Characteristics and Treatment Patterns of Patients with Haemophilia B Receiving Recombinant Coagulation Factor IX
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Sources
2.3. Outcome Measures
2.4. Statistical Analysis
3. Results
3.1. Patients’ Characteristics and Treatment Patterns
3.2. Prophylacxis Uptake and FIX Exposure
3.3. Patients’ Adherence
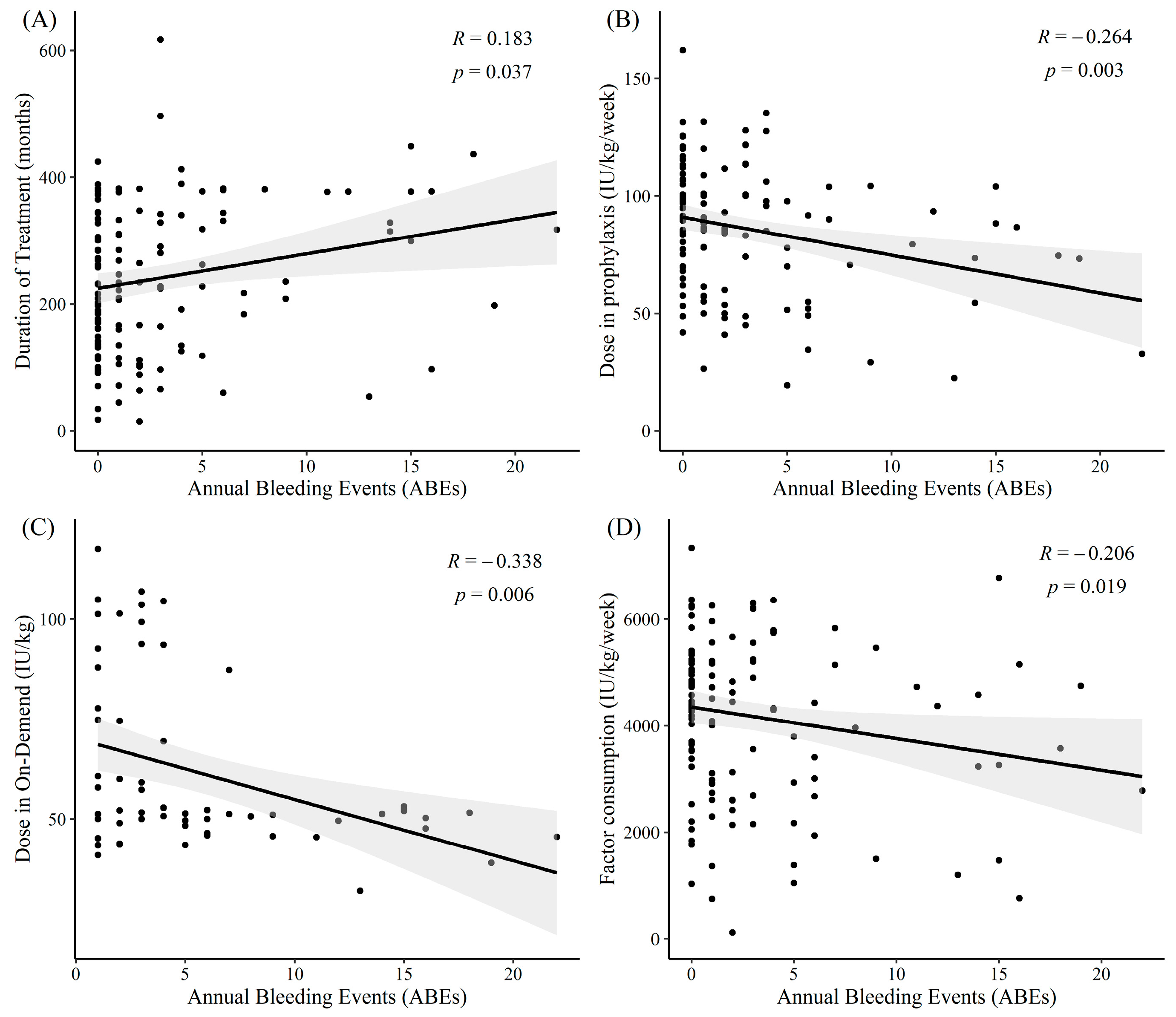
3.4. Annual Bleeding Events
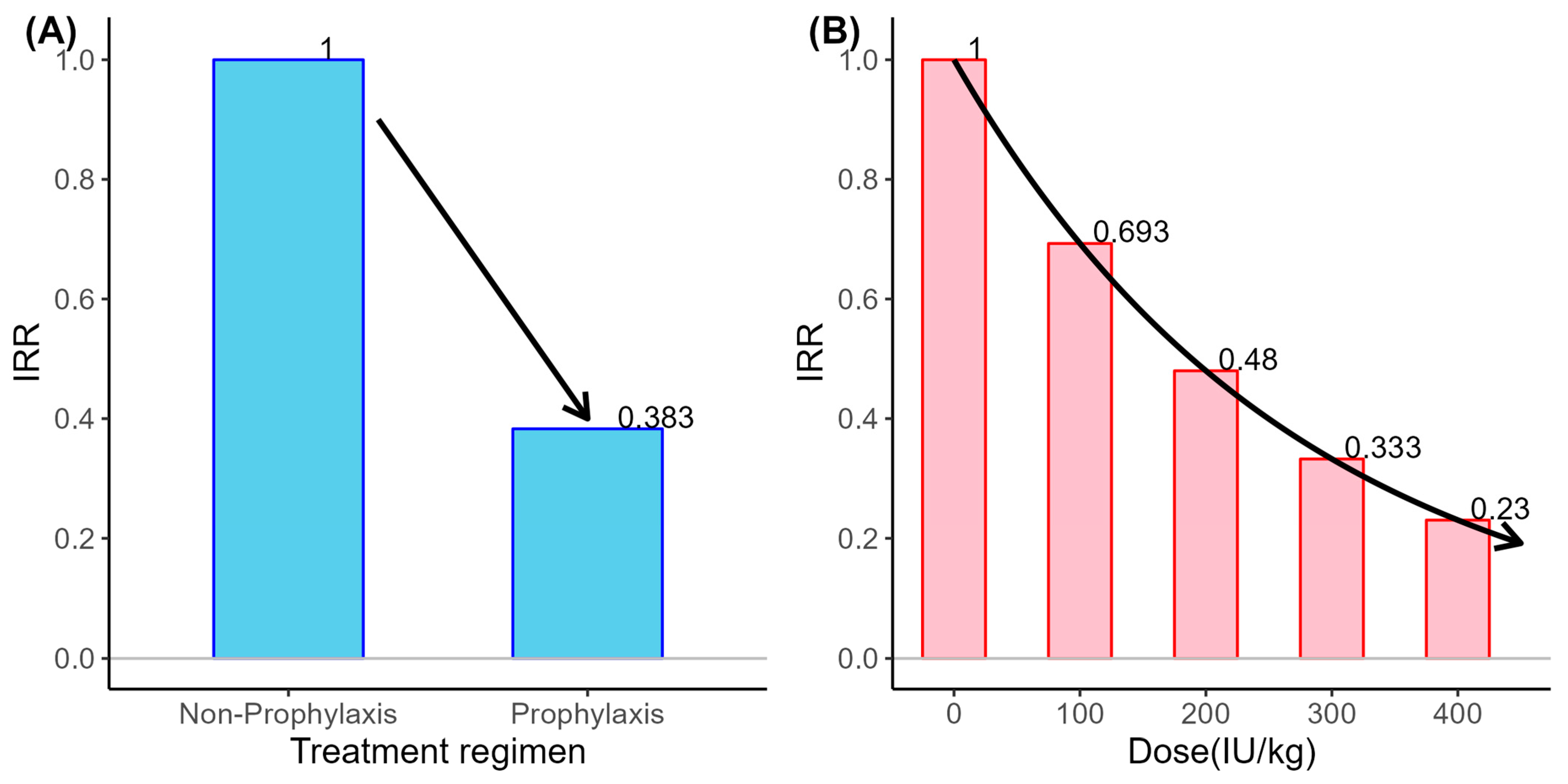
3.5. Influence of Treatment Pattern and Patients’ Adherence on Annual Bleeding Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Djambas Khayat, C. Once-weekly prophylactic dosing of recombinant factor IX improves adherence in hemophilia B. J. Blood Med. 2016, 7, 275–282. [Google Scholar] [CrossRef]
- Adam, M.P.; Mirzaa, G.M.; Pagon, R.A.; Wallace, S.E.; Bean, L.J.; Gripp, K.W.; Amemiya, A. GeneReviews® [Internet]; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Franchini, M.; Frattini, F.; Crestani, S.; Sissa, C.; Bonfanti, C. Treatment of hemophilia B: Focus on recombinant factor IX. Biol. Targets Ther. 2013, 7, 33–38. [Google Scholar] [CrossRef]
- Anguela, X.M.; High, K.A. Hemophilia B and gene therapy: A new chapter with etranacogene dezaparvovec. Blood Adv. 2024, 8, 1796–1803. [Google Scholar] [CrossRef]
- Soroka, A.B.; Feoktistova, S.G.; Mityaeva, O.N.; Volchkov, P.Y. Gene therapy approaches for the treatment of hemophilia B. Int. J. Mol. Sci. 2023, 24, 10766. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Santagostino, E.; Dougall, A.; Kitchen, S.; Sutherland, M.; Pipe, S.W.; Carcao, M.; Mahlangu, J.; Ragni, M.V.; Windyga, J. WFH guidelines for the management of hemophilia. Haemophilia 2020, 26, 1–158. [Google Scholar] [CrossRef]
- Hemophilia Guidelines Side-by-Side: ISTH vs. WFH. Available online: https://www.guidelinecentral.com/insights/hemophilia-side-by-side/ (accessed on 1 April 2025).
- Rezende, S.M.; Neumann, I.; Angchaisuksiri, P.; Awodu, O.; Boban, A.; Cuker, A.; Curtin, J.A.; Fijnvandraat, K.; Gouw, S.C.; Gualtierotti, R. International Society on Thrombosis and Haemostasis clinical practice guideline for treatment of congenital hemophilia A and B based on the Grading of Recommendations Assessment, Development, and Evaluation methodology. J. Thromb. Haemost. 2024, 22, 2629–2652. [Google Scholar] [CrossRef] [PubMed]
