Breathe Better After COVID: The Impact of a Two-Week Pulmonary Rehabilitation Program on Pulmonary Function, Inflammatory Markers, and Quality of Life in Post-COVID Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
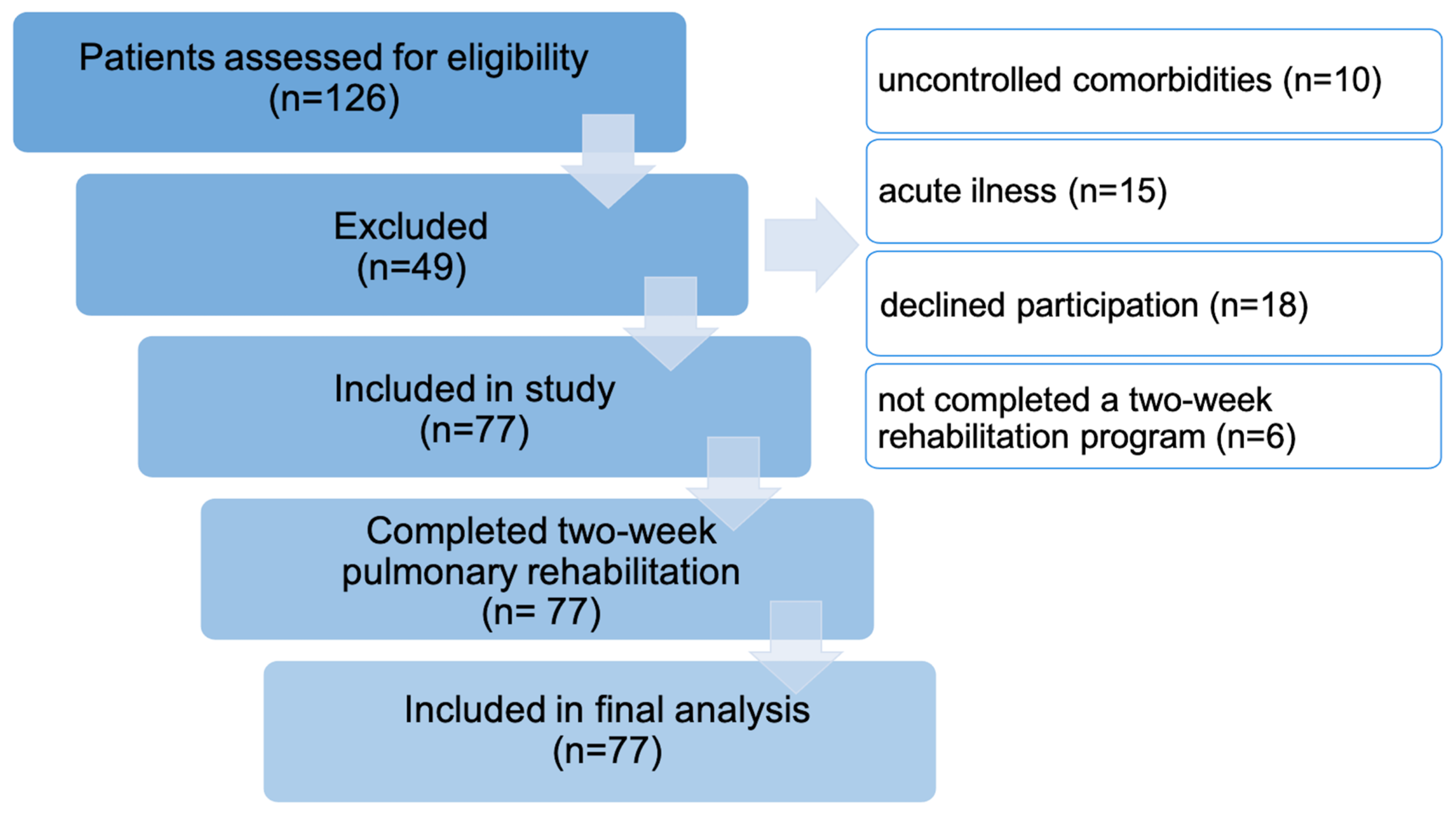
2.2. Participants
2.3. Intervention
2.4. Outcome Measures
2.4.1. Primary Outcomes
2.4.2. Spirometry
2.4.3. Body Plethysmography
2.4.4. Gasometry
2.4.5. Secondary Outcomes
2.5. Data Collection
2.6. Statistical Analysis
2.7. Ethical Considerations
3. Results
3.1. Participants Characteristics
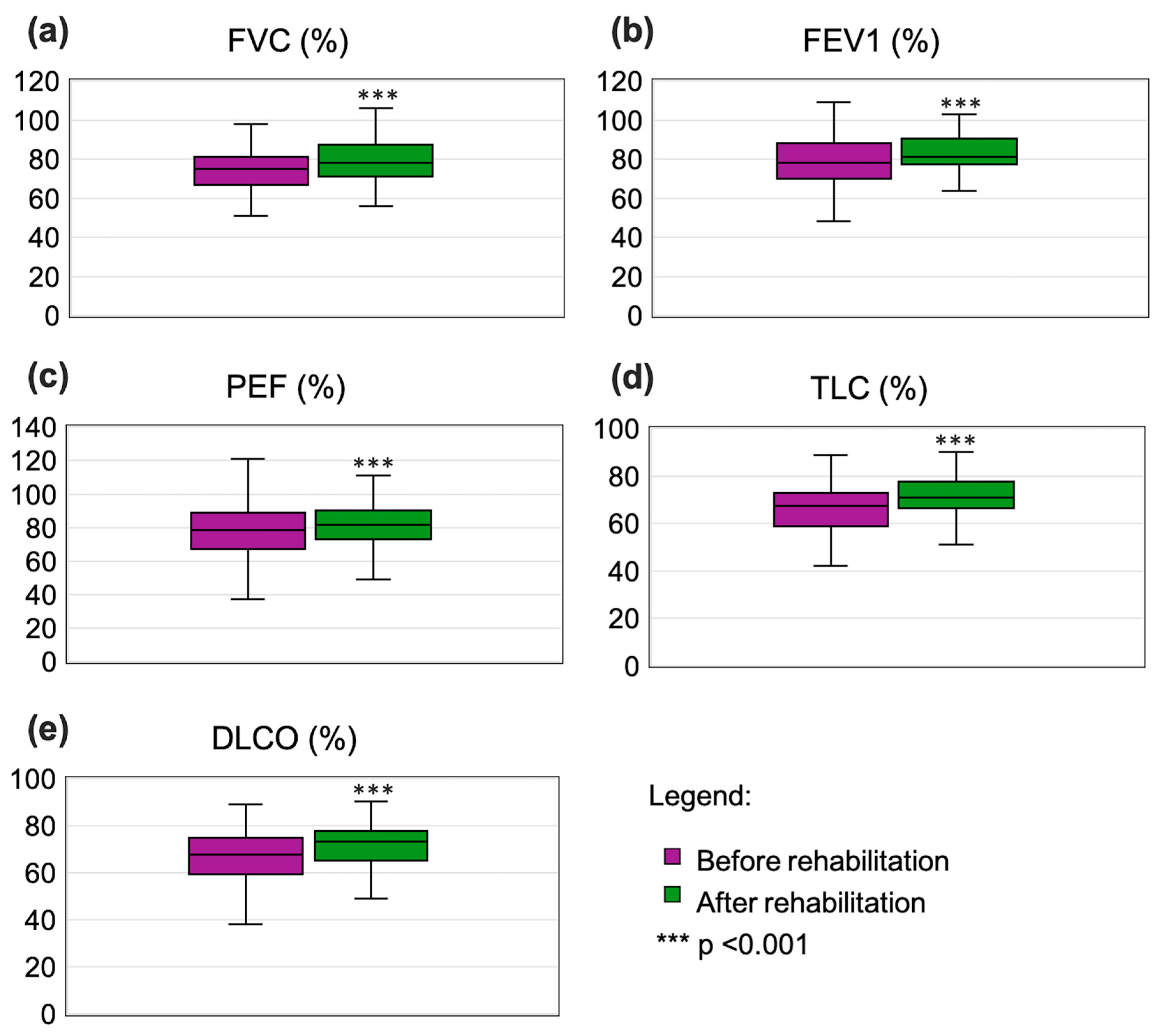
3.2. Pulmonary Function and Gas Exchange
3.3. Inflammatory Markers and Blood Parameters
3.4. Quality-of-Life Assessments
3.5. Key Factors Influencing Rehabilitation Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| COVID-19 | Coronavirus Disease 2019 |
| CRP | C-reactive Protein |
| DLCO | Diffusing Capacity of the Lung for Carbon Monoxide |
| FEV1 | Forced Expiratory Volume in One Second |
| FVC | Forced Vital Capacity |
| MEF | Maximum Expiratory Flow |
| PaCO2 | Partial Pressure of Carbon Dioxide |
| PaO2 | Partial Pressure of Oxygen |
| PEF | Peak Expiratory Flow |
| RMT | Respiratory Muscle Training |
| SF-36 | Short Form (36) Health Survey |
| TLC | Total Lung Capacity |
| WBC | White Blood Cells |
| WHOQOL-BREF | World Health Organization Quality of Life—BREF questionnaire |
