Hair Calcium Levels in Relation to Coronary Artery Disease Severity and Systemic Inflammation Markers: A Pilot Study
Abstract
1. Introduction
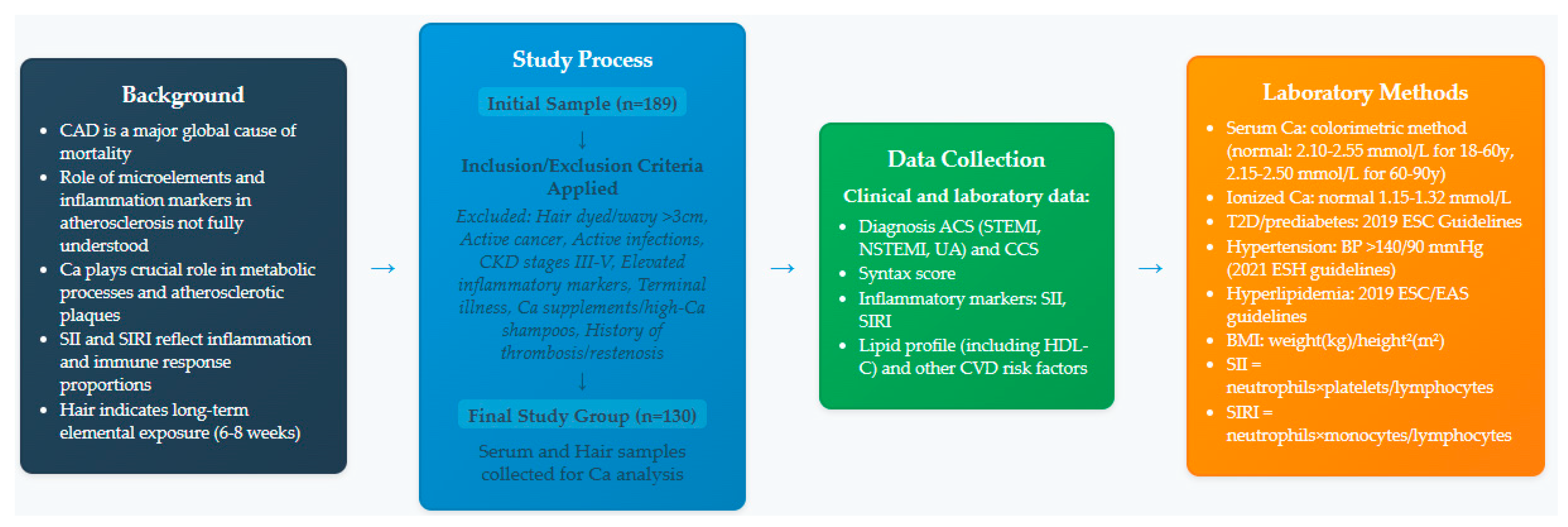
2. Materials and Methods
2.1. Study Population
2.2. Exclusion Criteria
2.3. ACS Diagnosis and Angiography
2.4. Laboratory Tests and Clinical Assessment
2.5. Analysis of Hair Samples
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
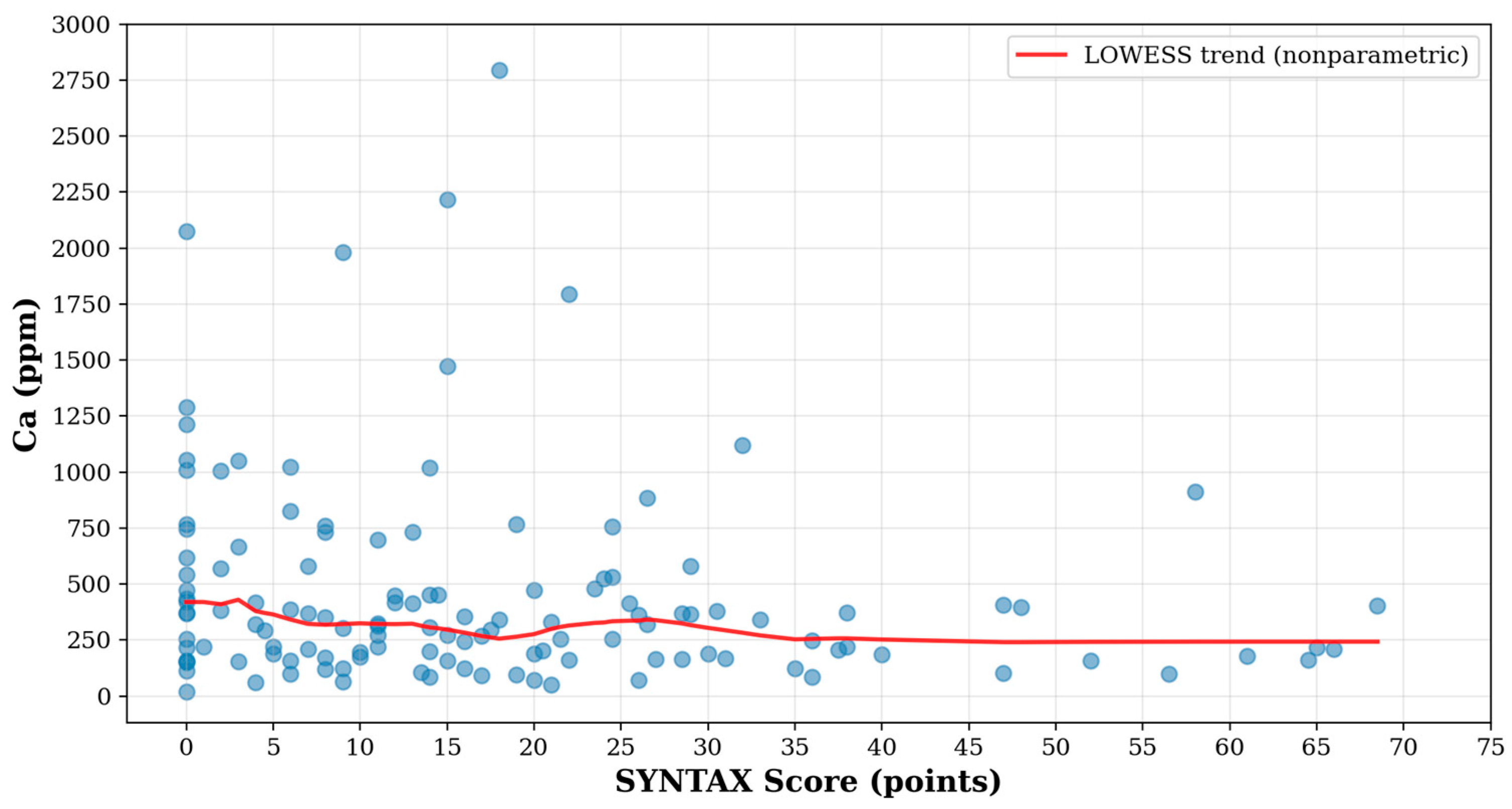
3.2. Association Between Ca Level and Severity of CAD
3.3. Difference in Ca Level Between Patients with Stable CAD and Patients with ACS
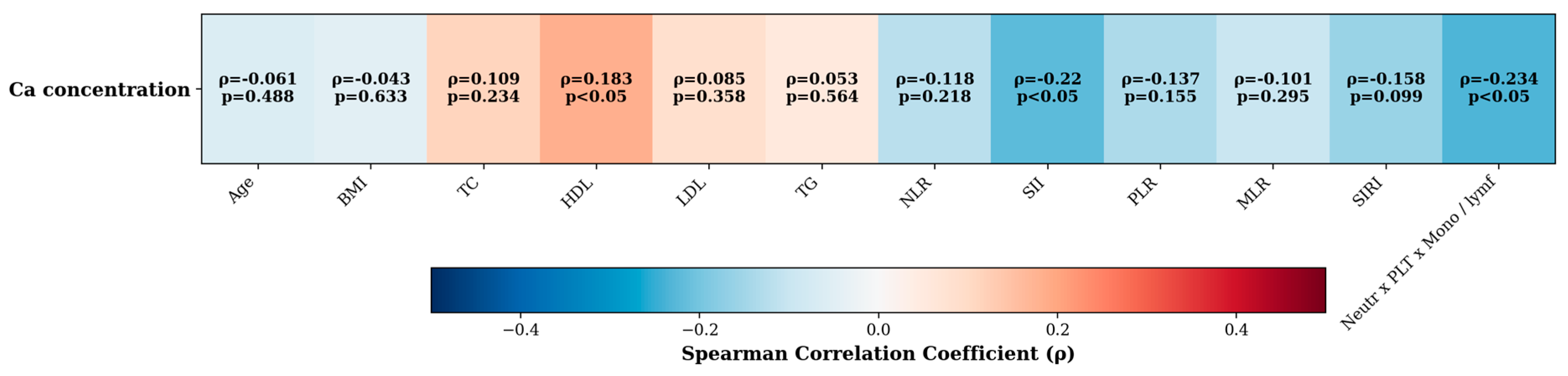
3.4. Correlation/Association Between Ca Level and Various Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, T.; Wang, P.; Wang, X.; Liu, Z.; Zhang, Z.; Zhang, Y.; Wang, Z.; Feng, Y.; Wang, Q.; Guo, X.; et al. Inflammation and Insulin Resistance in Diabetic Chronic Coronary Syndrome Patients. Nutrients 2023, 15, 2808. [Google Scholar] [CrossRef] [PubMed]
- Bauersachs, R.; Zeymer, U.; Brière, J.B.; Marre, C.; Bowrin, K.; Huelsebeck, M. Burden of Coronary Artery Disease and Peripheral Artery Disease: A Literature Review. Cardiovasc. Ther. 2019, 2019, 8295054. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Liu, Y.; Yuan, J. Evidence in Guidelines for Treatment of Coronary Artery Disease. Adv. Exp. Med. Biol. 2020, 1177, 37–73. [Google Scholar] [CrossRef]
- Sachinkumar, J.; Hegde, S.; Avinash; Madhusudhan. Serum Calcium Concentration its Association with Disease Severity in Critically Ill Patients Admitted to Intensive Care Unit. J. Assoc. Physicians India 2023, 71, 1. [Google Scholar]
- Wlaźlak, E.; Surkont, G.; Dunicz-Sokołowska, A.; Długaszek, M.; Radomska, K.; Stetkiewicz, T.; Graczyk, A. Analysis of calcium concentration in perimenopausal women hair. Menopause Rev./Przegląd Menopauzalny 2007, 6, 51–54. [Google Scholar]
- Çelik, B.; Nalçacıoğlu, H.; Karakükçü, Ç.; Aslaner, H.; Şahiner, Ü.M. Assessment of Hair Zinc in the School Children in Kayseri, Turkey. Biol. Trace Elem. Res. 2020, 196, 343–348. [Google Scholar] [CrossRef]
- Wongdee, K.; Chanpaisaeng, K.; Teerapornpuntakit, J.; Charoenphandhu, N. Intestinal Calcium Absorption. Compr. Physiol. 2021, 11, 2047–2073. [Google Scholar] [CrossRef]
- Reid, I.R.; Birstow, S.M.; Bolland, M.J. Calcium and Cardiovascular Disease. Endocrinol. Metab. 2017, 32, 339–349. [Google Scholar] [CrossRef]
- Mortazavi, C.M.; Hoyt, J.M.; Patel, A.; Chignalia, A.Z. The glycocalyx and calcium dynamics in endothelial cells. Curr. Top. Membr. 2023, 91, 21–41. [Google Scholar] [CrossRef]
- Thakore, P.; Earley, S. Transient Receptor Potential Channels and Endothelial Cell Calcium Signaling. Compr. Physiol. 2019, 9, 1249–1277. [Google Scholar] [CrossRef]
- Jiang, Y.; Xing, W.; Li, Z.; Zhao, D.; Xiu, B.; Xi, Y.; Bai, S.; Li, X.; Zhang, Z.; Zhang, W.; et al. The calcium-sensing receptor alleviates endothelial inflammation in atherosclerosis through regulation of integrin β1-NLRP3 inflammasome. FEBS J. 2025, 292, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Zanoli, L.; Briet, M.; Empana, J.P.; Cunha, P.G.; Mäki-Petäjä, K.M.; Protogerou, A.D.; Tedgui, A.; Touyz, R.M.; Schiffrin, E.L.; Spronck, B.; et al. Vascular consequences of inflammation: A position statement from the ESH Working Group on Vascular Structure and Function and the ARTERY Society. J. Hypertens. 2020, 38, 1682–1698. [Google Scholar] [CrossRef] [PubMed]
- Burdyga, T.; Borysova, L. Calcium signalling in pericytes. J. Vasc. Res. 2014, 51, 190–199. [Google Scholar] [CrossRef]
- Drabarek, B.; Dymkowska, D. Znaczenie jonów wapnia w śródbłonku naczyń [The role of calcium for functioning of the vascular endothelium]. Postep. Biochem. 2012, 58, 418–428. [Google Scholar]
- Moccia, F.; Tanzi, F.; Munaron, L. Endothelial remodelling and intracellular calcium machinery. Curr. Mol. Med. 2014, 14, 457–480. [Google Scholar] [CrossRef]
