Optimizing Airway Function Through Craniofacial and Cervical Manipulations and Emergency-Anesthesia Maneuvers: Applications in Airway Function Enhancement, Pneumonia, and Asthma—Narrative Review
Abstract
1. Introduction
2. Methods
3. Synthesis of the Literature
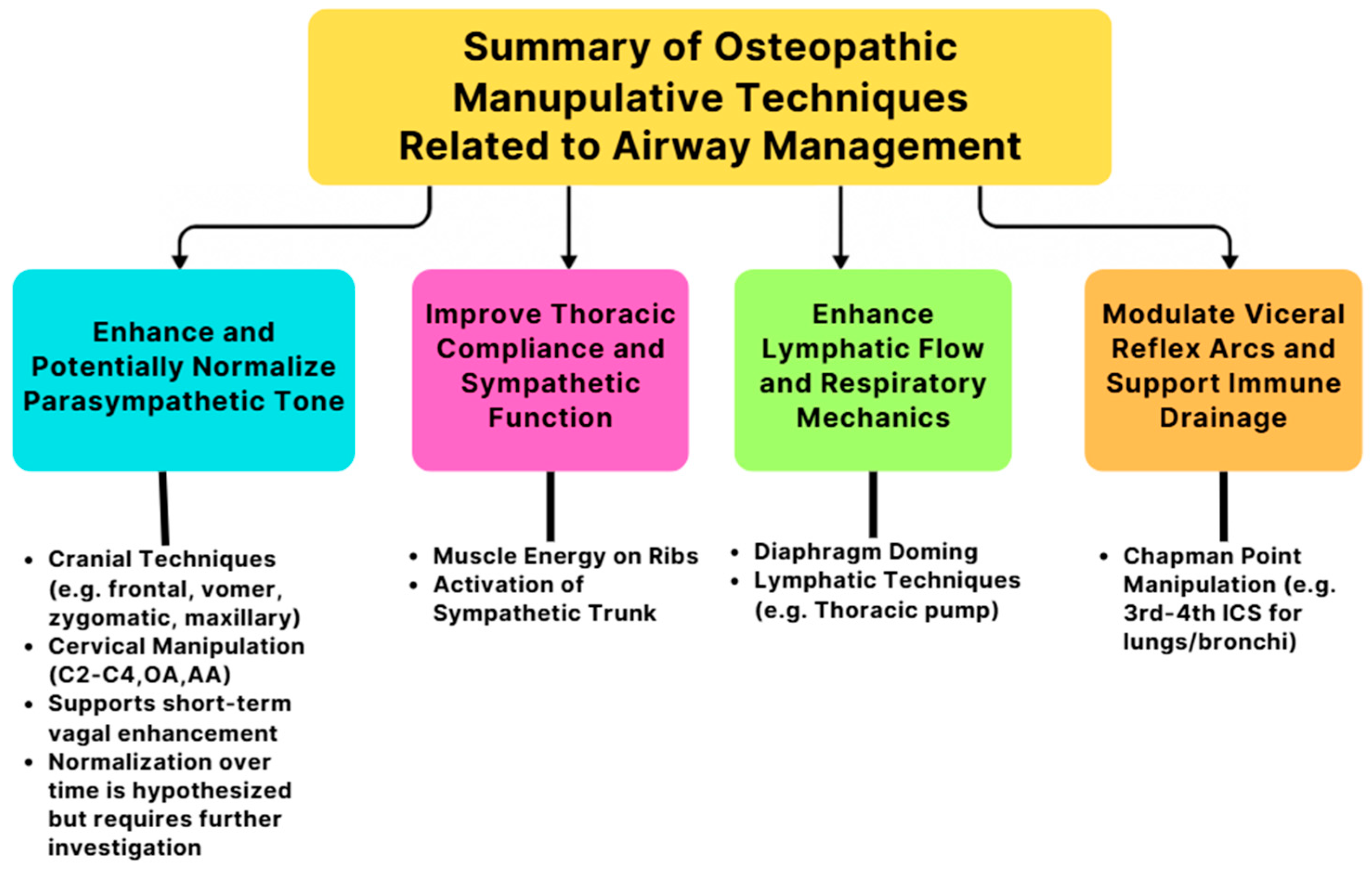
3.1. Osteopathic Manipulative Techniques and Physiological Mechanisms
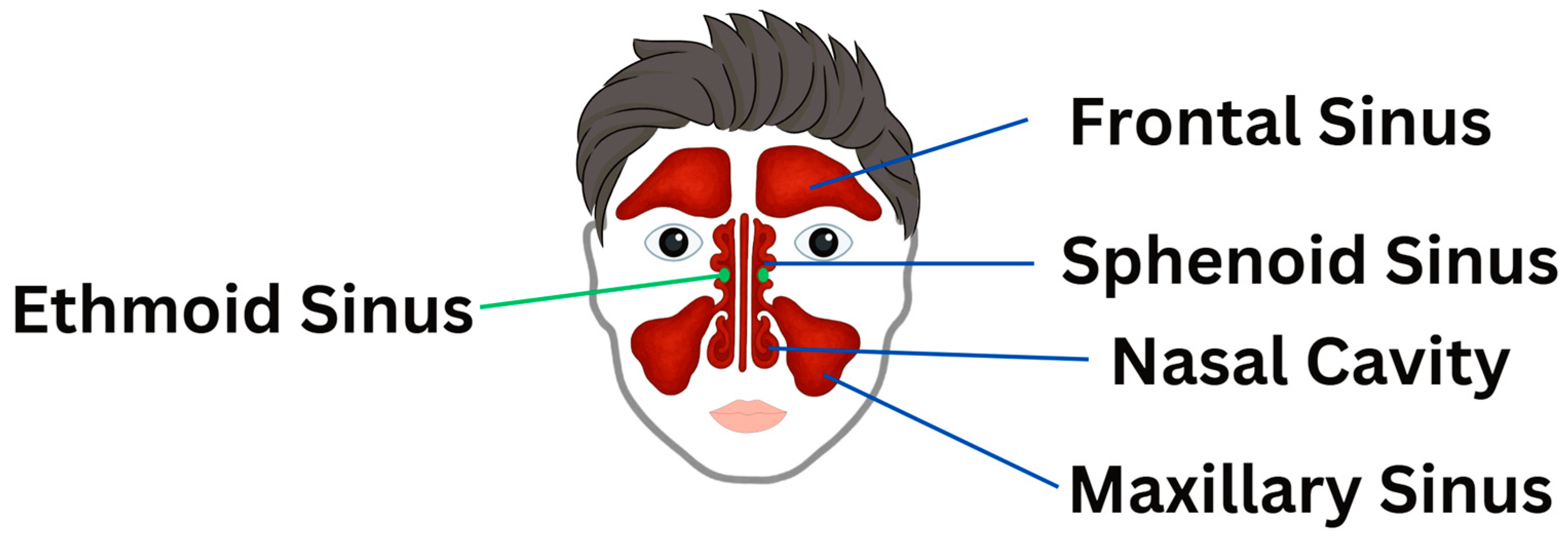
3.1.1. Airway Function Improvement
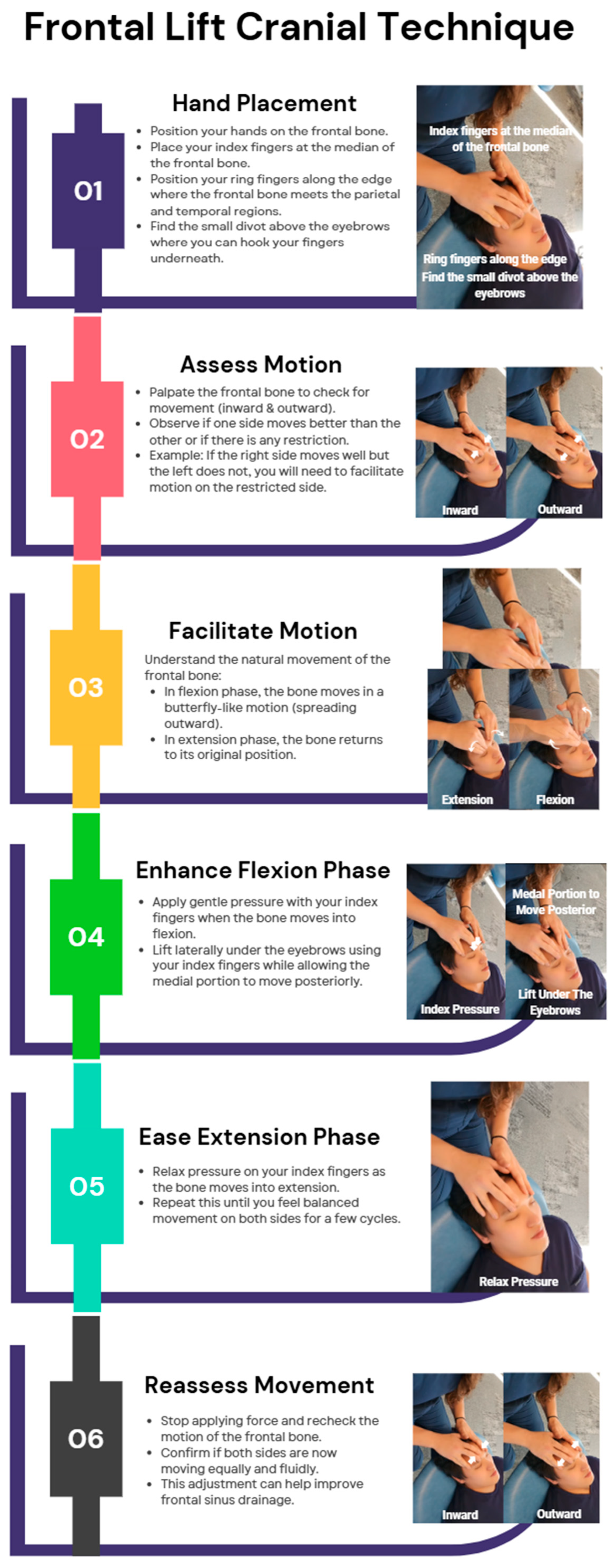
Frontal Bone Manipulation
Vomer Manipulation
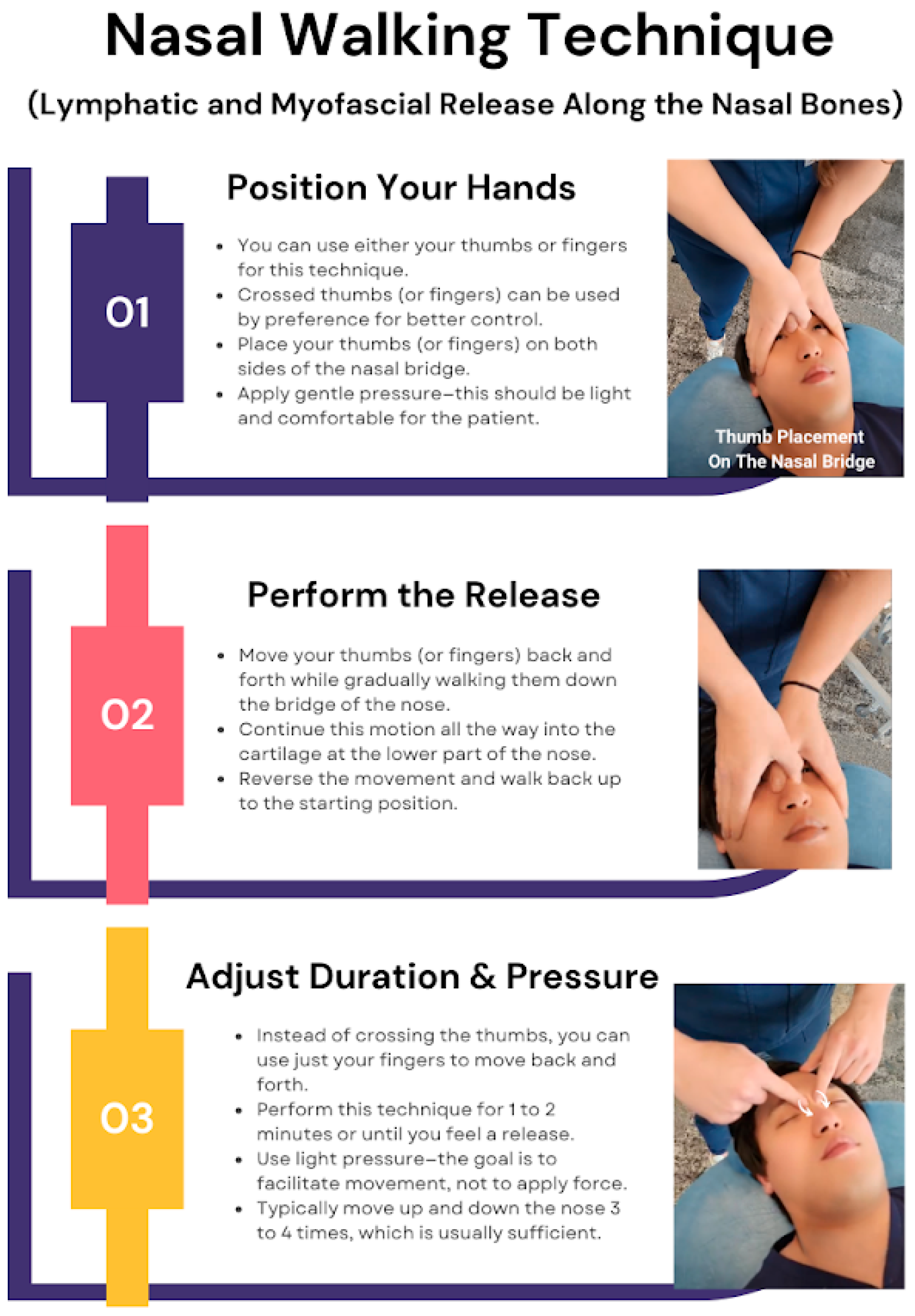
“Nasal Walking” Technique
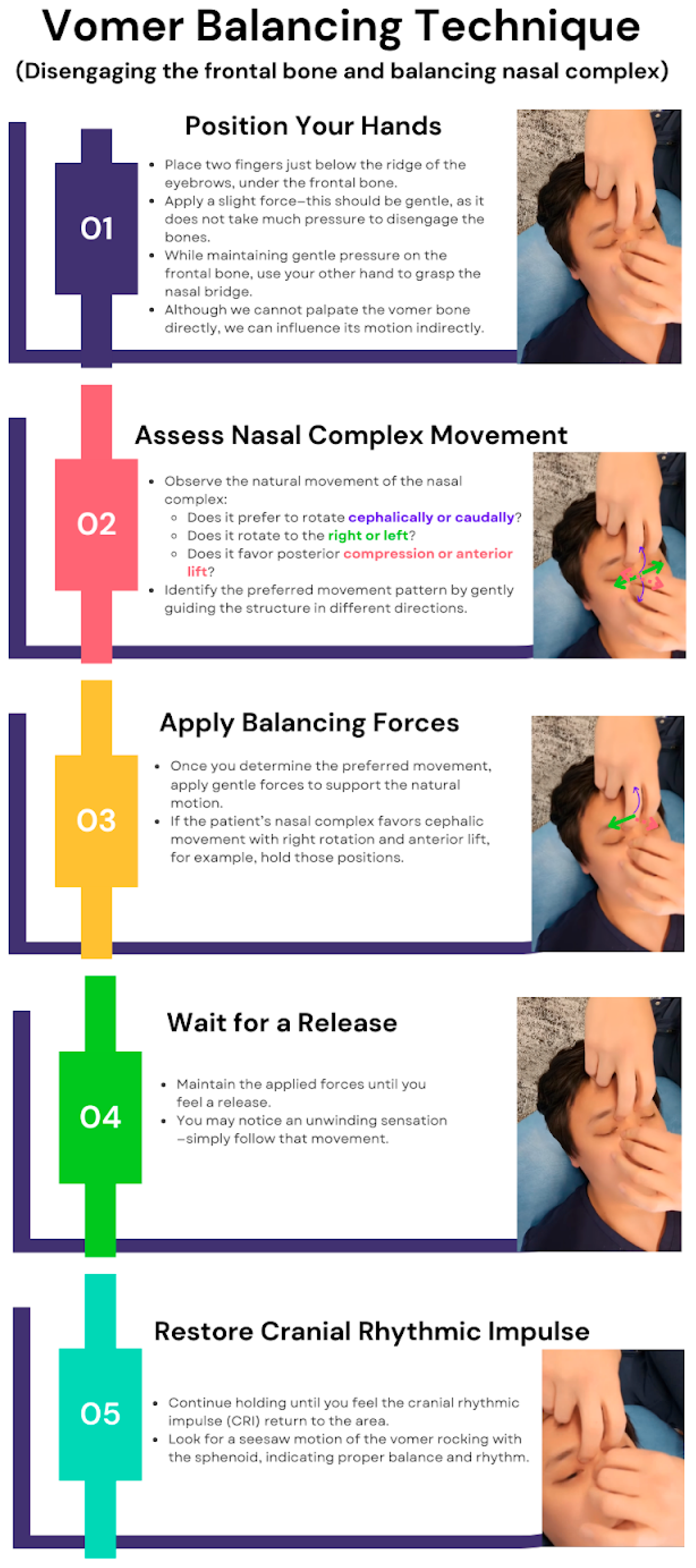
Vomer Balancing Technique
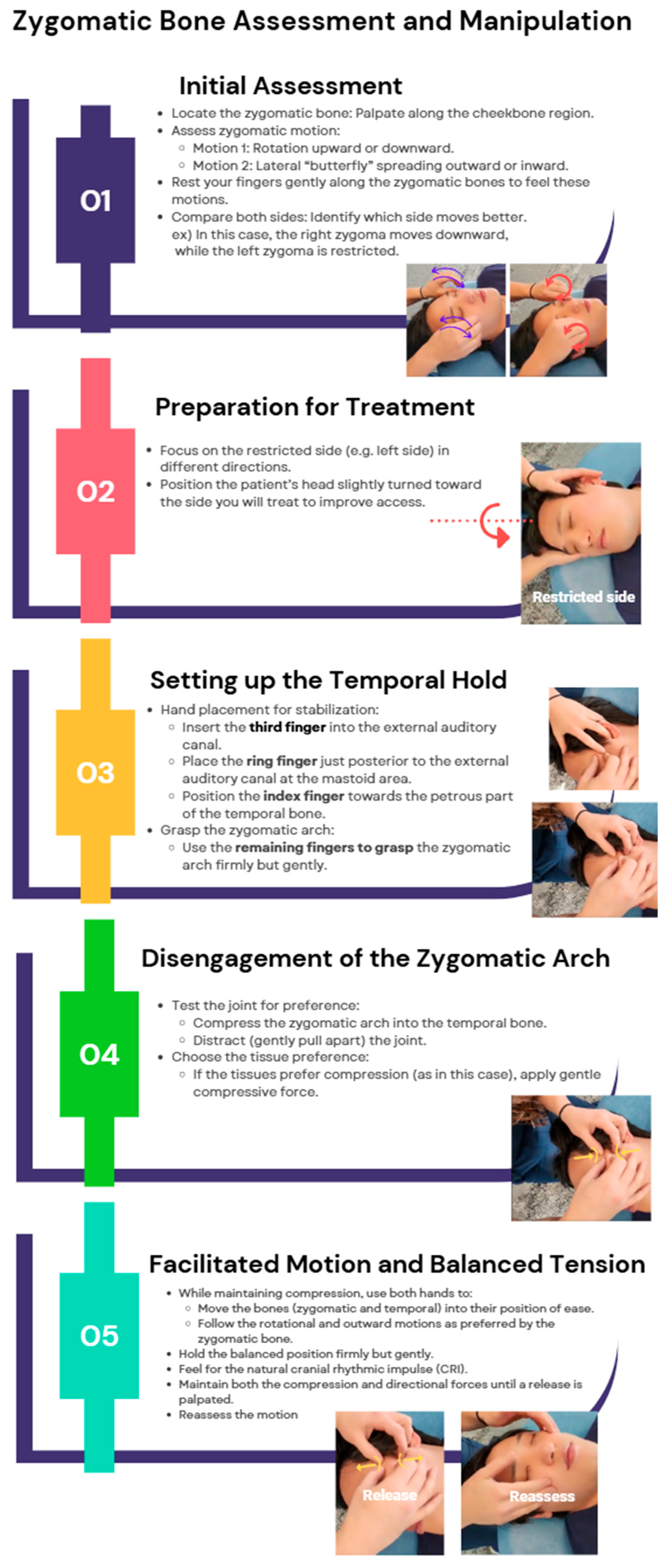
Zygomatic Bone Manipulation
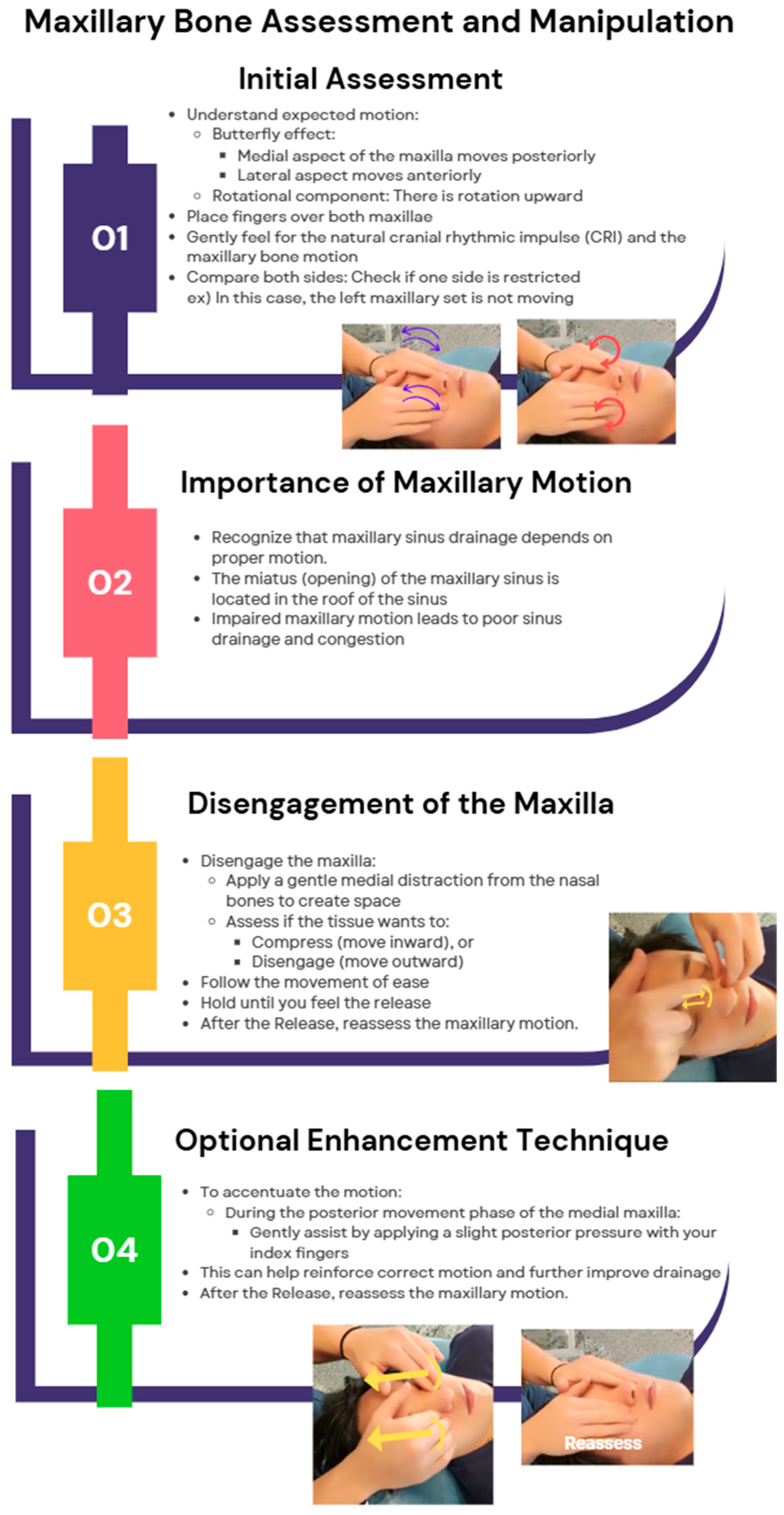
Maxillary Bone Manipulation
C2 and Cervical Manipulations