References
- Sivan, M.; Taylor, S. NICE guideline on long covid. BMJ 2020, 371, m4938. [Google Scholar] [CrossRef] [PubMed]
- Spruit, M.A.; Singh, S.J.; Garvey, C.; ZuWallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.C.; et al. An official American Thoracic Society/European Respiratory Society statement: Key concepts and advances in pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64. [Google Scholar] [CrossRef]
- Martínez-Pozas, O.; Meléndez-Oliva, E.; Rolando, L.M.; Rico, J.A.Q.; Corbellini, C.; Sánchez Romero, E.A. The pulmonary rehabilitation effect on long covid-19 syndrome: A systematic review and meta-analysis. Physiother. Res. Int. 2024, 29, e2077. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Oliva, E.; Martínez-Pozas, O.; Cuenca-Zaldívar, J.N.; Villafañe, J.H.; Jiménez-Ortega, L.; Sánchez-Romero, E.A. Efficacy of Pulmonary Rehabilitation in Post-COVID-19: A Systematic Review and Meta-Analysis. Biomedicines 2023, 11, 2213. [Google Scholar] [CrossRef] [PubMed]
- Kołodziej, M.; Wyszyńska, J.; Bal-Bocheńska, M. COVID-19: A New Challenge for Pulmonary Rehabilitation? J. Clin. Med. 2021, 10, 3361. [Google Scholar] [CrossRef]
- Gloeckl, R.; Leitl, D.; Jarosch, I.; Schneeberger, T.; Nell, C.; Stenzel, N.; Vogelmeier, C.F.; Kenn, K.; Koczulla, A.R. Benefits of pulmonary rehabilitation in COVID-19: A prospective observational cohort study. ERJ Open Res. 2021, 7, 00108-2021. [Google Scholar] [CrossRef]