- Hart, D.P.; Matino, D.; Astermark, J.; Dolan, G.; d’Oiron, R.; Hermans, C.; Jiménez-Yuste, V.; Linares, A.; Matsushita, T.; McRae, S. International consensus recommendations on the management of people with haemophilia B. Ther. Adv. Hematol. 2022, 13, 20406207221085202. [Google Scholar] [CrossRef] [PubMed]
- Thornburg, C.D.; Duncan, N.A. Treatment adherence in hemophilia. Patient Prefer. Adherence 2017, 11, 1677–1686. [Google Scholar] [CrossRef]
- Dover, S.; Blanchette, V.S.; Wrathall, D.; Pullenayegum, E.; Kazandjian, D.; Song, B.; Hawes, S.A.; Cloutier, S.; Rivard, G.E.; Klaassen, R.J. Hemophilia prophylaxis adherence and bleeding using a tailored, frequency-escalated approach: The Canadian Hemophilia Primary Prophylaxis Study. Res. Pract. Thromb. Haemost. 2020, 4, 318–325. [Google Scholar] [CrossRef]
- Armstrong, E.P.; Malone, D.C.; Krishnan, S.; Wessler, M.J. Adherence to clotting factors among persons with hemophilia A or B. Hematology 2015, 20, 148–153. [Google Scholar] [CrossRef]
- Schrijvers, L.; Uitslager, N.; Schuurmans, M.; Fischer, K. Barriers and motivators of adherence to prophylactic treatment in haemophilia: A systematic review. Haemophilia 2013, 19, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Saxena, K. Barriers and perceived limitations to early treatment of hemophilia. J. Blood Med. 2013, 4, 49–56. [Google Scholar] [CrossRef]
- Valentino, L.; Rusen, L.; Elezovic, I.; Smith, L.; Korth-Bradley, J.; Rendo, P. Multicentre, randomized, open-label study of on-demand treatment with two prophylaxis regimens of recombinant coagulation factor IX in haemophilia B subjects. Haemophilia 2014, 20, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Biss, T.; Chan, A.; Blanchette, V.; Iwenofu, L.; McLimont, M.; Carcao, M.; Association of Hemophilia Clinic Directors of Canada and the Canadian Association of Nurses in Hemophilia Care. The use of prophylaxis in 2663 children and adults with haemophilia: Results of the 2006 Canadian national haemophilia prophylaxis survey. Haemophilia 2008, 14, 923–930. [Google Scholar] [CrossRef]
