Predictive Factors for Response to Percutaneous Bleomycin in Lymphatic–Venous Malformations of the Head and Neck
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Final Diagnosis
2.3. Treatment Protocol of Bleomycin Sclerotherapy
2.4. Treatment Response
2.5. Data Collection
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, M.X.; Kamel, S.; Elsayes, K.M.; Guillerman, R.P.; Habiba, A.; Heng, L.; Revzin, M.; Mellnick, V.; Iacobas, I.; Chau, A. Vascular Anomaly Syndromes in the ISSVA Classification System: Imaging Findings and Role of Interventional Radiology in Management. RadioGraphics 2022, 42, 1598–1620. [Google Scholar] [CrossRef]
- Dubois, J.; Soulez, G.; Oliva, V.L.; Berthiaume, M.J.; Lapierre, C.; Therasse, E. Soft-tissue venous malformations in adult patients: Imaging and therapeutic issues. Radiographics 2001, 21, 1519–1531. [Google Scholar] [CrossRef]
- Mäkinen, T.; Boon, L.M.; Vikkula, M.; Alitalo, K. Lymphatic Malformations: Genetics, Mechanisms and Therapeutic Strategies. Circ. Res. 2021, 129, 136–154. [Google Scholar] [CrossRef]
- Cooke-Barber, J.; Kreimer, S.; Patel, M.; Dasgupta, R.; Jeng, M. Venous malformations. Semin. Pediatr. Surg. 2020, 29, 150976. [Google Scholar] [CrossRef] [PubMed]
- Bagga, B.; Goyal, A.; Das, A.; Bhalla, A.S.; Kandasamy, D.; Singhal, M.; Kairo, A. Clinicoradiologic predictors of sclerotherapy response in low-flow vascular malformations. J. Vasc. Surg. Venous Lymphat. Disord. 2021, 9, 209–219. [Google Scholar] [CrossRef]
- Mulligan, P.R.; Prajapati, H.J.; Martin, L.G.; Patel, T.H. Vascular anomalies: Classification, imaging characteristics and implications for interventional radiology treatment approaches. Br. J. Radiol. 2014, 87, 20130392. [Google Scholar] [CrossRef] [PubMed]
- Perkins, J.A.; Manning, S.C.; Tempero, R.M.; Cunningham, M.J.; Edmonds, J.L.; Hoffer, F.A.; Egbert, M.A. Lymphatic malformations: Review of current treatment. Otolaryngol.–Head Neck Surg. 2010, 142, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.W.; Mai, H.M.; Zhang, L.; Wang, Y.A.; Fan, X.D.; Su, L.X.; Qin, Z.P.; Yang, Y.W.; Jiang, Y.H.; Zhao, Y.F.; et al. Guidelines for the treatment of head and neck venous malformations. Int. J. Clin. Exp. Med. 2013, 6, 377–389. [Google Scholar]
- Churojana, A.; Mahiwan, L.; Songsaeng, D.; Khumtong, R.; Homsud, S. Predictive Factors for Successful Percutaneous Sclerotherapy of Venous and Lymphatic Malformations. ASEAN J. Radiol. 2013, 19, 29–42. [Google Scholar] [CrossRef]