- Ortega, M.A.; De Leon-Oliva, D.; Gimeno-Longas, M.J.; Boaru, D.L.; Fraile-Martinez, O.; García-Montero, C.; de Castro, A.V.; Barrena-Blázquez, S.; López-González, L.; Amor, S.; et al. Vascular Calcification: Molecular Networking, Pathological Implications and Translational Opportunities. Biomolecules 2024, 14, 275. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ozmen, H.; Akarsu, S.; Polat, F.; Cukurovali, A. The levels of calcium and magnesium, and of selected trace elements, in whole blood and scalp hair of children with growth retardation. Iran. J Pediatr. 2013, 23, 125–130. [Google Scholar] [PubMed] [PubMed Central]
- Mitsis, A.; Khattab, E.; Christodoulou, E.; Myrianthopoulos, K.; Myrianthefs, M.; Tzikas, S.; Ziakas, A.; Fragakis, N.; Kassimis, G. From Cells to Plaques: The Molecular Pathways of Coronary Artery Calcification and Disease. J. Clin. Med. 2024, 13, 6352. [Google Scholar] [CrossRef]
- Stone, P.H.; Libby, P.; Boden, W.E. Fundamental Pathobiology of Coronary Atherosclerosis and Clinical Implications for Chronic Ischemic Heart Disease Management-The Plaque Hypothesis: A Narrative Review. JAMA Cardiol. 2023, 8, 192–201. [Google Scholar] [CrossRef]
- Gholipour, A.; Zahedmehr, A.; Arabian, M.; Shakerian, F.; Maleki, M.; Oveisee, M.; Malakootian, M. MiR-6721-5p as a natural regulator of Meta-VCL is upregulated in the serum of patients with coronary artery disease. Non-Coding RNA Res. 2025, 10, 25–34. [Google Scholar] [CrossRef]
- Grigorieva, D.V.; Gorudko, I.V.; Shamova, E.V.; Terekhova, M.S.; Maliushkova, E.V.; Semak, I.V.; Cherenkevich, S.N.; Sokolov, A.V.; Timoshenko, A.V. Effects of recombinant human lactoferrin on calcium signaling and functional responses of human neutrophils. Arch. Biochem. Biophys. 2019, 675, 108122. [Google Scholar] [CrossRef]
- Hann, J.; Bueb, J.L.; Tolle, F.; Bréchard, S. Calcium signaling and regulation of neutrophil functions: Still a long way to go. J. Leukoc. Biol. 2020, 107, 285–297. [Google Scholar] [CrossRef]
- Fenninger, F.; Jefferies, W.A. What’s Bred in the Bone: Calcium Channels in Lymphocytes. J. Immunol. 2019, 202, 1021–1030. [Google Scholar] [CrossRef]
- Park, Y.J.; Yoo, S.A.; Kim, M.; Kim, W.U. The Role of Calcium-Calcineurin-NFAT Signaling Pathway in Health and Autoimmune Diseases. Front. Immunol. 2020, 11, 195. [Google Scholar] [CrossRef]
- Letizia, M.; Wang, Y.H.; Kaufmann, U.; Gerbeth, L.; Sand, A.; Brunkhorst, M.; Weidner, P.; Ziegler, J.F.; Böttcher, C.; Schlickeiser, S.; et al. Store-operated calcium entry controls innate and adaptive immune cell function in inflammatory bowel disease. EMBO Mol. Med. 2022, 14, e15687. [Google Scholar] [CrossRef]
- Kaschek, L.; Zöphel, S.; Knörck, A.; Hoth, M. A calcium optimum for cytotoxic T lymphocyte and natural killer cell cytotoxicity. Semin. Cell Dev. Biol. 2021, 115, 10–18. [Google Scholar] [CrossRef]
- Murthy, S.; Karkossa, I.; Schmidt, C.; Hoffmann, A.; Hagemann, T.; Rothe, K.; Seifert, O.; Anderegg, U.; von Bergen, M.; Schubert, K.; et al. Danger signal extracellular calcium initiates differentiation of monocytes into SPP1/osteopontin-producing macrophages. Cell Death Dis. 2022, 13, 53. [Google Scholar] [CrossRef]
- Zhao, Q.; Gong, Z.; Wang, J.; Fu, L.; Zhang, J.; Wang, C.; Miron, R.J.; Yuan, Q.; Zhang, Y. A Zinc- and Calcium-Rich Lysosomal Nanoreactor Rescues Monocyte/Macrophage Dysfunction under Sepsis. Adv. Sci. 2023, 10, e2205097. [Google Scholar] [CrossRef]
- Moz, S.; Lorenzin, M.; Ramonda, R.; Aneloni, V.; La Raja, M.; Plebani, M.; Basso, D. Emerging role of monocytes and their intracellular calcium pattern in spondyloarthritis. Clin. Chim. Acta Int. J. Clin. Chem. 2020, 500, 180–188. [Google Scholar] [CrossRef]
- Mammadova-Bach, E.; Gudermann, T.; Braun, A. Platelet Mechanotransduction: Regulatory Cross Talk Between Mechanosensitive Receptors and Calcium Channels. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 1339–1348. [Google Scholar] [CrossRef]
- Stalker, T.J.; Newman, D.K.; Ma, P.; Wannemacher, K.M.; Brass, L.F. Platelet signaling. In Antiplatelet Agents; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 59–85. [Google Scholar] [CrossRef]
- Yadav, P.; Beura, S.K.; Panigrahi, A.R.; Bhardwaj, T.; Giri, R.; Singh, S.K. Platelet-derived microvesicles activate human platelets via intracellular calcium mediated reactive oxygen species release. Blood Cells Mol. Dis. 2023, 98, 102701. [Google Scholar] [CrossRef] [PubMed]