3.1.2. Osteopathic Manipulative Treatments for Pneumonia—Thoracic Soft Tissue Techniques
Doming the Diaphragm to Facilitate Diaphragmatic Motion and Rib Raising
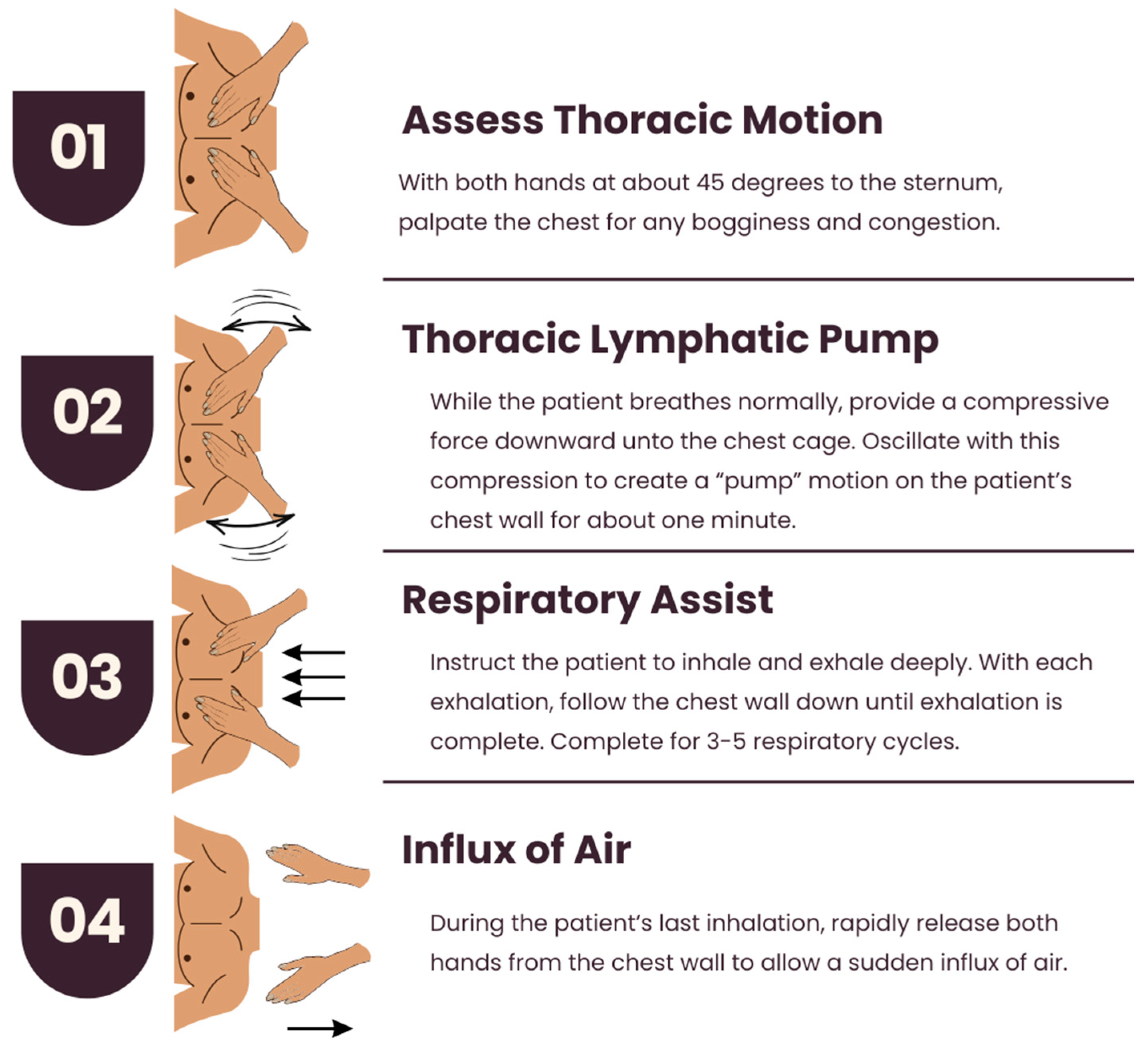
Thoracic Lymphatic Pump with Respiratory Assist
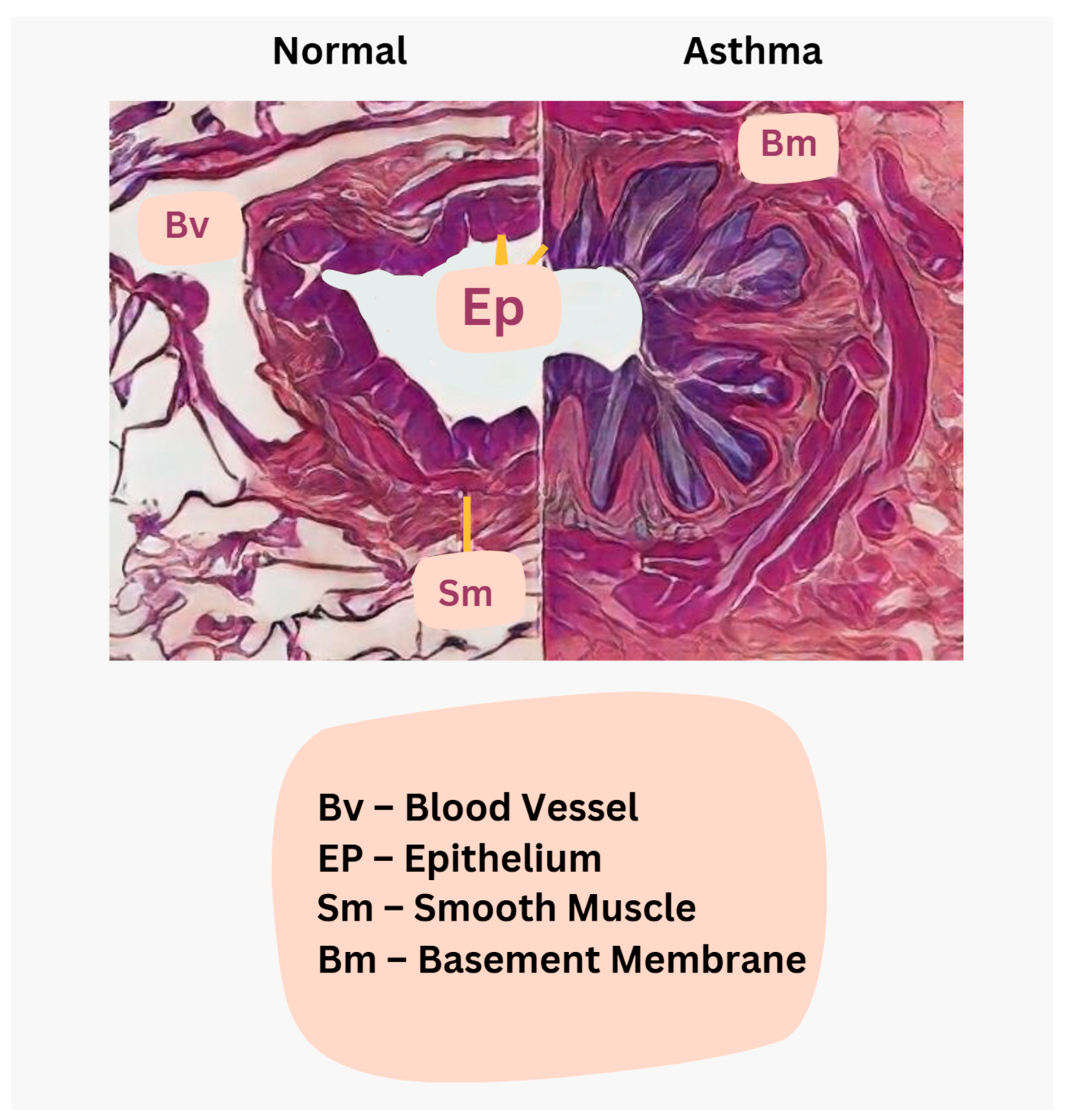
3.1.3. Osteopathic Manipulative Treatments for Asthma
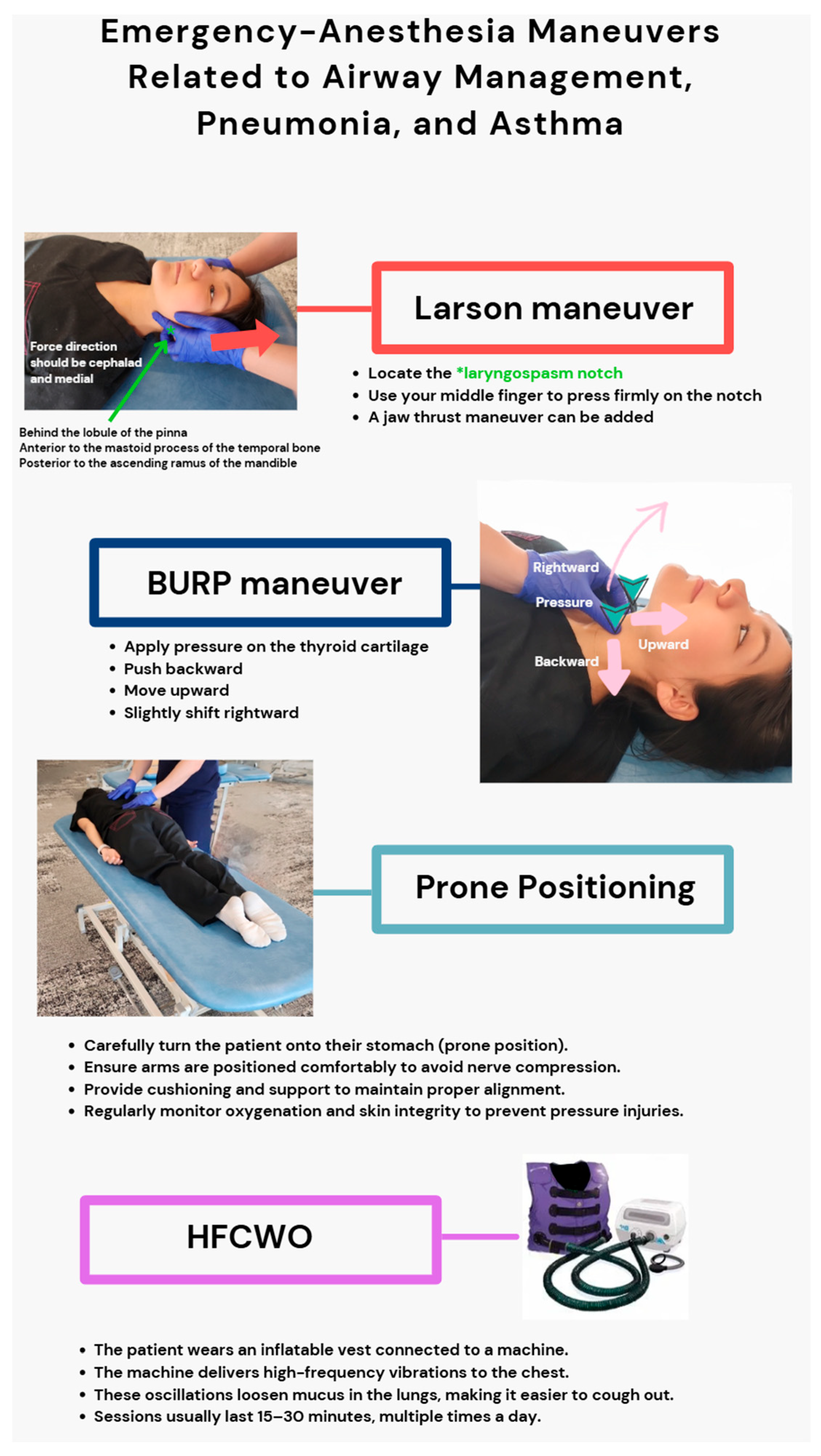
3.2. Emergency-Anesthesia Maneuvers Related to Airway Management, Pneumonia, and Asthma
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Atlantoaxial (Joint) |
| ARDS | Acute Respiratory Distress Syndrome |
| Bm | Basement Membrane |
| Bv | Blood Vessel |
| BURP | Backward, Upward, Rightward Pressure |
| CI | Confidence Interval |
| COPD | Chronic Obstructive Pulmonary Disease |
| cpm | Cycles Per Minute |
| CRI | Cranial Rhythmic Impulse |