- Martínez-Pozas, O.; Corbellini, C.; Cuenca-Zaldívar, J.N.; Meléndez-Oliva, E.; Sinatti, P.; Sánchez Romero, E.A. Effectiveness of telerehabilitation versus face-to-face pulmonary rehabilitation on physical function and quality of life in people with post COVID-19 condition: A systematic review and network meta-analysis. Eur. J. Phys. Rehabil. Med. 2024, 60, 868–877. [Google Scholar] [CrossRef]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Bajraktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef]
- Graham, B.L.; Brusasco, V.; Burgos, F.; Cooper, B.G.; Jensen, R.; Kendrick, A.; MacIntyre, N.R.; Thompson, B.R.; Wanger, J. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur. Respir. J. 2017, 49, 1600016. [Google Scholar] [CrossRef]
- Lim, H.J.; Lee, S.Y.; Choi, H.J. Evaluation of the Accuracy of Cr and BUN Using the ABL90 FLEX PLUS Blood Gas Analyzer and the Equivalence of Candidate Specimens for Assessment of Renal Function. J. Clin. Med. 2023, 12, 1940. [Google Scholar] [CrossRef]
- Anthony, F. Polish WHOQOL-BREF. Available online: https://www.who.int/tools/whoqol/whoqol-bref/docs/default-source/publishing-policies/whoqol-bref/polish_whoqol-bref71589543-d0e3-40cd-8e0a-cd171454a339 (accessed on 22 January 2021).