- Bani Hani, D.A.; Alshraideh, J.A.; Saleh, A.; Alduraidi, H.; Alwahadneh, A.A.; Al-Zaiti, S.S. Lymphocyte-based inflammatory markers: Novel predictors of significant coronary artery disease. Heart Lung J. Crit. Care 2024, 70, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, Q.; Wang, R.; Ji, H.; Chen, Y.; Quan, X.; Zhang, C. Systemic Immune-Inflammatory Index Predicts Clinical Outcomes for Elderly Patients with Acute Myocardial Infarction Receiving Percutaneous Coronary Intervention. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 9690–9701. [Google Scholar] [CrossRef]
- Hu, B.; Yang, X.R.; Xu, Y.; Sun, Y.F.; Sun, C.; Guo, W.; Zhang, X.; Wang, W.M.; Qiu, S.J.; Zhou, J.; et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2014, 20, 6212–6222. [Google Scholar] [CrossRef]
- Erdoğan, M.; Erdöl, M.A.; Öztürk, S.; Durmaz, T. Systemic immune-inflammation index is a novel marker to predict functionally significant coronary artery stenosis. Biomark. Med. 2020, 14, 1553–1561. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, T.; Chen, L.; Jin, T.; Sheng, Y.; Wu, G.; Zong, G. Systemic immune-inflammation index predicts the severity of coronary stenosis in patients with coronary heart disease. Coron. Artery Dis. 2021, 32, 715–720. [Google Scholar] [CrossRef]
- Candemir, M.; Kiziltunç, E.; Nurkoç, S.; Şahinarslan, A. Relationship Between Systemic Immune-Inflammation Index (SII) and the Severity of Stable Coronary Artery Disease. Angiology 2021, 72, 575–581. [Google Scholar] [CrossRef]
- Li, Q.; Ma, X.; Shao, Q.; Yang, Z.; Wang, Y.; Gao, F.; Zhou, Y.; Yang, L.; Wang, Z. Prognostic Impact of Multiple Lymphocyte-Based Inflammatory Indices in Acute Coronary Syndrome Patients. Front. Cardiovasc. Med. 2022, 9, 811790. [Google Scholar] [CrossRef]
- Yang, Y.L.; Wu, C.H.; Hsu, P.F.; Chen, S.C.; Huang, S.S.; Chan, W.L.; Lin, S.J.; Chou, C.Y.; Chen, J.W.; Pan, J.P.; et al. Systemic immune-inflammation index (SII) predicted clinical outcome in patients with coronary artery disease. Eur. J. Clin. Investig. 2020, 50, e13230. [Google Scholar] [CrossRef]
- Seo, M.; Yamada, T.; Morita, T.; Furukawa, Y.; Tamaki, S.; Iwasaki, Y.; Kawasaki, M.; Kikuchi, A.; Kawai, T.; Ikeda, I.; et al. P589 Prognostic value of systemic immune-inflammation index in patients with chronic heart failure. Eur. Heart J. 2018, 39 (Suppl. 1), ehy564-P589. [Google Scholar] [CrossRef]
- Urbanowicz, T.; Michalak, M.; Al-Imam, A.; Olasińska-Wiśniewska, A.; Rodzki, M.; Witkowska, A.; Haneya, A.; Buczkowski, P.; Perek, B.; Jemielity, M. The Significance of Systemic Immune-Inflammatory Index for Mortality Prediction in Diabetic Patients Treated with Off-Pump Coronary Artery Bypass Surgery. Diagnostics 2022, 12, 634. [Google Scholar] [CrossRef] [PubMed]
- Tosu, A.R.; Kalyoncuoglu, M.; Biter, H.İ.; Cakal, S.; Selcuk, M.; Çinar, T.; Belen, E.; Can, M.M. Prognostic Value of Systemic Immune-Inflammation Index for Major Adverse Cardiac Events and Mortality in Severe Aortic Stenosis Patients after TAVI. Medicina 2021, 57, 588. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Jung, J.; Ahn, Y.; Oh, J. Systemic Immune-Inflammation Index Predicted Short-Term Outcomes in Patients Undergoing Isolated Tricuspid Valve Surgery. J. Clin. Med. 2021, 10, 4147. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhang, J.; Liu, T.; Yin, Z.; Jin, Y.; Han, J.; Guo, Z.; Wang, H. The systemic-immune-inflammation index predicts the recurrence of atrial fibrillation after cryomaze concomitant with mitral valve surgery. BMC Cardiovasc. Disord. 2022, 22, 45. [Google Scholar] [CrossRef]
- Chao, B.; Ju, X.; Zhang, L.; Xu, X.; Zhao, Y. A Novel Prognostic Marker Systemic Inflammation Response Index (SIRI) for Operable Cervical Cancer Patients. Front. Oncol. 2020, 10, 766. [Google Scholar] [CrossRef]
- Jin, Z.; Wu, Q.; Chen, S.; Gao, J.; Li, X.; Zhang, X.; Zhou, Y.; He, D.; Cheng, Z.; Zhu, Y.; et al. The Associations of Two Novel Inflammation Indexes, SII and SIRI with the Risks for Cardiovascular Diseases and All-Cause Mortality: A Ten-Year Follow-Up Study in 85,154 Individuals. J. Inflamm. Res. 2021, 14, 131–140. [Google Scholar] [CrossRef]