- Ullman, M.; Zhang, Q.; Grosse, S.; Recht, M.; Soucie, J.; Hemophilia Treatment Center Network Investigators. Prophylaxis use among males with haemophilia B in the United States. Haemophilia 2017, 23, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K. Prophylaxis for adults with haemophilia: One size does not fit all. Blood Transfus. 2012, 10, 169. [Google Scholar] [PubMed]
- Curtis, R.; Roberts, J.C.; Crook, N.; Decker-Palmer, M.; Khainar, R.; Baker, J.R.; Ullman, M.; Koerper, M.A.; Wu, J.; Nichol, M.B. Trends in prescribing practices for management of haemophilia: 1999–2021. Haemophilia 2023, 29, 761–769. [Google Scholar] [CrossRef]
- Malec, L.M.; Cheng, D.; Witmer, C.M.; Jaffray, J.; Kouides, P.A.; Haley, K.M.; Sidonio, R.F., Jr.; Johnson, K.; Recht, M.; White, G. The impact of extended half-life factor concentrates on prophylaxis for severe hemophilia in the United States. Am. J. Hematol. 2020, 95, 960–965. [Google Scholar] [CrossRef]
- Castaman, G. The benefits of prophylaxis in patients with hemophilia B. Expert Rev. Hematol. 2018, 11, 673–683. [Google Scholar] [CrossRef]
- Sidonio, R.F.; Malec, L. Hemophilia B (factor IX deficiency). Hematol. Oncol. Clin. 2021, 35, 1143–1155. [Google Scholar] [CrossRef]
- Horava, S.D.; Peppas, N.A. Recent advances in hemophilia B therapy. Drug Deliv. Transl. Res. 2017, 7, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Kavakli, K.; Smith, L.; Kuliczkowski, K.; Korth-Bradley, J.; You, C.; Fuiman, J.; Zupančić-Šalek, S.; Abdul Karim, F.; Rendo, P. Once-weekly prophylactic treatment vs. on-demand treatment with nonacog alfa in patients with moderately severe to severe haemophilia B. Haemophilia 2016, 22, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Burke, T.; Asghar, S.; O’Hara, J.; Sawyer, E.K.; Li, N. Clinical, humanistic, and economic burden of severe hemophilia B in the United States: Results from the CHESS US and CHESS US+ population surveys. Orphanet J. Rare Dis. 2021, 16, 143. [Google Scholar] [CrossRef] [PubMed]