- Lippi, G.; Tripodi, A.; Simundic, A.M.; Favaloro, E.J. International survey on D-dimer test reporting: A call for standardization. Semin. Thromb. Hemost. 2015, 41, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Khatcheressian, J.; Lyman, G.H.; Ozer, H.; Armitage, J.O.; Balducci, L.; Bennett, C.L.; Cantor, S.B.; Crawford, J.; Cross, S.J.; et al. 2006 update of recommendations for the use of white blood cell growth factors: An evidence-based clinical practice guideline. J. Clin. Oncol. 2006, 24, 3187–3205. [Google Scholar] [CrossRef] [PubMed]
- Negrini, F.; de Sire, A.; Andrenelli, E.; Lazzarini, S.G.; Patrini, M.; Ceravolo, M.G. Rehabilitation and COVID-19: A rapid living systematic review 2020 by Cochrane Rehabilitation Field. Update as of October 31st, 2020. Eur. J. Phys. Rehabil. Med. 2021, 57, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Boutou, A.K.; Georgopoulou, A.; Pitsiou, G.; Stanopoulos, I.; Kontakiotis, T.; Kioumis, I. Changes in the respiratory function of COVID-19 survivors during follow-up: A novel respiratory disorder on the rise? Int. J. Clin. Pract. 2021, 75, e14301. [Google Scholar] [CrossRef]
- Okan, F.; Okan, S.; Duran Yücesoy, F. Evaluating the Efficiency of Breathing Exercises via Telemedicine in Post-Covid-19 Patients: Randomized Controlled Study. Clin. Nurs. Res. 2022, 31, 771–781. [Google Scholar] [CrossRef]
- Del Corral, T.; Fabero-Garrido, R.; Plaza-Manzano, G.; Fernández-de-Las-Peñas, C.; Navarro-Santana, M.; López-de-Uralde-Villanueva, I. Home-based respiratory muscle training on quality of life and exercise tolerance in long-term post-COVID-19: Randomized controlled trial. Ann. Phys. Rehabil. Med. 2023, 66, 101709. [Google Scholar] [CrossRef]
- Donohue, J.F. Minimal clinically important differences in COPD lung function. Copd 2005, 2, 111–124. [Google Scholar] [CrossRef]
- Jones, P.W.; Beeh, K.M.; Chapman, K.R.; Decramer, M.; Mahler, D.A.; Wedzicha, J.A. Minimal clinically important differences in pharmacological trials. Am. J. Respir. Crit. Care Med. 2014, 189, 250–255. [Google Scholar] [CrossRef]
- du Bois, R.M.; Weycker, D.; Albera, C.; Bradford, W.Z.; Costabel, U.; Kartashov, A.; King, T.E., Jr.; Lancaster, L.; Noble, P.W.; Sahn, S.A.; et al. Forced vital capacity in patients with idiopathic pulmonary fibrosis: Test properties and minimal clinically important difference. Am. J. Respir. Crit. Care Med. 2011, 184, 1382–1389. [Google Scholar] [CrossRef]