- Han, K.; Shi, D.; Yang, L.; Wang, Z.; Li, Y.; Gao, F.; Liu, Y.; Ma, X.; Zhou, Y. Prognostic value of systemic inflammatory response index in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Ann. Med. 2022, 54, 1667–1677. [Google Scholar] [CrossRef]
- Karadeniz, F.Ö.; Karadeniz, Y.; Altuntaş, E. Systemic immune-inflammation index, and neutrophilto-lymphocyte and platelet-to-lymphocyte ratios can predict clinical outcomes in patients with acute coronary syndrome. Cardiovasc. J. Afr. 2024, 35, 82–88. [Google Scholar] [CrossRef]
- Qi, Q.; Zhuang, L.; Shen, Y.; Geng, Y.; Yu, S.; Chen, H.; Liu, L.; Meng, Z.; Wang, P.; Chen, Z. A novel systemicinflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer 2016, 122, 2158–2167. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, Z.; Wei, J.; Luo, C. Association of systemic immune inflammation index and system inflammation response index with clinical risk of acute myocardial infarction. Front. Cardiovasc. Med. 2023, 10, 1248655. [Google Scholar] [CrossRef]
- Ye, Z.; Hu, T.; Wang, J.; Xiao, R.; Liao, X.; Liu, M.; Sun, Z. Systemic immune-inflammation index as a potential biomarker of cardiovascular diseases: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2022, 9, 933913. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zheng, L. Associations between SII, SIRI, and cardiovascular disease in obese individuals: A nationwide cross-sectional analysis. Front. Cardiovasc. Med. 2024, 11, 1361088. [Google Scholar] [CrossRef] [PubMed]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e18–e114. [Google Scholar] [CrossRef]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Bortnick, A.E.; Shitole, S.G.; Hashim, H.; Khullar, P.; Park, M.; Weinreich, M.; Seibert, S.; Rauch, J.; Weisz, G.; Kizer, J.R. Residual SYNTAX II Score and long-term outcomes post-ST-elevation myocardial infarction in an urban US cohort: The Montefiore STEMI Registry. Coron. Artery Dis. 2022, 33, 206–212. [Google Scholar] [CrossRef]
- Rafaeli, I.R.; Kireeva, A.Y.; Rogatova, A.N.; Azarov, A.V.; Semitko, S.P. Prognostic Value of Residual Coronary Artery Lesions on the SYNTAX Scale in Patients with Acute Myocardial Infarction without ST Segment Elevation in the Mid-Term Period. Kardiologiia 2021, 61, 36–43. [Google Scholar] [CrossRef]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; de Pinho, R.M.; Albini, F.L.; Boivin, J.M.; et al. 2024 European Society of Hypertension clinical practice guidelines for the management of arterial hypertension. Eur. J. Intern. Med. 2024, 126, 1–15. [Google Scholar] [CrossRef]
- Afify, H.; Lee, H.L.; Soliman, E.Z.; Singleton, M.J. Prognostic significance of body mass index-adjusted criteria for left ventricular hypertrophy. J. Clin. Hypertens. 2020, 22, 1476–1483. [Google Scholar] [CrossRef]
- Li, W.; Li, S.; Shang, Y.; Zhuang, W.; Yan, G.; Chen, Z.; Lyu, J. Associations between dietary and blood inflammatory indices and their effects on cognitive function in elderly Americans. Front. Neurosci. 2023, 17, 1117056. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Britto, J.E.; de la Fuente, F.; Meitín, J.J.; Marrero, M.; Yero, M.; Guski, H. Coronary atherosclerosis and chemical trace elements in the hair. A canonical correlation study of autopsy subjects, using an atherometric system and the X-ray fluorescence analysis. Zentralblatt Pathologie. 1993, 139, 61–65. [Google Scholar]
- Jin, Y.; He, L.; Wang, Q.; Chen, Y.; Ren, X.; Tang, H.; Song, X.; Ding, L.; Qi, Q.; Huang, Z.; et al. Serum calcium levels are not associated with coronary heart disease. Vasc. Health Risk Manag. 2013, 9, 517–520. [Google Scholar] [CrossRef]
- Rasouli, M.; Kiasari, A.M. Serum calcium and phosphorus associate with the occurrence and severity of angiographically documented coronary heart disease, possibly through correlation with atherogenic (apo)lipoproteins. Clin. Chem. Lab. Med. 2006, 44, 43–50. [Google Scholar] [CrossRef]
- Bacsó, J.; Lusztig, G.; Pál, A.; Uzonyi, I. Comparative investigation of some mineral elements in the aortic wall and the calcium concentration in hair. Exp. Pathol. 1986, 29, 119–125. [Google Scholar] [CrossRef]