| CR(s) | Chapman’s Reflex(es) |
| CRS | Chronic Rhinosinusitis |
| CSF | Cerebrospinal Fluid |
| CW | Clockwise |
| CCW | Counterclockwise |
| DO | Doctor of Osteopathy |
| Ep | Epithelium |
| FVC | Forced Vital Capacity |
| FEV1 | Forced Expiratory Volume in 1 Second |
| FVC/FEV1 | Ratio of Forced Vital Capacity to Forced Expiratory Volume in 1 Second |
| FEF 25–75% | Forced Expiratory Flow at 25–75% of Pulmonary Volume |
| HFCWO | High-Frequency Chest Wall Oscillation |
| HLVA | High-Velocity, Low-Amplitude |
| HRV | Heart Rate Variability |
| ICS | Intercostal Space |
| Ig | Immunoglobulin |
| IL | Interleukin |
| ILCs | Innate Lymphoid Cells |
| ME | Muscle Energy |
| MFR | Myofascial Release |
| OMT | Osteopathic Manipulative Treatment |
| OA | Occipitoatlantal (Joint) |
| OA-D | Occipitoatlantal Decompression |
| PRM | Primary Respiratory Mechanism |
| PFT | Pulmonary Function Test |
| QoL | Quality of Life |
| RCT | Randomized Controlled Trial |
| SD | Student Doctor |
| Sm | Smooth Muscle |
| T3 | Thoracic Vertebra 3 |
| Th | T Helper (cell) |
References
- Torres, A.; Blasi, F.; Dartois, N.; Akova, M. Which individuals are at increased risk of pneumococcal disease and why? Impact of COPD, asthma, smoking, diabetes, and/or chronic heart disease on community-acquired pneumonia and invasive pneumococcal disease: Table 1. Thorax 2015, 70, 984–989. [Google Scholar] [CrossRef]
- Akgün, K.M.; Crothers, K.; Pisani, M. Epidemiology and management of common pulmonary diseases in older persons. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 276–291. [Google Scholar] [CrossRef]