- Wasserman, K.; Hansen, J.E.; Sue, D.Y.; Stringer, W.W.; Sietsema, K.E.; Sun, X.G.; Whipp, B.J. Principles of Exercice Testing and Interpretation. Including Pathophysiology and Clinical Applications, 5th ed.; Wolters Kluwer, L.W. Wilkins: Philadelphia, PA, USA; Baltimore, MD, USA; New York, NY, USA; London, UK; Buenos Aires, Argentina; Hong Kong, China; Sydney, Australia; Tokyo, Japan, 2012; pp. 151–175. [Google Scholar]
- Rivas, E.; Arismendi, E.; Agustí, A.; Sanchez, M.; Delgado, S.; Gistau, C.; Wagner, P.D.; Rodriguez-Roisin, R. Ventilation/Perfusion distribution abnormalities in morbidly obese subjects before and after bariatric surgery. Chest 2015, 147, 1127–1134. [Google Scholar] [CrossRef]
- Hermann, M.; Pekacka-Egli, A.M.; Witassek, F.; Baumgaertner, R.; Schoendorf, S.; Spielmanns, M. Feasibility and Efficacy of Cardiopulmonary Rehabilitation After COVID-19. Am. J. Phys. Med. Rehabil. 2020, 99, 865–869. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Shang, Y.M.; Song, W.B.; Li, Q.Q.; Xie, H.; Xu, Q.F.; Jia, J.L.; Li, L.M.; Mao, H.L.; Zhou, X.M.; et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine 2020, 25, 100463. [Google Scholar] [CrossRef] [PubMed]
- Polastri, M.; Nava, S.; Clini, E.; Vitacca, M.; Gosselink, R. COVID-19 and pulmonary rehabilitation: Preparing for phase three. Eur. Respir. J. 2020, 55, 2001822. [Google Scholar] [CrossRef] [PubMed]
- McDonald, L.T. Healing after COVID-19: Are survivors at risk for pulmonary fibrosis? Am. J. Physiol. Lung Cell Mol. Physiol. 2021, 320, L257–L265. [Google Scholar] [CrossRef] [PubMed]
- Guler, S.A.; Ebner, L.; Aubry-Beigelman, C.; Bridevaux, P.O.; Brutsche, M.; Clarenbach, C.; Garzoni, C.; Geiser, T.K.; Lenoir, A.; Mancinetti, M.; et al. Pulmonary function and radiological features 4 months after COVID-19: First results from the national prospective observational Swiss COVID-19 lung study. Eur. Respir. J. 2021, 57, 2003690. [Google Scholar] [CrossRef]
- Wong, A.W.; Shah, A.S.; Johnston, J.C.; Carlsten, C.; Ryerson, C.J. Patient-reported outcome measures after COVID-19: A prospective cohort study. Eur. Respir. J. 2020, 56, 2003276. [Google Scholar] [CrossRef]
- Connor, J.; Madhavan, S.; Mokashi, M.; Amanuel, H.; Johnson, N.R.; Pace, L.E.; Bartz, D. Health risks and outcomes that disproportionately affect women during the COVID-19 pandemic: A review. Soc. Sci. Med. 2020, 266, 113364. [Google Scholar] [CrossRef]
- Sharma, P.; Goswami, S. Pulmonary Tele-Rehabilitation in Patients (Post COVID-19) with Respiratory Complications: A Randomized Controlled Trial. Indian J. Physiother. Occup. Ther. 2022, 16, 182–189. [Google Scholar] [CrossRef]