- Noone, D.; Pedra, G.; Asghar, S.; O’Hara, J.; Sawyer, E.K.; Li, N.N. Prophylactic treatment in people with severe hemophilia B in the US: An analysis of real-world healthcare system costs and clinical outcomes. Blood 2019, 134, 2118. [Google Scholar] [CrossRef]
| Demographics and Clinical Characteristics | Treatment Patterns | ||
|---|---|---|---|
| Mean ± SD or n (%) | Mean ± SD or n (%) | ||
| Age (years) | 31.56 ± 16.99 | Treatment duration (months) | 241.20 ± 116.43 |
| Gender, n (%) | Half-life, n (%) | ||
| Male | 129 (99.23%) | Standard half-life | 122 (93.85%) |
| Female | 1 (0.77%) | Extended half-life | 8 (6.15%) |
| Height (cm) | 165.74 ± 16.79 | Switch | 0 (0.00%) |
| Weight (kg) | 67.69 ± 19.43 | Treatment regimen, n (%) | |
| BMI (kg/m2) | 23.97 ± 4.33 | Prophylaxis | 47 (36.15%) |
| Disease duration (months) | 294.22 ± 146.41 | Non-prophylaxis | 83 (63.85%) |
| Severity, n (%) | Prophylactic dose (IU/kg/week) | n = 126 86.26 ± 26.84 | |
| Severe | 105 (80.77%) | Prophylaxis | n = 47 106.58 ± 13.42 |
| Moderate | 23 (17.69%) | Non-prophylaxis | n = 79 74.17 ± 25.52 |
| Mild | 2 (1.54%) | On-demand dose (IU/kg) | n = 64 61.27 ± 21.23 |
| Factor IX activity level (%) | n = 109 9.13 ± 13.64 | Prophylaxis | n = 14 69.97 ± 25.10 |
| Non-prophylaxis | n = 50 58.83 ± 19.61 | ||
| Factor consumption (IU/kg/week) | 79.99 ± 29.10 | ||
| Prophylaxis | 105.04 ± 11.60 | ||
| Non-prophylaxis | 65.81 ± 26.34 | ||
| (A) Proportions of Patients Receiving Prophylaxis by Age and Severity | |||||||
| Subgroup | Prophylaxis (n = 47) | Non-Prophylaxis (n = 83) | |||||
| n (%) | n (%) | ||||||
| Age | 0–11 | 6 (40%) | 9 (60%) | ||||
| 12–17 | 9 (69.23%) | 4 (30.77%) | |||||
| ≥18 | 32 (31.37%) | 70 (68.63%) | |||||
| (B) Weekly FIX Exposure | |||||||
| Treatment Group | Prophylaxis | Non-Prophylaxis | |||||
| Prophylactic Dose (IU/kg/week) | Factor Consumption (IU/kg/week) | Prophylactic Dose (IU/kg/week) | Factor consumption (IU/kg/week) | ||||
| n (%) | Mean ± SD | Mean ± SD | n (%) | Mean ± SD | Mean ± SD | ||
| Mild | 0 (0%) | - | - | 2 (100%) | 91.47 ± 48.18 | 80.03 ± 28.85 | |