- Cao, J.; Liu, J.; Zhang, X.; Wang, Z. A systematic review and network meta-analysis of the effectiveness of sclerotherapy for venous malformation. J. Vasc. Surg. Venous Lymphat. Disord. 2023, 11, 210–218. [Google Scholar] [CrossRef]
- Finitsis, S.; Faiz, K.; Linton, J.; Shankar, J.J.S. Bleomycin for Head and Neck Venolymphatic Malformations: A Systematic Review. Can. J. Neurol. Sci. 2021, 48, 365–371. [Google Scholar] [CrossRef]
- Horbach, S.E.R.; Rigter, I.M.; Smitt, J.H.S.; Reekers, J.A.; Spuls, P.I.; van der Horst, C. Intralesional Bleomycin Injections for Vascular Malformations: A Systematic Review and Meta-Analysis. Plast. Reconstr. Surg. 2016, 137, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Abdelaty, M.H.; Badran, A.I.; Aborahma, A.M.; Elheniedy, M.A.; Kamhawy, A.H. Intralesional injection of bleomycin in the management of low flow vascular malformations: Results and factors affecting the outcome. J. Vasc. Surg. Venous Lymphat. Disord. 2024, 12, 101694. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Osuga, K.; Maeda, N.; Higashihara, H.; Hamada, K.; Hashimoto, N.; Uehara, S.; Tomiyama, N. Percutaneous sclerotherapy for venous malformations in the extremities: Clinical outcomes and predictors of patient satisfaction. Springerplus 2014, 3, 520. [Google Scholar] [CrossRef]
- Wu, Z.; Zou, Y.; Fu, R.; Jin, P.; Yuan, H. A nomogram for predicting sclerotherapy response for treatment of lymphatic malformations in children. Eur. J. Med. Res. 2022, 27, 209. [Google Scholar] [CrossRef]
- Rijswijk, C.S.P.v.; Linden, E.v.d.; Woude, H.-J.v.d.; Baalen, J.M.v.; Bloem, J.L. Value of Dynamic Contrast-Enhanced MR Imaging in Diagnosing and Classifying Peripheral Vascular Malformations. Am. J. Roentgenol. 2002, 178, 1181–1187. [Google Scholar] [CrossRef]
- Schmidt, V.F.; Masthoff, M.; Czihal, M.; Cucuruz, B.; Häberle, B.; Brill, R.; Wohlgemuth, W.A.; Wildgruber, M. Imaging of peripheral vascular malformations—Current concepts and future perspectives. Mol. Cell. Pediatr. 2021, 8, 19. [Google Scholar] [CrossRef]