- de Mol, M.; Visser, S.; Aerts, J.; Lodder, P.; de Vries, J.; den Oudsten, B.L. Satisfactory results of a psychometric analysis and calculation of minimal clinically important differences of the World Health Organization quality of life-BREF questionnaire in an observational cohort study with lung cancer and mesothelioma patients. BMC Cancer 2018, 18, 1173. [Google Scholar] [CrossRef]
- Ali, A.M.; Rostam, H.M.; Fatah, M.H.; Noori, C.M.; Ali, K.M.; Tawfeeq, H.M. Serum troponin, D-dimer, and CRP level in severe coronavirus (COVID-19) patients. Immun. Inflamm. Dis. 2022, 10, e582. [Google Scholar] [CrossRef]
- Che, K.; Zeng, Z.; Hong, C.; Peng, D.; Liu, A.; He, Y. Association between serum C-reactive protein (CRP) and Omicron variant COVID-19 pneumonia in cancer patients: A multicenter cross-sectional study at the end of 2022 in China. Medicine 2024, 103, e36965. [Google Scholar] [CrossRef] [PubMed]
- Tartibian, B.; Khayat, S.M.A.; Maleki, B.H.; Chehrazi, M. The Effects of Exercise Training on Recovery of Biochemical and Hematological Outcomes in Patients Surviving COVID-19: A Randomized Controlled Assessor-Blinded Trial. Sports Med Open. 2022, 8, 152. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.M.; Behera, D.; Mohapatra, A.K.; Mohapatra, S.K.; Jagaty, S.K.; Banu, P.; Pattanaik, A.P.; Mohapatra, I.; Patro, S. Supervised hospital based pulmonary rehabilitation outcome in long COVID-experience from a tertiary care hospital. J. Fam. Med. Prim. Care 2022, 11, 7875–7881. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Nie, J.; Wang, H.; Zhao, Q.; Xiong, Y.; Deng, L.; Song, S.; Ma, Z.; Mo, P.; Zhang, Y. Characteristics of Peripheral Lymphocyte Subset Alteration in COVID-19 Pneumonia. J. Infect. Dis. 2020, 221, 1762–1769. [Google Scholar] [CrossRef]


| N | % | Female | Male | |||||
|---|---|---|---|---|---|---|---|---|
| Gender | Female | 30 | 39 | N | % | N | % | |
| Male | 47 | 61 | ||||||
| Residence | City/town | 49 | 63.6 | 21 | 70 | 28 | 59.6 | χ2 = 0.86; p(χ2) > 0.05 |
| Countryside | 28 | 36.4 | 9 | 30 | 19 | 40.4 | ||
| Education | Primary | 1 | 1.3 | 0 | 0 | 1 | 2.1 | χ2 = 3.579; p(χ2) > 0.05 |
| Vocational | 16 | 20.8 | 6 | 20 | 10 | 21.3 | ||
| Middle | 33 | 42.9 | 10 | 33.3 | 23 | 48.9 | ||
| High | 27 | 35.1 | 14 | 46.7 | 13 | 27.7 | ||
| Work | White collar | 22 | 28.6 | 13 | 43.3 | 9 | 19.1 | F = 5.138; p(F) > 0.05 |
| Blue collar | 47 | 61.0 | 15 | 50 | 32 | 68.1 | ||
| Mixed white and blue collar | 8 | 10.4 | 2 | 6.7 | 6 | 12.8 | ||
| Body mass category | Normal | 13 | 16.9 | 6 | 20 | 7 | 14.9 | χ2 = 8.585; p(χ2) < 0.05 |
| Overweight | 41 | 53.2 | 10 | 33.3 | 31 | 66 | ||
| Obesity | 23 | 29.9 | 14 | 46.7 | 9 | 19.1 | ||