- Cutler, M.J.; Holland, B.S.; Stupski, B.A.; Gamber, R.G.; Smith, M.L. Cranial manipulation can alter sleep latency and sympathetic nerve activity in humans: A pilot study. J. Altern. Complement. Med. 2005, 11, 103–108. [Google Scholar] [CrossRef]
- An, H.J.; Kim, A.Y.; Park, S.J. Immediate Effects of Diaphragmatic Breathing with Cervical Spine Mobilization on the Pulmonary Function and Craniovertebral Angle in Patients with Chronic Stroke. Medicina 2021, 57, 826. [Google Scholar] [CrossRef]
- Noll, D.R.; Degenhardt, B.F.; Morley, T.F.; Blais, F.X.; Hortos, K.A.; Hensel, K.; Johnson, J.C.; Pasta, D.J.; Stoll, S.T. Efficacy of osteopathic manipulation as an adjunctive treatment for hospitalized patients with pneumonia: A randomized controlled trial. Osteopath. Med. Prim. Care 2010, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Chu, E.C.P.; Yun, S.; Huang, K.H.K. Cervicogenic Angina and Dyspnea Secondary to Cervical Radiculopathy. Cureus 2023, 15, e37515. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Arsalan, A.; Zafar, H.; Ahmad, A.; Hanif, A. Effects of breathing reeducation on cervical and pulmonary outcomes in patients with non specific chronic neck pain: A double blind randomized controlled trial. PLoS ONE 2022, 17, e0273471. [Google Scholar] [CrossRef] [PubMed]
- Bath, M.; Nguyen, A.; Bordoni, B. Physiology, Chapman’s Points. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Sukhera, J. Narrative Reviews: Flexible, Rigorous, and Practical. J. Grad. Med. Educ. 2022, 14, 414–417. [Google Scholar] [CrossRef]
- Sutherland, W.G. The cranial bowl. 1944. J. Osteopath. Med. 2000, 100, 568–573. Available online: https://www.researchgate.net/publication/12269791_The_cranial_bowl_1944 (accessed on 18 June 2025).