- MacPherson, A.; Bacsó, J. Relationship of hair calcium concentration to incidence of coronary heart disease. Sci. Total Environ. 2000, 255, 11–19. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, S.J.; Kim, K.N.; Cho, D.Y.; Kim, Y.S.; Kim, B.T. Coronary Calcification Is Reversely Related with Bone and Hair Calcium: The Relationship among Different Calcium Pools in Body. J. Bone Metab. 2016, 23, 191–197. [Google Scholar] [CrossRef]
- Afridi, H.I.; Kazi, T.G.; Kazi, N.; Kandhro, G.A.; Baig, J.A.; Shah, A.Q.; Kolachi, N.F.; Wadhwa, S.K.; Khan, S.; Shah, F. Potassium, calcium, magnesium, and sodium levels in biological samples of Pakistani myocardial infarction patients at different stages as related to controls. Clin. Lab. 2010, 56, 427–439. [Google Scholar]
- Urbanowicz, T.; Hanć, A.; Frąckowiak, J.; Białasik-Misiorny, M.; Radek, Z.; Krama, M.; Filipiak, K.J.; Krasińska-Płachta, A.; Iwańczyk, S.; Kowalewski, M.; et al. What Can We Learn from the Scalp Hair’s Trace Element Content? The Relationship with the Advancement of Coronary Artery Disease. J. Clin. Med. 2024, 13, 5260. [Google Scholar] [CrossRef]
- Song, C.H.; Barrett-Connor, E.; Chung, J.H.; Kim, S.H.; Kim, K.S. Associations of calcium and magnesium in serum and hair with bone mineral density in premenopausal women. Biol. Trace Elem. Res. 2007, 118, 1–9. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, S.H.; Cho, D.Y.; Kim, K.M.; Lee, D.J.; Kim, B.T. Hair calcium concentration is associated with calcium intake and bone mineral density. International journal for vitamin and nutrition research. Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung. J. Int. Vitaminol. Nutr. 2013, 83, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Skalny, A.V.; Korobeinikova, T.V.; Aschner, M.; Paoliello, M.M.B.; Lu, R.; Skalny, A.A.; Mazaletskaya, A.L.; Tinkov, A.A. Hair and Serum Trace Element and Mineral Levels Profiles in Women with Premenopausal and Postmenopausal Osteoporosis. Biol. Trace Elem. Res. 2024, 202, 3886–3899. [Google Scholar] [CrossRef] [PubMed]
- Lopes, N.H.M. The Interface between Osteoporosis and Atherosclerosis in Postmenopausal Women. Arq. Bras. Cardiol. 2018, 110, 217–218. [Google Scholar] [CrossRef] [PubMed]
- Szekanecz, Z.; Raterman, H.G.; Pethő, Z.; Lems, W.F. Common mechanisms and holistic care in atherosclerosis and osteoporosis. Arthritis Res. Ther. 2019, 21, 15. [Google Scholar] [CrossRef]
- Qu, L.; Zuo, X.; Yu, J.; Duan, R.; Zhao, B. Association of inflammatory markers with all-cause mortality and cardiovascular mortality in postmenopausal women with osteoporosis or osteopenia. BMC Women’s Health 2023, 23, 487. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, X.; Sun, T.; Huang, X.; Ma, M.; Yang, S.; Zhou, Y. Prognostic value of systemic immune-inflammation index in CAD patients: Systematic review and meta-analyses. Eur. J. Clin. Investig. 2024, 54, e14100. [Google Scholar] [CrossRef]
- Stroope, C.; Nettersheim, F.S.; Coon, B.; Finney, A.C.; Schwartz, M.A.; Ley, K.; Rom, O.; Yurdagul, A., Jr. Dysregulated cellular metabolism in atherosclerosis: Mediators and therapeutic opportunities. Nat. Metab. 2024, 6, 617–638. [Google Scholar] [CrossRef]
- Koenen, R.R.; Binder, C.J. Platelets and coagulation factors: Established and novel roles in atherosclerosis and atherothrombosis. Atherosclerosis 2020, 307, 78–79. [Google Scholar] [CrossRef]
- Kessler, T.; Schunkert, H.; von Hundelshausen, P. Novel Approaches to Fine-Tune Therapeutic Targeting of Platelets in Atherosclerosis: A Critical Appraisal. Thromb. Haemost. 2020, 120, 1492–1504. [Google Scholar] [CrossRef]
- Huilcaman, R.; Venturini, W.; Fuenzalida, L.; Cayo, A.; Segovia, R.; Valenzuela, C.; Brown, N.; Moore-Carrasco, R. Platelets, a Key Cell in Inflammation and Atherosclerosis Progression. Cells 2022, 11, 1014. [Google Scholar] [CrossRef]
- Momi, S.; Falcinelli, E.; Petito, E.; Ciarrocca Taranta, G.; Ossoli, A.; Gresele, P. Matrix metalloproteinase-2 on activated platelets triggers endothelial PAR-1 initiating atherosclerosis. Eur. Heart J. 2022, 43, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Li, N. Platelets as an inter-player between hyperlipidaemia and atherosclerosis. J. Intern. Med. 2024, 296, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Martinez Bravo, G.; Annarapu, G.; Carmona, E.; Nawarskas, J.; Clark, R.; Novelli, E.; Mota Alvidrez, R.I. Platelets in Thrombosis and Atherosclerosis: A Double-Edged Sword. Am. J. Pathol. 2024, 194, 1608–1621. [Google Scholar] [CrossRef] [PubMed]
