomography in Surgical Patients with Colorectal Malignancy: Comparison of Agreement Between Methods. Nutr. Cancer 2020, 72, 1074–1083. [Google Scholar] [CrossRef]
- Patidar, K.R.; Peng, J.L.; Pike, F.; Orman, E.S.; Glick, M.; Kettler, C.D.; Nephew, L.D.; Desai, A.P.; Nair, K.; Khan, B.A. Associations Between Mean Arterial Pressure and Poor ICU Outcomes in Critically Ill Patients With Cirrhosis: Is 65 The Sweet Spot? Crit. Care Med. 2020, 48, e753–e760. [Google Scholar] [CrossRef]
- Mallik, M.; Singhai, A.; Khadanga, S.; Ingle, V. The Significant Morbidity and Mortality Indicators in Patients of Cirrhosis. Cureus 2022, 14, e21226. [Google Scholar] [CrossRef]
- Chirapongsathorn, S.; Bunraksa, W.; Chaiprasert, A.; Punpanich, D.; Supasyndh, O.; Kamath, P.S. Adding C-reactive protein and procalcitonin to the model of end-stage liver disease score improves mortality prediction in patients with complications of cirrhosis. J. Gastroenterol. Hepatol. 2018, 33, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Perálvarez, M.L.; de la Rosa, G.; Gómez-Orellana, A.M.; Aguilera, M.V.; Pascual Vicente, T.; Pereira, S.; Ortiz, M.L.; Pagano, G.; Suarez, F.; Grande, R.G.; et al. GEMA-Na and MELD 3.0 severity scores to address sex disparities for accessing liver transplantation: A nationwide retrospective cohort study. EClinicalMedicine 2024, 74, 102737. [Google Scholar] [CrossRef] [PubMed]
- Bhakta, D.; Patel, M.; Ma, T.W.; Boutté, J.; Sarmast, N.; Asrani, S.K. Model for End-Stage Liver Disease Lactate Score and Prediction of Inpatient Mortality in Critically Ill Patients with Cirrhosis. Liver Transpl. 2021, 27, 1861–1864. [Google Scholar] [CrossRef]
- Deng, Y.; Lin, L.; Fan, X.; Cui, B.; Hou, L.; Zhao, T.; Hou, J.; Mao, L.; Wang, X.; Zhao, W.; et al. Incorporation of frailty estimated by gait speed within MELD-Na and the predictive potential for mortality in cirrhosis. Ther. Adv. Chronic Dis. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Cullaro, G.; Verna, E.C.; Duarte-Rojo, A.; Kappus, M.R.; Ganger, D.R.; Rahimi, R.S.; Boyarsky, B.; Segev, D.L.; McAdams-DeMarco, M.; Ladner, D.P.; et al. Frailty and the Risk of Acute Kidney Injury Among Patients With Cirrhosis. Hepatol. Commun. 2022, 6, 910–919. [Google Scholar] [CrossRef]
- Montano-Loza, A.J.; Duarte-Rojo, A.; Meza-Junco, J.; Baracos, V.E.; Sawyer, M.B.; Pang, J.X.; Beaumont, C.; Esfandiari, N.; Myers, R.P. Inclusion of Sarcopenia within MELD (MELD-Sarcopenia) and the Prediction of Mortality in Patients with Cirrhosis. Clin. Transl. Gastroenterol. 2015, 6, e102. [Google Scholar] [CrossRef]
- Tapper, E.B.; Zhang, P.; Garg, R.; Nault, T.; Leary, K.; Krishnamurthy, V.; Su, G.L. Body composition predicts mortality and decompensation in compensated cirrhosis patients: A prospective cohort study. JHEP Rep. 2019, 2, 100061. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Taylor, T.J.; Kramer, J.R.; Cao, Y.; Smith, D.; Gifford, A.L.; El-Serag, H.B.; Naik, A.D.; Asch, S.M. Development, Validation, and Evaluation of a Simple Machine Learning Model to Predict Cirrhosis Mortality. JAMA Netw. Open 2020, 3, e2023780. [Google Scholar] [CrossRef] [PubMed]