| Smoking cigarettes | Yes, regularly | 24 | 31.2 | 6 | 20 | 18 | 38.3 | F = 7.261; p(F) < 0.05 |
| Yes, occasionally | 5 | 6.5 | 0 | 0 | 5 | 10.6 | ||
| No | 48 | 62.3 | 24 | 80 | 24 | 51.1 | ||
| Parameter | Before Rehabilitation | After Rehabilitation | Wilcoxon’s Test | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | Me | Min | Max | Q1 | Q3 | M | SD | Me | Min | Max | Q1 | Q3 | ||
| Pulmonary function | |||||||||||||||
| FVC %norm | 73.43 | 12.09 | 75.00 | 50.90 | 102.30 | 67.00 | 81.10 | 79.14 | 10.63 | 78.40 | 56.00 | 106.00 | 71.00 | 88.00 | Z = −7.386; p < 0.001 |
| FEV1%norm | 78.91 | 12.87 | 78.20 | 48.20 | 109.30 | 70.00 | 88.20 | 83.97 | 11.31 | 80.50 | 52.00 | 112.30 | 77.30 | 90.70 | Z = −6.386; p < 0.001 |
| FEV1/FVC%norm | 101.51 | 8.25 | 102.0 | 77.70 | 123.30 | 98.00 | 106.90 | 103.14 | 13.00 | 103.70 | 11.90 | 123.30 | 99.50 | 109.00 | Z = −5.367; p < 0.001 |
| PEF%norm | 80.52 | 17.78 | 78.70 | 37.20 | 143.00 | 67.00 | 89.00 | 82.60 | 15.98 | 81.00 | 49.00 | 137.00 | 73.00 | 90.00 | Z = −4.468; p < 0.001 |
| MEF75%norm | 75.72 | 17.85 | 75.00 | 40.60 | 133.00 | 65.00 | 83.40 | 76.92 | 16.23 | 77.00 | 46.00 | 123.40 | 67.00 | 85.00 | Z = −4.599; p < 0.001 |
| MEF50%norm | 71.69 | 15.96 | 70.50 | 21.40 | 123.70 | 64.00 | 77.40 | 74.08 | 15.31 | 71.80 | 38.00 | 120.50 | 65.70 | 81.00 | Z = −5.449; p < 0.001 |
| MEF25%norm | 65.50 | 15.04 | 65.80 | 27.30 | 102.00 | 55.00 | 73.00 | 68.44 | 16.49 | 67.00 | 32.00 | 136.00 | 56.70 | 76.40 | Z = −5.601; p < 0.001 |
| TLC%norm | 67.31 | 11.63 | 67.30 | 42.00 | 101.00 | 58.90 | 73.00 | 77.70 | 51.23 | 71.00 | 6.00 | 505.90 | 66.40 | 79.00 | Z = −6.416; p < 0.001 |
| DLCO%norm | 66.30 | 11.69 | 67.80 | 38.00 | 89.00 | 59.50 | 75.00 | 78.70 | 56.62 | 74.00 | 49.00 | 561.50 | 65.90 | 78.00 | Z = −6.842; p < 0.001 |
| Inflammatory markers | |||||||||||||||
| CRP (mg/dL) | 8.41 | 6.05 | 8.90 | 0.09 | 27.40 | 3.40 | 11.30 | 2.32 | 2.35 | 1.32 | 0.03 | 9.50 | 0.62 | 3.13 | Z = −7.525; p < 0.001 |
| D-Dimer (ng/mL) | 2273.00 | 2554.91 | 1722.20 | 98.50 | 15,889.70 | 876.60 | 2464.30 | 492.99 | 1017.91 | 203.44 | 11.70 | 8597.20 | 115.00 | 453.30 | Z = −7.451; p < 0.001 |
| WBC × 103/uL | 10.57 | 4.63 | 9.50 | 3.49 | 21.20 | 6.78 | 14.29 | 12.09 | 6.76 | 10.01 | 2.75 | 27.33 | 6.84 | 17.23 | Z = −1.765; p > 0.05 |
| Arterial blood gases | |||||||||||||||
| PaCO2 (mmHg) | 35.38 | 5.08 | 35.00 | 21.20 | 47.30 | 33.00 | 38.90 | 38.35 | 3.52 | 38.0 | 24.9 | 49.00 | 36.60 | 40.00 | Z = −5.174; p < 0.001 |