- Döring, Y.; Soehnlein, O.; Weber, C. Neutrophil Extracellular Traps in Atherosclerosis and Atherothrombosis. Circ. Res. 2017, 120, 736–743. [Google Scholar] [CrossRef]
- Nording, H.; Baron, L.; Langer, H.F. Platelets as therapeutic targets to prevent atherosclerosis. Atherosclerosis 2020, 307, 97–108. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Li, J. Neutrophil extracellular traps: A catalyst for atherosclerosis. Mol. Cell. Biochem. 2024, 479, 3213–3227. [Google Scholar] [CrossRef]
- Gu, C.; Pang, B.; Sun, S.; An, C.; Wu, M.; Wang, N.; Yuan, Y.; Liu, G. Neutrophil extracellular traps contributing to atherosclerosis: From pathophysiology to clinical implications. Exp. Biol. Med. 2023, 248, 1302–1312. [Google Scholar] [CrossRef]
- Smith, C.; Damås, J.K.; Otterdal, K.; Øie, E.; Sandberg, W.J.; Yndestad, A.; Waehre, T.; Scholz, H.; Endresen, K.; Olofsson, P.S.; et al. Increased levels of neutrophil-activating peptide-2 in acute coronary syndromes: Possible role of platelet-mediated vascular inflammation. J. Am. Coll. Cardiol. 2006, 48, 1591–1599. [Google Scholar] [CrossRef]
- Hedrick, C.C. Lymphocytes in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 253–257. [Google Scholar] [CrossRef]
- Xu, L.; Chen, F.; Fan, W.; Saito, S.; Cao, D. The role of γδT lymphocytes in atherosclerosis. Front. Immunol. 2024, 15, 1369202. [Google Scholar] [CrossRef]
- Saigusa, R.; Winkels, H.; Ley, K. T cell subsets and functions in atherosclerosis. Nat. Rev. Cardiol. 2020, 17, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Xu, Y.; Li, Y.; Shi, J.; Zhang, X.; You, Y.; Sun, Q.; Zhai, H.; Hu, Y. A bibliometric analysis of T cell and atherosclerosis. Front. Immunol. 2022, 13, 948314. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, S.; Zernecke, A. CD8+ T Cells in Atherosclerosis. Cells 2020, 10, 37. [Google Scholar] [CrossRef]
- Kobiyama, K.; Saigusa, R.; Ley, K. Vaccination against atherosclerosis. Curr. Opin. Immunol. 2019, 59, 15–24. [Google Scholar] [CrossRef]
- Kyaw, T.; Peter, K.; Li, Y.; Tipping, P.; Toh, B.H.; Bobik, A. Cytotoxic lymphocytes and atherosclerosis: Significance, mechanisms and therapeutic challenges. Br. J. Pharmacol. 2017, 174, 3956–3972. [Google Scholar] [CrossRef]
- Tok, D.; Ekizler, F.A.; Tak, B.T. The relation between apical thrombus formation and systemic immune-inflammation index in patients with acute anterior myocardial infarction. Medicine 2022, 101, e32215. [Google Scholar] [CrossRef]
- Keskin, M.; Öcal, L.; Cerşit, S.; Yılmaz, C.; Küp, A.; Çelik, M.; Doğan, S.; Koyuncu, A.; Kaya, A.; Turkmen, M.M. The Predictive Role of a Novel Risk Index in Patients Undergoing Carotid Artery Stenting: Systemic Immune-Inflammation Index. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2021, 30, 105955. [Google Scholar] [CrossRef]
- Dziedzic, E.A.; Gąsior, J.S.; Tuzimek, A.; Paleczny, J.; Junka, A.; Dąbrowski, M.; Jankowski, P. Investigation of the Associations of Novel Inflammatory Biomarkers-Systemic Inflammatory Index (SII) and Systemic Inflammatory Response Index (SIRI)-With the Severity of Coronary Artery Disease and Acute Coronary Syndrome Occurrence. Int. J. Mol. Sci. 2022, 23, 9553. [Google Scholar] [CrossRef]
- Rajakumar, H.K.; Coimbatore Sathyabal, V.; Vasanthan, M.; Dasarathan, R. The predictive role of Systemic Inflammation Response Index (SIRI), Neutrophil-Lymphocyte Ratio (NLR), and Platelet-Lymphocyte Ratio (PLR) in the prognosis of acute coronary syndrome in a tertiary care hospital. Heliyon 2024, 10, e39029. [Google Scholar] [CrossRef]
- Fan, W.; Wei, C.; Liu, Y.; Sun, Q.; Tian, Y.; Wang, X.; Liu, J.; Zhang, Y.; Sun, L. The Prognostic Value of Hematologic Inflammatory Markers in Patients With Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention. Clin. Appl. Thromb./Hemost. Off. J. Int. Acad. Clin. Appl. Thromb./Hemost. 2022, 28, 10760296221146183. [Google Scholar] [CrossRef]