- Maharshi, S.; Sharma, B.C.; Srivastava, S. Malnutrition in cirrhosis increases morbidity and mortality. J. Gastroenterol. Hepatol. 2015, 30, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.; Mazumder, N.R.; Ladner, D.P.; Foraker, R.E. Predicting mortality among patients with liver cirrhosis in electronic health records with machine learning. PLoS ONE 2021, 16, e0256428. [Google Scholar] [CrossRef]
- Chang, K.V.; Chen, J.D.; Wu, W.T.; Huang, K.C.; Han, D.S. Association of loss of muscle mass with mortality in liver cirrhosis without or before liver transplantation: A systematic review and meta-analysis. Medicine 2019, 98, e14373. [Google Scholar] [CrossRef]
| Variable | Mean | SD | p Value | OR | p Value (ULR) | |
|---|---|---|---|---|---|---|
| Age (years) | Expired | 53.77 | 9.293 | 0.006 | 1.073 | 0.025 |
| Survived | 44.25 | 13.252 | ||||
| HG (kg) | Expired | 16.51 | 8.682 | 0.013 | 0.913 | 0.025 |
| Survived | 24.14 | 10.517 | ||||
| MAMC | Expired | 21.06 | 2.831 | 0.522 | 0.964 | 0.749 |
| Survived | 21.33 | 2.725 | ||||
| SMI | Expired | 41.03 | 6.708 | 0.634 | 0.985 | 0.671 |
| Survived | 42.16 | 9.120 | ||||
| AMAP | Expired | 76.85 | 16.582 | 0.093 | 0.940 | 0.093 |
| Survived | 82.39 | 8.343 | ||||
| WBC | Expired | 6.38 | 2.288 | 0.475 | 0.061 | 0.576 |
| Survived | 5.90 | 2.944 | ||||
| Hemoglobin | Expired | 11.49 | 1.706 | 0.313 | 0.858 | 0.314 |
| Survived | 12.14 | 2.167 | ||||
| Platelets | Expired | 122.87 | 115.416 | 0.587 | 0.998 | 0.580 |
| Survived | 142.65 | 115.865 | ||||
| PTT | Expired | 40.45 | 5.750 | 0.930 | 0.997 | 0.938 |
| Survived | 40.62 | 7.438 | ||||
| INR | Expired | 1.62 | 0.462 | 0.764 | 0.881 | 0.806 |
| Survived | 1.67 | 0.676 | ||||
| BUN | Expired | 20.77 | 10.305 | 0.170 | 1.024 | 0.267 |
| Survived | 15.98 | 13.080 | ||||
| Cr | Expired | 0.90 | 0.234 | 0.756 | 0.659 | 0.755 |
| Survived | 0.93 | 0.244 | ||||
| Total protein | Expired | 6.67 | 0.702 | 0.242 | 0.662 | 0.290 |
| Survived | 6.94 | 0.867 | ||||
| Albumin | Expired | 3.09 | 0.461 | 0.049 | 0.477 | 0.130 |
| Survived | 3.43 | 0.753 | ||||
| AST | Expired | 93.23 | 79.840 | 0.740 | 1.001 | 0.744 |
| Survived | 84.77 | 85.633 | ||||
| ALT | Expired | 55.77 | 39.220 | 0.239 | 0.995 | 0.495 |
| Survived | 79.40 | 120.052 | ||||
| Total bilirubin | Expired | 4.19 | 2.994 | 0.628 | 0.984 | 0.756 |
| Survived | 4.83 | 7.272 | ||||
| Direct bilirubin | Expired | 1.86 | 2.195 | 0.490 | 0.956 | 0.630 |
| Survived | 2.46 | 4.301 | ||||
| Alkaline Phosphatase | Expired | 382.52 | 227.033 | 0.906 | 1.000 | 0.927 |
| Survived | 392.18 | 367.072 | ||||