- Lintonbon, D. A Study to Investigate the Effect of “Frontal Lift” Osteopathic Manipulative Technique (OMT) in Patients with Chronic Sinusitis. Am. J. Biomed. Sci. Res. 2019, 3, 213–222. Available online: https://biomedgrid.com/fulltext/volume3/a-study-to-investigate-the-effect-of-frontal-lift-osteopathic-manipulative-technique.000665.php (accessed on 8 March 2025). [CrossRef]
- Lee-Wong, M.; Karagic, M.; Doshi, A.; Gomez, S.; Resnick, D. An Osteopathic Approach to Chronic Sinusitis. J. Allergy Ther. 2011, 2, 109. [Google Scholar] [CrossRef]
- Mehra, M.; Companion, N.; Yao, S.; Ventimiglia, M. The Effect of Osteopathic Manipulative Treatment on Acute Post COVID-19 Anosmia and Ageusia Symptoms: A Case Report. AAO J. 2024, 34, 10–14. [Google Scholar] [CrossRef]
- Baisakhiya, N. Efficacy of Osteopathic Manipulative Treatment in the Patient of Chronic Rhinosinusitis: A Case Report. Int. J. Clin. Rhinol. 2020, 11, 58–60. [Google Scholar] [CrossRef]
- Saini, S.; Singhal, S.; Prakash, S. Airway management in maxillofacial trauma. J. Anaesthesiol. Clin. Pharmacol. 2021, 37, 319–327. [Google Scholar] [CrossRef] [PubMed]
- King, H.H. Osteopathic Manipulation Shown to Improve Upper Airway Stabilization. J. Osteopath. Med. 2018, 118, 348–349. [Google Scholar] [CrossRef]
- Giles, P.D.; Hensel, K.L.; Pacchia, C.F.; Smith, M.L. Suboccipital Decompression Enhances Heart Rate Variability Indices of Cardiac Control in Healthy Subjects. J. Altern. Complement. Med. 2013, 19, 92–96. [Google Scholar] [CrossRef]
- Dalgleish, A.S.; Kania, A.M.; Stauss, H.M.; Jelen, A.Z. Occipitoatlantal decompression and noninvasive vagus nerve stimulation slow conduction velocity through the atrioventricular node in healthy participants. J. Osteopath. Med. 2021, 121, 349–359. [Google Scholar] [CrossRef]
- Rasmussen, T.R.; Meulengracht, K.C. Direct measurement of the rhythmic motions of the human head identifies a third rhythm. J. Bodyw. Mov. Ther. 2021, 26, 24–29. [Google Scholar] [CrossRef]
- Yao, S.; Hassani, J.; Gagne, M.; George, G.; Gilliar, W. Osteopathic Manipulative Treatment as a Useful Adjunctive Tool for Pneumonia. J. Vis. Exp. 2014, 50687. [Google Scholar] [CrossRef]
- Schend, J.; Rowane, M.; Sanan, N.; Hostoffer, S.R. An Osteopathic Modular Approach to Asthma: A Narrative Review. J. Osteopath. Med. 2020, 120, 774–782. [Google Scholar] [CrossRef]
- Jones, L.M.; Regan, C.; Wolf, K.; Bryant, J.; Rakowsky, A.; Pe, M.; Snyder, D.A. Effect of osteopathic manipulative treatment on pulmonary function testing in children with asthma. J. Osteopath. Med. 2021, 121, 589–596. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H. The immunology of asthma. Nat. Immunol. 2015, 16, 45–56. [Google Scholar] [CrossRef]
- Hough, K.P.; Curtiss, M.L.; Blain, T.J.; Liu, R.M.; Trevor, J.; Deshane, J.S.; Thannickal, V.J. Airway Remodeling in Asthma. Front. Med. 2020, 7, 191. [Google Scholar] [CrossRef] [PubMed]
- Strada, M.; Galli, M.; Cattaneo, R. Objective measurement of Cranial Rhythmic Impulse (CRI): Visual analysis of an observational case series. J. Bodyw. Mov. Ther. 2025, 44, 220–244. [Google Scholar] [CrossRef]
- Rowane, M.; Shankar, A.; Nagireddi, S.; Kalkat, A.; Hammes, C.; Kohli, E.; Callahan, M.; Hostoffer, R. The effect of osteopathic manipulative treatment on chronic rhinosinusitis. J. Osteopath. Med. 2025, 125, 299–304. [Google Scholar] [CrossRef]
- Stępnik, J.; Kędra, A.; Czaprowski, D. Short-term effect of osteopathic manual techniques (OMT) on respiratory function in healthy individuals. PLoS ONE 2020, 15, e0235308. [Google Scholar] [CrossRef] [PubMed]
- Zanotti, E.; Berardinelli, P.; Bizzarri, C.; Civardi, A.; Manstretta, A.; Rossetti, S.; Fracchia, C. Osteopathic manipulative treatment effectiveness in severe chronic obstructive pulmonary disease: A pilot study. Complement. Ther. Med. 2012, 20, 16–22. [Google Scholar] [CrossRef]