- Blagov, A.V.; Markin, A.M.; Bogatyreva, A.I.; Tolstik, T.V.; Sukhorukov, V.N.; Orekhov, A.N. The Role of Macrophages in the Pathogenesis of Atherosclerosis. Cells 2023, 12, 522. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Chen, G.; Wu, F.; Cao, Y.; Yang, F.; You, T.; Liu, C.; Li, M.; Hu, S.; Ren, L.; et al. Endothelial CCRL2 induced by disturbed flow promotes atherosclerosis via chemerin-dependent β2 integrin activation in monocytes. Cardiovasc. Res. 2023, 119, 1811–1824. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Lichtman, A.H. Monocyte-Macrophages and T Cells in Atherosclerosis. Immunity 2017, 47, 621–634. [Google Scholar] [CrossRef]
- Dong, Z.; Hou, L.; Luo, W.; Pan, L.H.; Li, X.; Tan, H.P.; Wu, R.D.; Lu, H.; Yao, K.; Mu, M.D.; et al. Myocardial infarction drives trained immunity of monocytes, accelerating atherosclerosis. Eur. Heart J. 2024, 45, 669–684. [Google Scholar] [CrossRef]
- Groh, L.; Keating, S.T.; Joosten, L.A.B.; Netea, M.G.; Riksen, N.P. Monocyte and macrophage immunometabolism in atherosclerosis. Semin. Immunopathol. 2018, 40, 203–214. [Google Scholar] [CrossRef]
- Beerman, R.W.; Matty, M.A.; Au, G.G.; Looger, L.L.; Choudhury, K.R.; Keller, P.J.; Tobin, D.M. Direct In Vivo Manipulation and Imaging of Calcium Transients in Neutrophils Identify a Critical Role for Leading-Edge Calcium Flux. Cell Rep. 2015, 13, 2107–2117. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.; Hansson, G. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef]
- Li-Sha, G.; Li, L.; De-Pu, Z.; Zhe-Wei, S.; Xiaohong, G.; Guang-Yi, C.; Jia, L.; Jia-Feng, L.; Maoping, C.; Yue-Chun, L. Corrigendum: Ivabradine Treatment Reduces Cardiomyocyte Apoptosis in a Murine Model of Chronic Viral Myocarditis. Front. Pharmacol. 2019, 10, 1126. [Google Scholar] [CrossRef]
- Sluiter, T.J.; van Buul, J.D.; Huveneers, S.; Quax, P.H.A.; de Vries, M.R. Endothelial Barrier Function and Leukocyte Transmigration in Atherosclerosis. Biomedicines 2021, 9, 328. [Google Scholar] [CrossRef]
- Zhang, X.; Hong, S.; Qi, S.; Liu, W.; Zhang, X.; Shi, Z.; Chen, W.; Zhao, M.; Yin, X. NLRP3 Inflammasome Is Involved in Calcium-Sensing Receptor-Induced Aortic Remodeling in SHRs. Mediat. Inflamm. 2019, 2019, 6847087. [Google Scholar] [CrossRef]
- Pazár, B.; Ea, H.K.; Narayan, S.; Kolly, L.; Bagnoud, N.; Chobaz, V.; Roger, T.; Lioté, F.; So, A.; Busso, N. Basic calcium phosphate crystals induce monocyte/macrophage IL-1β secretion through the NLRP3 inflammasome in vitro. J. Immunol. 2011, 186, 2495–2502. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Manson, J.E.; Sesso, H.D. Calcium intake and risk of cardiovascular disease: A review of prospective studies and randomized clinical trials. Am. J. Cardiovasc. Drugs Drugs Devices Other Interv. 2012, 12, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, E.W.; Nordestgaard, L.T.; Frikke-Schmidt, R. HDL Cholesterol and Non-Cardiovascular Disease: A Narrative Review. Int. J. Mol. Sci. 2021, 22, 4547. [Google Scholar] [CrossRef] [PubMed]
- Vanaelst, B.; Huybrechts, I.; Michels, N.; Flórez, M.R.; Aramendía, M.; Balcaen, L.; Resano, M.; Vanhaecke, F.; Bammann, K.; Bel-Serrat, S.; et al. Hair minerals and metabolic health in Belgian elementary school girls. Biol. Trace Elem. Res. 2013, 151, 335–343. [Google Scholar] [CrossRef]
- Song, Z.; Wang, Y.; Zhang, F.; Yao, F.; Sun, C. Calcium Signaling Pathways: Key Pathways in the Regulation of Obesity. Int. J. Mol. Sci. 2019, 20, 2768. [Google Scholar] [CrossRef]
- Bergamaschi, L.; Arangalage, D.; Maurizi, N.; Pizzi, C.; Valgimigli, M.; Iglesias, J.F.; Landi, A.; Leo, L.A.; Eeckhout, E.; Schwitter, J.; et al. Hepatic T1 mapping as a novel cardio-hepatic axis imaging biomarker early after ST-elevation myocardial infarction. Eur. Heart J.—Cardiovasc. Imaging 2025, 26, 229–238. [Google Scholar] [CrossRef]
| Variable | All Patients | Stable CAD (N = 65, 50%) | ACS (N = 65, 50%) | p * |
|---|---|---|---|---|
| Number (♀/♂) | 36/94 | 20/45 | 16/49 | 0.433 |
| Age (years) | 65 (52–85) | 63 (52–84) | 66 (48–87) | 0.333 |
| BMI (kg/m2) | 28 (23–37) | 28 (23–38) | 28 (22–35) | 0.592 |
| t2DM (yes/pre-diabetes/no) | 41/7/82 | 23/3/39 | 18/4/43 | 0.623 |
| TC (mg/dL) | 165 (99–260) | 159 (118–251) | 168 (94–294) | 0.160 |