| Na | Expired | 135.25 | 4.996 | 0.022 | 0.874 | 0.030 |
| Survived | 138.83 | 4.893 | ||||
| K | Expired | 4.24 | 0.232 | 0.993 | 0.994 | 0.993 |
| Survived | 4.24 | 0.585 | ||||
| Variable | Range of Variable | * | Rank | Assigned Point | Linear Interpolation Equation | |
|---|---|---|---|---|---|---|
| HG | −0.0956 | 51 to 2 by 5 | 4.6844 | 1 | 0 assigned to HG = 51 100 × (4.6844/4.6844) = 100 assigned to HG = 2 | Point = 104.08 − 2.0408 × HG |
| SMI | 0.0351 | 22 to 63 by 5 | 1.4391 | 5 | 0 assigned to SMI = 22 100 × (1.4391/4.6844) = 30.72 assigned to SMI = 63 | Point = −16.48 + 0.7493 × SMI |
| Average MAP | −0.0308 | 123 to 50 by 10 | 2.2484 | 3 | 0 assigned to AMAP = 123 100 × (2.2484/4.6844) = 48.00 assigned to AMAP = 50 | Point = 80.88 − 0.6575 × AMAP |
| Na | −0.1324 | 147 to 123 by 2 | 3.1776 | 2 | 0 assigned to Na = 147 100 × (3.1776/4.6844) = 67.83 assigned to Na = 123 | Point = 415.46 − 2.8263 × Sodium |
| Total bilirubin | −0.0520 | 37.02 to 0.42 by 5 | 1.9032 | 4 | 0 assigned to total bilirubin = 147 100 × (1.9032/4.6844) = 40.63 assigned to total bilirubin = 0.42 | Point = 40.75 − 0.2772 × Total bilirubin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosseini-Asl, S.M.K.; Masoumi, S.J.; Rashidizadeh, G.; Hassani, A.H.; Mehrabani, G.; Ebrahimi, V.; Malek-Hosseini, S.A.; Nikeghbalian, S.; Shakibafard, A. Sina Score as a New Machine Learning-Derived Online Prediction Model of Mortality for Cirrhotic Patients Awaiting Liver Transplantation: A Prospective Cohort Study. J. Clin. Med. 2025, 14, 4559. https://doi.org/10.3390/jcm14134559
Hosseini-Asl SMK, Masoumi SJ, Rashidizadeh G, Hassani AH, Mehrabani G, Ebrahimi V, Malek-Hosseini SA, Nikeghbalian S, Shakibafard A. Sina Score as a New Machine Learning-Derived Online Prediction Model of Mortality for Cirrhotic Patients Awaiting Liver Transplantation: A Prospective Cohort Study. Journal of Clinical Medicine. 2025; 14(13):4559. https://doi.org/10.3390/jcm14134559
Chicago/Turabian StyleHosseini-Asl, Seyed Mohammad Kazem, Seyed Jalil Masoumi, Ghazaleh Rashidizadeh, Amir Hossein Hassani, Golnoush Mehrabani, Vahid Ebrahimi, Seyed Ali Malek-Hosseini, Saman Nikeghbalian, and Alireza Shakibafard. 2025. "Sina Score as a New Machine Learning-Derived Online Prediction Model of Mortality for Cirrhotic Patients Awaiting Liver Transplantation: A Prospective Cohort Study" Journal of Clinical Medicine 14, no. 13: 4559. https://doi.org/10.3390/jcm14134559
APA StyleHosseini-Asl, S. M. K., Masoumi, S. J., Rashidizadeh, G., Hassani, A. H., Mehrabani, G., Ebrahimi, V., Malek-Hosseini, S. A., Nikeghbalian, S., & Shakibafard, A. (2025). Sina Score as a New Machine Learning-Derived Online Prediction Model of Mortality for Cirrhotic Patients Awaiting Liver Transplantation: A Prospective Cohort Study. Journal of Clinical Medicine, 14(13), 4559. https://doi.org/10.3390/jcm14134559