- Bouwman, F.C.M.; Kooijman, S.S.; Verhoeven, B.H.; Schultze Kool, L.J.; van der Vleuten, C.J.M.; Botden, S.; de Blaauw, I. Lymphatic malformations in children: Treatment outcomes of sclerotherapy in a large cohort. Eur. J. Pediatr. 2021, 180, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Helal, H.A.; Mahmoud, N.A. Effect of foam and liquid bleomycin in the management of venous malformations in head and neck region: A comparative study. J. Plast. Reconstr. Aesthet. Surg. 2020, 73, 90–97. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, W.; Li, X. Safety and efficacy of surgery combined with bleomycin irrigation for complex cervical-facial lymphatic malformations of children. Int. J. Pediatr. Otorhinolaryngol. 2020, 128, 109724. [Google Scholar] [CrossRef]
- Yang, X.; Chen, H.; Gu, H.; Jin, Y.; Hu, L.; Hua, C.; Wang, Y.; Sun, Y.; Yu, W.; Lin, X. Interim results of bleomycin-polidocanol foam sclerotherapy as a highly efficient technique for venous malformations. J. Vasc. Surg. Venous Lymphat. Disord. 2020, 8, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.; Kunz, R.; Brozek, J.; Alonso-Coello, P.; Montori, V.; Akl, E.A.; Djulbegovic, B.; Falck-Ytter, Y.; et al. GRADE guidelines: 4. Rating the quality of evidence--study limitations (risk of bias). J. Clin. Epidemiol. 2011, 64, 407–415. [Google Scholar] [CrossRef]
- Henzler, T.; Vogler, N.; Lange, B.; Dally, F.; Meyer, M.; Schoenberg, S.O.; Sadick, M. Low dose time-resolved CT-angiography in pediatric patients with venous malformations using 3rd generation dual-source CT: Initial experience. Eur. J. Radiol. Open 2016, 3, 216–222. [Google Scholar] [CrossRef]
- Cahill, A.M.; Nijs, E.L. Pediatric vascular malformations: Pathophysiology, diagnosis, and the role of interventional radiology. Cardiovasc. Interv. Radiol. 2011, 34, 691–704. [Google Scholar] [CrossRef]
- Yılmaz, H.; Yılmaz, Ö.; Çamlıdağ, İ.; Belet, Ü.; Akan, H. Single center experience with intralesional bleomycin sclerotherapy for lymphatic malformations. Jpn. J. Radiol. 2017, 35, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, K.; Bauman, N.; Chun, R.H.; Darrow, D.H.; Grimmer, J.F.; Perkins, J.A.; Richter, G.T.; Shin, J.J.; Shivaram, G.M.; Sidell, D.R.; et al. Standardized outcome and reporting measures in pediatric head and neck lymphatic malformations. Otolaryngol.–Head Neck Surg. 2015, 152, 948–953. [Google Scholar] [CrossRef]
- Nevesny, F.; Chevallier, O.; Falvo, N.; Guillen, K.; Malakhia, A.; Pellegrinelli, J.; Comby, P.O.; Bonniaud, B.; Midulla, M.; Loffroy, R. Bleomycin for Percutaneous Sclerotherapy of Venous and Lymphatic Malformations: A Retrospective Study of Safety, Efficacy and Mid-Term Outcomes in 26 Patients. J. Clin. Med. 2021, 10, 1302. [Google Scholar] [CrossRef] [PubMed]