| Moderate | 4 (20%) | 104.38 ± 16.10 | 100.17 ± 14.84 | 16 (80%) | 72.40 ± 22.03 | 53.66 ± 31.41 | |
| Severe | 43 (41.35%) | 106.78 ± 13.35 | 105.49 ± 11.37 | 61 (58.65%) | 74.06 ± 25.99 | 69.07 ± 23.76 | |
| Variables of Patients’ Adherence | |
|---|---|
| Adherence status, n (%) | |
| Adherent | 97 (74.62%) |
| Non-adherent | 33 (25.38%) |
| Number of non-adherents | 4.00 ± 2.32 |
| Types of non-adherence, n (%) | n = 33 |
| Overdose injection per administration, n (%) | 12 (36.36%) |
| Under-dose injection per administration, n (%) | 5 (15.15%) |
| Over-frequent administration, n (%) | 9 (27.27%) |
| Under-frequent administration, n (%) | 19 (57.58%) |
| Reasons for non-adherence, n (%) | n = 33 |
| Lack of time, n (%) | 7 (21.21%) |
| Too frequent administration, n (%) | 3 (9.09%) |
| Feel worsening of disease, n (%) | 5 (15.15%) |
| Feel getting better of disease, n (%) | 2 (6.06%) |
| Occurrence of bleeding, n (%) | 13 (39.39%) |
| Too expensive, n (%) | 1 (3.03%) |
| Forgetfulness, n (%) | 4 (12.12%) |
| Lack of dosage, n (%) | 0 (0.00%) |
| Tiredness from injections, n (%) | 12 (36.36%) |
| Others, n (%) | 5 (15.15%) |
| Total (n = 130) | Prophylaxis (n = 47) | Non-Prophylaxis (n = 83) | p-Value | |
|---|---|---|---|---|
| (A) Bleeding event severity | ||||
| Bleeding severity, cases (%) | 396 (100.00%) | 69 (100.00%) | 327 (100.00%) | <0.001 † |
| Mild | 41 (10.35%) | 21 (30.43%) | 20 (6.12%) | |
| Moderate | 346 (87.37%) | 43 (62.32%) | 303 (92.66%) | |
| Severe | 9 (2.27%) | 5 (7.25%) | 4 (1.22%) | |
| (B) ABEs per patient | ||||
| Recorded bleeding events per patient during observation, Mean ± SD | 3.05 ± 4.71 | 1.47 ± 2.91 | 3.94 ± 5.29 | <0.001 § |
| ABEs = 0, n (%) | 52 (40.00%) | 29 (61.70%) | 23 (27.71%) | |
| ABEs > 0 and ≤3, n (%) | 44 (33.85%) | 12 (25.53%) | 32 (38.55%) | |
| ABEs > 3 and ≤6, n (%) | 16 (12.31%) | 2 (4.26%) | 14 (16.87%) | |
| ABEs > 6, n (%) | 18 (13.85%) | 4 (8.51%) | 14 (16.87%) | |
| (C) ABEs by adherence | ||||
| ABEs according to patients’ adherence | 0.308 ‡ | |||
| Adherent | 97 (74.62%) 2.75 ± 4.52 | 38 (80.85%) 1.37 ± 3.04 | 59 (71.08%) 3.64 ± 5.08 | <0.001 § |