| HDL (mg/dL) | 46 (29–68) | 46 (32–67) | 47 (29–68) | 0.365 |
| LDL (mg/dL) | 89 (33–189) | 77 (41–170) | 101 (33–206) | 0.009 |
| TG (mg/dL) | 110 (64–245) | 122 (72–281) | 99 (48–189) | 0.006 |
| Hyperlipidemia (yes/no) | 53/67 | 28/33 | 25/34 | 0.697 |
| Hypertension (yes/no) | 112/18 | 55/10 | 57/8 | 0.612 |
| Smoking (active/former/no) | 38/17/75 | 17/15/33 | 21/2/42 | 0.003 |
| Previous MI (yes/no) | 38/52 | 22/43 | 16/49 | 0.247 |
| Syntax score | 14 (0–57) | 9 (0–47) | 15 (0–61) | 0.011 |
| NLR | 2.3 (1.2–5.3) | 2.1 (1.1–5.0) | 2.4 (1.2–5.5) | 0.232 |
| MLR | 0.35 (0.19–0.71) | 0.34 (0.18–0.71) | 0.36 (0.21–0.77) | 0.756 |
| PLR | 105 (51–207) | 95 (41–207) | 119 (61–212) | 0.003 |
| SII | 502 (186–1429) | 434 (166–1210) | 547 (195–1464) | 0.028 |
| SIRI | 1.63 (0.74–5.55) | 1.63 (0.75–5.77) | 1.63 (0.72–5.55) | 0.779 |
| Neutro × PLT × Mono/Lym | 350 (124–1644) | 314 (124–1611) | 404 (123–2032) | 0.298 |
| Ca (ppm) | 321 (85–1290) | 340 (70–1290) | 315 (96–1121) | 0.392 |
| Variable | Stable CAD | STEMI | NSTEMI | UA | p * |
|---|---|---|---|---|---|
| Number (♀/♂) | 20/45 | 7/24 | 5/14 | 4/11 | 0.864 |
| Age (years) | 63 (52–84) | 61 (45–89) | 74 (56–84) | 66 (59–87) | 0.079 |
| BMI (kg/m2) | 28 (23–38) | 28 (19–35) | 27 (21–45) | 29 (22–36) | 0.496 |
| t2DM (yes/pre-diabetes/no) | 23/3/39 | 8/2/21 | 4/2/13 | 6/0/9 | 0.701 |
| TC (mg/dL) | 159 (118–251) | 169 (135–283) | 161 (70–245) | 167 (94–311) | 0.113 |
| HDL (mg/dL) | 46 (32–67) | 47 (30–68) | 43 (29–68) | 46 (26–67) | 0.439 |
| LDL (mg/dL) | 77 (41–170) | 105 (63–188) | 96 (27–175) | 102 (33–228) | 0.024 |
| TG (mg/dL) | 122 (72–281) | 105 (68–205) | 91 (43–157) | 101 (43–189) | 0.023 |
| Hyperlipidemia (yes/no) | 28/33 | 13/16 | 5/11 | 7/7 | 0.716 |
| Hypertension (yes/no) | 55/10 | 24/7 | 18/1 | 15/0 | 0.127 |
| Smoking (active/former/no) | 17/15/33 | 13/1/17 | 5/0/14 | 3/1/11 | 0.023 |
| Previous MI (yes/no) | 22/43 | 6/25 | 5/14 | 5/10 | 0.507 |
| Syntax score | 9 (0–47) | 15 (2–52) | 22 (0–69) | 13 (0–36) | 0.042 |
| NLR | 2.1 (1.1–5.0) | 2.4 (1.2–8.1) | 2.2 (1.1–3.1) | 2.7 (1.8–3.9) | 0.201 |
| MLR | 0.34 (0.18–0.71) | 0.39 (0.21–0.91) | 0.34 (0.21–0.56) | 0.33 (0.21–0.55) | 0.614 |
| PLR | 95 (41–207) | 123 (61–223) | 109 (61–190) | 126 (51–160) | 0.022 |
| SII | 434 (166–1210) | 539 (241–2338) | 522 (186–1464) | 587 (337–865) | 0.117 |
| SIRI | 1.63 (0.75–5.77) | 1.66 (0.74–7.41) | 1.46 (0.54–4.35) | 1.52 (0.97–3.42) | 0.871 |
| Neutro × PLT × mono/lym | 314 (124–1611) | 427 (123–2032) | 368 (95–2532) | 352 (168–770) | 0.646 |
| Ca (ppm) | 340 (70–1290) | 322 (96–1793) | 217 (71–581) | 380 (165–1121) | 0.225 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziedzic, E.A.; Czernicka, A.; Gąsior, J.S.; Szamreta-Siwicka, A.; Wodejko-Kucharska, B.; Maciński, P.; Arbaszewska, A.; Adler, K.; Osiecki, A.; Kochman, W. Hair Calcium Levels in Relation to Coronary Artery Disease Severity and Systemic Inflammation Markers: A Pilot Study. J. Clin. Med. 2025, 14, 4537. https://doi.org/10.3390/jcm14134537
Dziedzic EA, Czernicka A, Gąsior JS, Szamreta-Siwicka A, Wodejko-Kucharska B, Maciński P, Arbaszewska A, Adler K, Osiecki A, Kochman W. Hair Calcium Levels in Relation to Coronary Artery Disease Severity and Systemic Inflammation Markers: A Pilot Study. Journal of Clinical Medicine. 2025; 14(13):4537. https://doi.org/10.3390/jcm14134537
Chicago/Turabian StyleDziedzic, Ewelina A., Aleksandra Czernicka, Jakub S. Gąsior, Anna Szamreta-Siwicka, Beata Wodejko-Kucharska, Paweł Maciński, Anna Arbaszewska, Konrad Adler, Andrzej Osiecki, and Wacław Kochman. 2025. "Hair Calcium Levels in Relation to Coronary Artery Disease Severity and Systemic Inflammation Markers: A Pilot Study" Journal of Clinical Medicine 14, no. 13: 4537. https://doi.org/10.3390/jcm14134537
APA StyleDziedzic, E. A., Czernicka, A., Gąsior, J. S., Szamreta-Siwicka, A., Wodejko-Kucharska, B., Maciński, P., Arbaszewska, A., Adler, K., Osiecki, A., & Kochman, W. (2025). Hair Calcium Levels in Relation to Coronary Artery Disease Severity and Systemic Inflammation Markers: A Pilot Study. Journal of Clinical Medicine, 14(13), 4537. https://doi.org/10.3390/jcm14134537