- Som, P.M.; Curtin, H.D. Head and Neck Imaging: Expert Consult—Online and Print; Elsevier Health Sciences: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Alsadah, S.A.; Alshiha, W.S.; Assiri, N.; Alnasser, H. Facial venous malformation with phleboliths. J. Pediatr. Surg. Case Rep. 2020, 59, 101402. [Google Scholar] [CrossRef]
- Sun, J.; Wang, C.; Li, J.; Song, D.; Guo, L. The efficacy of bleomycin sclerotherapy in the treatment of lymphatic malformations: A review and meta-analysis. Braz. J. Otorhinolaryngol. 2023, 89, 101285. [Google Scholar] [CrossRef]
- Puig, S.; Aref, H.; Chigot, V.; Bonin, B.; Brunelle, F. Classification of venous malformations in children and implications for sclerotherapy. Pediatr. Radiol. 2003, 33, 99–103. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Horbach, S.E.R.; van de Ven, J.S.; Nieuwkerk, P.T.; Spuls, P.I.; van der Horst, C.; Reekers, J.A. Patient-Reported Outcomes of Bleomycin Sclerotherapy for Low-Flow Vascular Malformations and Predictors of Improvement. Cardiovasc. Interv. Radiol. 2018, 41, 1494–1504. [Google Scholar] [CrossRef] [PubMed]
- Legiehn, G.M.; Heran, M.K. Classification, diagnosis, and interventional radiologic management of vascular malformations. Orthop. Clin. N. Am. 2006, 37, 435–474. [Google Scholar] [CrossRef]
- Bashir, U.; Shah, S.; Jeph, S.; O’Keeffe, M.; Khosa, F. Magnetic Resonance (MR) Imaging of Vascular Malformations. Pol. J. Radiol. 2017, 82, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, A.H.; Mulliken, J.B.; Fishman, S.J.; Quatrano, N.A.; Zurakowski, D.; Greene, A.K. Lymphatic malformation: Risk of progression during childhood and adolescence. J. Craniofac. Surg. 2012, 23, 149–152. [Google Scholar] [CrossRef]
- Dubois, J.; Garel, L. Imaging and therapeutic approach of hemangiomas and vascular malformations in the pediatric age group. Pediatr. Radiol. 1999, 29, 879–893. [Google Scholar] [CrossRef]
- Zhou, Q.; Zheng, J.W.; Mai, H.M.; Luo, Q.F.; Fan, X.D.; Su, L.X.; Wang, Y.A.; Qin, Z.P. Treatment guidelines of lymphatic malformations of the head and neck. Oral Oncol. 2011, 47, 1105–1109. [Google Scholar] [CrossRef]