| PaO2 (mmHg) | 58.35 | 11.85 | 59.40 | 32.00 | 89.00 | 52.60 | 66.00 | 76.92 | 6.65 | 67.00 | 48.70 | 87.00 | 65.00 | 71.30 | Z = −6.441; p < 0.001 |
| Quality-of-life domains | |||||||||||||||
| Physical | 41.8 | 11.2 | 41.0 | 13.0 | 69.0 | 34.5 | 50.0 | 56.1 | 7.9 | 56.0 | 31.0 | 75.0 | 50.0 | 63.0 | Z = −6.906; p < 0.001 |
| Psychological | 53.2 | 11.7 | 56.0 | 19.0 | 75.0 | 44.0 | 63.0 | 59.9 | 8.2 | 56.0 | 44.0 | 81.0 | 56.0 | 69.0 | Z = −4.669; p < 0.001 |
| Social relationships | 64 | 20.4 | 69.0 | 19.0 | 94.0 | 50.0 | 81.0 | 65.5 | 19.5 | 69.0 | 25.0 | 94.0 | 50.0 | 81.0 | Z = −1.656; p > 0.05 |
| Environmental | 55.6 | 13.9 | 56.0 | 19.0 | 81.0 | 44.0 | 63.0 | 64.8 | 11.5 | 63.0 | 38.0 | 94.0 | 56.0 | 75.0 | Z = −5.909; p < 0.001 |
| Effect | Factor | Unstandardized Coefficients | Standardized Coefficients | t | p | |
|---|---|---|---|---|---|---|
| B | Std. Error | Beta | ||||
| Rehabilitation effect— physical domain | no factors in the model | |||||
| Rehabilitation effect— psychological domain | Gender | −8.43 | 2.30 | −0.40 | −3.66 | <0.001 |
| Rehabilitation effect— social relationships domain | CRP (mg/dL) | 1.23 | 0.34 | 0.42 | 3.64 | <0.001 |
| d-dimer (ng/mL) | 0.00 | 0.00 | −0.42 | −3.60 | <0.001 | |
| Rehabilitation effect— environmental domain | Gender | −8.00 | 2.26 | −0.39 | −3.53 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bal-Bocheńska, M.; Wyszyńska, J.; Kołodziej, M. Breathe Better After COVID: The Impact of a Two-Week Pulmonary Rehabilitation Program on Pulmonary Function, Inflammatory Markers, and Quality of Life in Post-COVID Syndrome. J. Clin. Med. 2025, 14, 4533. https://doi.org/10.3390/jcm14134533
Bal-Bocheńska M, Wyszyńska J, Kołodziej M. Breathe Better After COVID: The Impact of a Two-Week Pulmonary Rehabilitation Program on Pulmonary Function, Inflammatory Markers, and Quality of Life in Post-COVID Syndrome. Journal of Clinical Medicine. 2025; 14(13):4533. https://doi.org/10.3390/jcm14134533
Chicago/Turabian StyleBal-Bocheńska, Monika, Justyna Wyszyńska, and Magdalena Kołodziej. 2025. "Breathe Better After COVID: The Impact of a Two-Week Pulmonary Rehabilitation Program on Pulmonary Function, Inflammatory Markers, and Quality of Life in Post-COVID Syndrome" Journal of Clinical Medicine 14, no. 13: 4533. https://doi.org/10.3390/jcm14134533
APA StyleBal-Bocheńska, M., Wyszyńska, J., & Kołodziej, M. (2025). Breathe Better After COVID: The Impact of a Two-Week Pulmonary Rehabilitation Program on Pulmonary Function, Inflammatory Markers, and Quality of Life in Post-COVID Syndrome. Journal of Clinical Medicine, 14(13), 4533. https://doi.org/10.3390/jcm14134533