| Non-adherent | 33 (25.38%) 3.91 ± 5.22 | 9 (19.15%) 1.89 ± 2.37 | 24 (28.92%) 4.67 ± 5.81 | 0.211 § |
| Predictors | Annual ABEs (Negative Binomial) | Monthly Bleeds (GLMM) | ||
|---|---|---|---|---|
| Exponentiated Estimates (95% CI) | p-Value | Exponentiated Estimates (95% CI) | p-Value | |
| Treatment regimen | ||||
| Non-prophylaxis | ref | |||
| Prophylaxis | 0.3830 (0.182–0.805) | 0.011 | 0.634 (0.315–1.275) | 0.201 |
| Dose (100 IU/kg) † | - | - | 0.693 (0.606–0.792) | <0.001 |
| Patients’ adherence | ||||
| Non-adherent | ref | |||
| Adherent | 0.703 (0.383–1.292) | 0.257 | 0.689 (0.361–1.313) | 0.257 |
| Age (years) | 1.011 (0.989–1.033) | 0.341 | 1.005 (0.981–1.030) | 0.663 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.-S.; Hwang, T.J.; Park, S.K.; Choi, E.J.; Park, J.A.; Baek, H.J.; Lyu, C.J.; Lee, J.H.; Kim, M.K.; Kim, J.Y.; et al. Characteristics and Treatment Patterns of Patients with Haemophilia B Receiving Recombinant Coagulation Factor IX. J. Clin. Med. 2025, 14, 4555. https://doi.org/10.3390/jcm14134555
Park Y-S, Hwang TJ, Park SK, Choi EJ, Park JA, Baek HJ, Lyu CJ, Lee JH, Kim MK, Kim JY, et al. Characteristics and Treatment Patterns of Patients with Haemophilia B Receiving Recombinant Coagulation Factor IX. Journal of Clinical Medicine. 2025; 14(13):4555. https://doi.org/10.3390/jcm14134555
Chicago/Turabian StylePark, Young-Shil, Tai Ju Hwang, Sang Kyu Park, Eun Jin Choi, Jeong A Park, Hee Jo Baek, Chuhl Joo Lyu, Jae Hee Lee, Mi Kyung Kim, Ji Yoon Kim, and et al. 2025. "Characteristics and Treatment Patterns of Patients with Haemophilia B Receiving Recombinant Coagulation Factor IX" Journal of Clinical Medicine 14, no. 13: 4555. https://doi.org/10.3390/jcm14134555
APA StylePark, Y.-S., Hwang, T. J., Park, S. K., Choi, E. J., Park, J. A., Baek, H. J., Lyu, C. J., Lee, J. H., Kim, M. K., Kim, J. Y., Lee, S. A., Park, B., Kim, D.-H., Chung, S. B., Nam, C.-M., Lee, Y., & Yoo, K. Y. (2025). Characteristics and Treatment Patterns of Patients with Haemophilia B Receiving Recombinant Coagulation Factor IX. Journal of Clinical Medicine, 14(13), 4555. https://doi.org/10.3390/jcm14134555