- Esposito, F.; Ferrara, D.; Di Serafino, M.; Diplomatico, M.; Vezzali, N.; Giugliano, A.M.; Colafati, G.S.; Zeccolini, M.; Tomà, P. Classification and ultrasound findings of vascular anomalies in pediatric age: The essential. J. Ultrasound 2019, 22, 13–25. [Google Scholar] [CrossRef]
- Sheng, L.; Yu, Z.; Li, S.; Cao, W.; Jiang, Z. Bleomycin sclerotherapy for large diffuse microcystic lymphatic malformations. Gland. Surg. 2021, 10, 1865–1873. [Google Scholar] [CrossRef]
- Kulungowski, A.M.; Patel, M. Lymphatic malformations. Semin. Pediatr. Surg. 2020, 29, 150971. [Google Scholar] [CrossRef]
- Burrows, P.E.; Mitri, R.K.; Alomari, A.; Padua, H.M.; Lord, D.J.; Sylvia, M.B.; Fishman, S.J.; Mulliken, J.B. Percutaneous sclerotherapy of lymphatic malformations with doxycycline. Lymphat. Res. Biol. 2008, 6, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Deveikis, J.P. Percutaneous ethanol sclerotherapy for vascular malformations in the head and neck. Arch. Facial Plast. Surg. 2005, 7, 322–325. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, Y.; Li, W.; Shi, C. Effectiveness and safety of polidocanol for the treatment of hemangiomas and vascular malformations: A meta-analysis. Dermatol. Ther. 2018, 31, e12568. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Guo, Y.; Chen, X.; Lei, S. Effectiveness and Safety of Ethanol for the Treatment of Venous Malformations: A Meta-Analysis. Dermatol. Surg. 2020, 46, 1514–1518. [Google Scholar] [CrossRef]
- Wiegand, S.; Dietz, A.; Wichmann, G. Efficacy of sirolimus in children with lymphatic malformations of the head and neck. Eur. Arch. Otorhinolaryngol. 2022, 279, 3801–3810. [Google Scholar] [CrossRef]
- Jin, Y.; Lin, X.; Chen, H.; Li, W.; Hu, X.; Ma, G.; Zhu, L.; Sun, M.; Yang, C.; Wang, W. Craniofacial venous malformations: Magnetic resonance imaging features that predict treatment outcome. J. Oral Maxillofac. Surg. 2009, 67, 2388–2396. [Google Scholar] [CrossRef] [PubMed]
- Caty, V.; Kauffmann, C.; Dubois, J.; Mansour, A.; Giroux, M.F.; Oliva, V.; Piché, N.; Therasse, E.; Soulez, G. Clinical validation of semi-automated software for volumetric and dynamic contrast enhancement analysis of soft tissue venous malformations on magnetic resonance imaging examination. Eur. Radiol. 2014, 24, 542–551. [Google Scholar] [CrossRef]
- Shen, Y.-C.; Wang, D.-M.; Yang, X.-T.; Wang, Z.-F.; Wen, M.-Z.; Han, Y.-F.; Zheng, L.-Z.; Di, R.-Y.; Jiang, C.-Y.; Wang, J.-B.; et al. Novel radiopaque ethanol injection: Physicochemical properties, animal experiments, and clinical application in vascular malformations. Mil. Med. Res. 2024, 11, 39. [Google Scholar] [CrossRef]
- Mazoyer, E.; Enjolras, O.; Bisdorff, A.; Perdu, J.; Wassef, M.; Drouet, L. Coagulation disorders in patients with venous malformation of the limbs and trunk: A case series of 118 patients. Arch. Dermatol. 2008, 144, 861–867. [Google Scholar] [CrossRef]

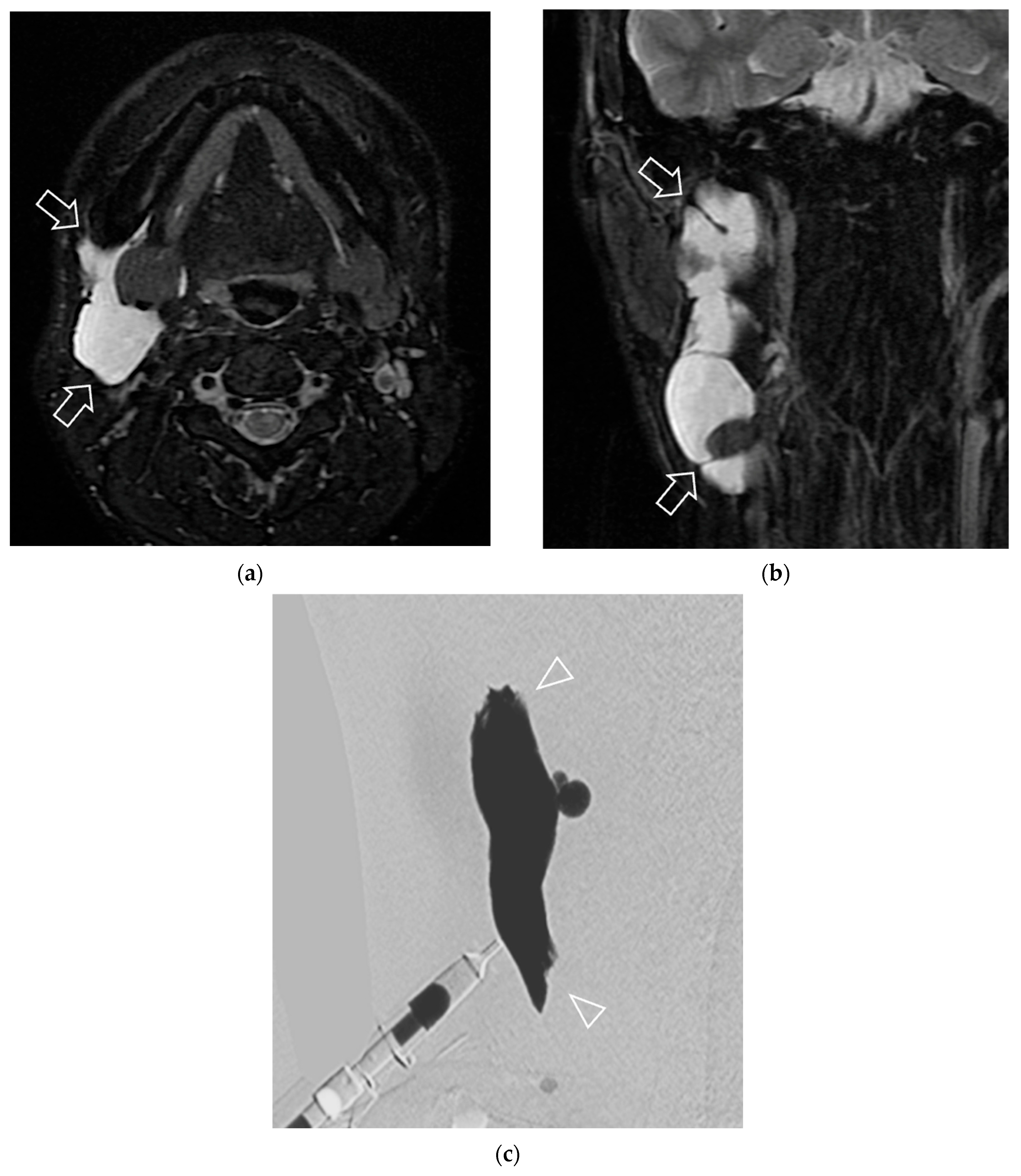


| Characteristics | Number of Patients (n = 80 Patients/85 Lesions) | p-Value ** | |
|---|---|---|---|
| Non-Responders (n = 40) | Responders * (n = 45) | ||
| Age at first treatment (years) | 14.5 (6.5–38) | 14 (3–36) | 0.13 |
| Female | 19 (47.50%) | 29 (64.44%) | 0.11 |
| Location | 0.002 | ||
| Neck | 5 (12.50%) | 20 (44.44%) | |
| Cheek | 14 (35%) | 14 (31.11%) | |
| Scalp | 4 (10%) | 3 (6.67%) | |
| Lip | 6 (15%) | 2 (4.44%) | |
| Eyelid and orbit | 4 (10%) | 1 (2.22%) | |
| Others *** | 5 (12.50%) | 5 (11.12%) | |
| Concerning symptom | 0.001 | ||
| Mass | 32 (80%) | 43 (95.56%) | |
| Pain | 4 (10%) | 0 | |
| Others **** | 4 (10%) | 3 (6.67%) | |
| Change in size by physical examination with or without imaging | <0.001 | ||
| Worsening | 6 (15%) | 0 | |
| No change | 18 (45%) | 1 (2.22%) | |
| Size reduction < 50% | 14 (35%) | 2 (4.44%) | |
| Size reduction > 50% ***** | 0 | 43 (95.56%) | |
| Variables | Response | Univariable Analysis | Multivariable Analysis * | |||
|---|---|---|---|---|---|---|
| Non-Responders (n = 40) | Responders (n = 45) | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Location at neck | 5 (12.50%) | 20 (44.44%) | 4 (1.17–13.66) | 0.03 | 3.09 (0.53–17.98) | 0.21 |
| Type of LVM | ||||||
| Pure venous malformation | 27 (67.50%) | 15 (33.33%) | ||||
| Pure lymphatic malformation | 5 (12.50%) | 17 (37.78%) | 6.12 (1.88–19.92) | 0.004 | 3.43 (0.40–14.85) | 0.34 |
| Mixed | 8 (20.00%) | 13 (28.89%) | 2.93 (0.99–8.64) | 0.052 | 1.54 (0.30–7.84) | 0.60 |
| Imaging features | ||||||
| Pre-treatment volume (mL) ** | 31.57 (14.79–55.27) | 35.81 (15.04–66.20) | 1.00 (0.99–1.00) | 0.64 | 1.00 (0.99–1.01) | 0.81 |
| Depth of lesion *** | ||||||
| Superficial | 12 (30.00%) | 6 (13.33%) | ||||
| Deep | 13 (32.50%) | 24 (53.33%) | 3.69 (1.00–10.0) | 0.03 | 3.74 (0.66–21.25) | 0.14 |
| Mixed | 15 (37.50%) | 15 (33.33%) | 2.00 (0.59–6.73) | 0.26 | 0.59 (0.09–3.95) | 0.59 |
| Component in LM | (n = 13) | (n = 30) | ||||
| Microcystic | 5 (38.46%) | 2 (6.67%) | ||||
| Macrocystic | 8 (61.54%) | 28 (93.33%) | 10 (1.54–64.75) | 0.016 | 3.74 (0.66–21.25) | 0.14 |
| Presence of venous ectasia | 7 (17.50%) | 5 (11.11%) | 0.59 (0.17–2.03) | 0.40 | 0.31 (0.02–5.22) | 0.42 |
| Presence of fluid level/ hemorrhage | 0 | 3 (6.67%) | 1.05 (0.68–1.62) | 0.83 | * | * |
| Presence of phlebolith | 15 (37.50%) | 9 (20.00%) | 2.46 (0.93–6.51) | 0.077 | 0.26 (0.04–1.60) | 0.15 |
| Pattern of contrast opacification on dynamic digital radiographic images | ||||||
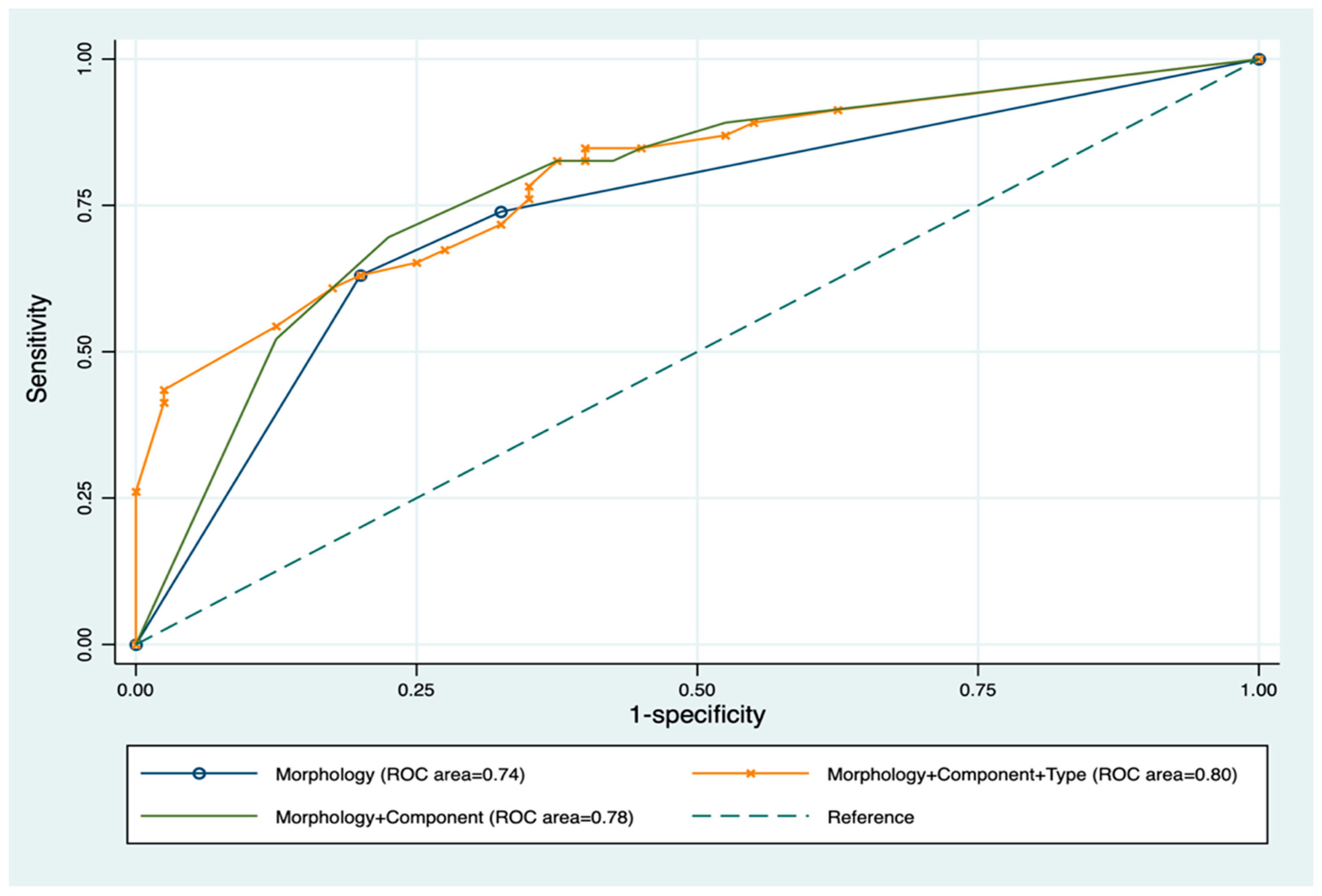
| Morphology | ||||||
| Spongy | 27 (67.50%) | 11 (24.44%) | ||||
| Cavitary | 8 (20.00%) | 29 (64.44%) | 8.90 (3.11–25.45) | <0.001 | 5.90 (1.05–33.05) | 0.04 |
| Mixed | 5 (12.50%) | 5 (11.11%) | 3.63 (0.84–15.70) | 0.09 | 5.18 (0.57–47.55) | 0.15 |
| Type of venous drainage **** | ||||||
| Type I | 25 (62.50%) | 32 (71.11%) | ||||
| Type II | 10 (25.00%) | 11 (24.44%) | 0.86 (0.35–2.34) | 0.77 | 4.30 (0.94–19.75) | 0.06 |
| Type III | 5 (12.50%) | 2 (4.44%) | 0.31 (0.06–1.75) | 0.19 | 3.08 (0.15–63.46) | 0.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanthawang, T.; Hirun, Y.; Unsrisong, K.; Vongsfak, J.; Vuthiwong, W. Predictive Factors for Response to Percutaneous Bleomycin in Lymphatic–Venous Malformations of the Head and Neck. J. Clin. Med. 2025, 14, 4505. https://doi.org/10.3390/jcm14134505
Kanthawang T, Hirun Y, Unsrisong K, Vongsfak J, Vuthiwong W. Predictive Factors for Response to Percutaneous Bleomycin in Lymphatic–Venous Malformations of the Head and Neck. Journal of Clinical Medicine. 2025; 14(13):4505. https://doi.org/10.3390/jcm14134505
Chicago/Turabian StyleKanthawang, Thanat, Yuttapol Hirun, Kittisak Unsrisong, Jirapong Vongsfak, and Withawat Vuthiwong. 2025. "Predictive Factors for Response to Percutaneous Bleomycin in Lymphatic–Venous Malformations of the Head and Neck" Journal of Clinical Medicine 14, no. 13: 4505. https://doi.org/10.3390/jcm14134505
APA StyleKanthawang, T., Hirun, Y., Unsrisong, K., Vongsfak, J., & Vuthiwong, W. (2025). Predictive Factors for Response to Percutaneous Bleomycin in Lymphatic–Venous Malformations of the Head and Neck. Journal of Clinical Medicine, 14(13), 4505. https://doi.org/10.3390/jcm14134505