- Mériaux, F.; Stubbe, L.; Guyon, A. Physiological Mechanisms Underlying the Primary Respiratory Mechanism (PRM) and Cranial Rhythmic Impulse (CRI) in Osteopathy: A Systematic Review. Healthcare 2024, 12, 2503. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.S.; Witt, P.L. The Controversy of Cranial Bone Motion. J. Orthop. Sports Phys. Ther. 1997, 26, 95–103. [Google Scholar] [CrossRef]
- Hill, G.E.D.; Zundel, M.T.; Pagel, P.S. Larson’s Maneuver to Facilitate Endotracheal Intubation Using Videolaryngoscopy. J. Cardiothorac. Vasc. Anesth. 2019, 33, 883–884. [Google Scholar] [CrossRef]
- Yu, T.; Wu, R.R.; Longhini, F.; Wang, B.; Wang, M.F.; Yang, F.F.; Hua, F.Z.; Yao, W.D.; Jin, X.J. The “BURP” maneuver improves the glottic view during laryngoscopy but remains a difficult procedure. J. Int. Med. Res. 2020, 48, 0300060520925325. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Heo, S.K.; Cheon, S.U.; Ryu, S.A. The effects of backward, upward, rightward pressure maneuver for intubation using the OptiscopeTM: A retrospective study. Anesth. Pain. Med. 2021, 16, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Brusatori, S.; D’Albo, R.; Maj, R.; Velati, M.; Zinnato, C.; Gattarello, S.; Lombardo, F.; Fratti, I.; Romitti, F.; et al. Prone position: How understanding and clinical application of a technique progress with time. Anesthesiol. Perioper. Sci. 2023, 1, 3. [Google Scholar] [CrossRef]
- Harris, R.S.; Winkler, T.; Musch, G.; Vidal Melo, M.F.; Schroeder, T.; Tgavalekos, N.; Venegas, J.G. The prone position results in smaller ventilation defects during bronchoconstriction in asthma. J. Appl. Physiol. 2009, 107, 266–274. [Google Scholar] [CrossRef][Green Version]
- Bose, S.; Jun, J.; Diette, G.B. High-Frequency Chest Wall Oscillation Successful in Controlling Refractory Asthma. J. Asthma 2013, 50, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Chuang, M.L.; Chou, Y.L.; Lee, C.Y.; Huang, S.F. Instantaneous responses to high-frequency chest wall oscillation in patients with acute pneumonic respiratory failure receiving mechanical ventilation. Medicine 2017, 96, e5912. [Google Scholar] [CrossRef]
- Yan, Y.; Bao, J.; Cai, S.; Zhong, X.; Geng, B.; Liang, J.; Deng, Z.; Chen, Z.; Qin, Z.; Hu, H.; et al. The effects of prolonged prone positioning on response and prognosis in patients with acute respiratory distress syndrome: A retrospective cohort study. J. Intensive Care 2025, 13, 24. [Google Scholar] [CrossRef]

| Author (Year) | Technique Studied | Condition/Focus | Study Type | Key Outcomes |
|---|---|---|---|---|
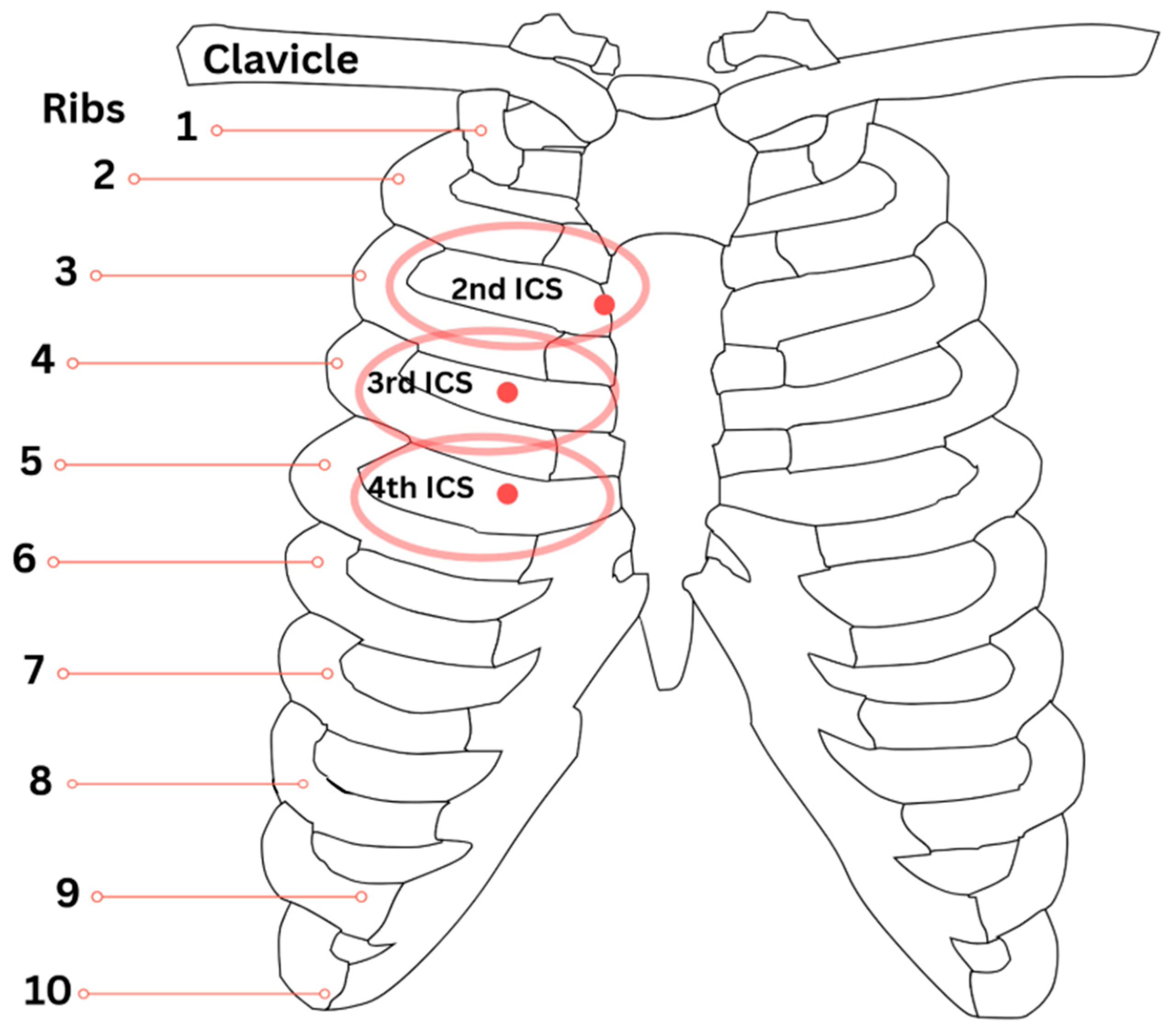
| Bath et al. (2023) [8] | Chapman’s Reflex Points | Sinus drainage, respiratory lymphatic support | Review | Described neuro-lymphatic reflexes; highlighted 2nd rib points for sinus drainage, 3rd and 4th ICS points for sinus and pulmonary drainage |
| Lintonbon (2019) [11] | Frontal lift OMT | Chronic Sinusitis | Case Study | Improved sinus drainage and cranial mobility |
| Lee-Wong et al. (2011) [12] | Sinus OMTs | Chronic Sinusitis | Observational | Statistically significant relief (p = 0.0012) |
| Mehra et al. (2024) [13] | OMT including vomer manipulation | Post-COVID anosmia & ageusia | Case Report | Sense of smell and taste improvement |
| Baisakhiya (2020) [14] | Vomer and Sinus manipulation | Chronic Rhinosinusitis (CRS) | Case Report | Improved nasal airflow, sinus drainage |
| Saini et al. (2021) [15] | Airway management in maxillofacial trauma | Maxillofacial trauma, airway stabilization | Review/Perspective | Facilitated airway management, facial structural alignment; no direct OMT technique evaluated |
| King (2018) [16] | OMT for upper airway stabilization | General airway function | Review/Perspective | Noted improvement in airway stability |
| Giles et al. (2013) [17] | Suboccipital decompression | Vagal tone, Heart Rate Variability (HRV) | RCT | Increased HRV, improved autonomic balance |
| Dalgleish et al. (2021) [18] | Occipitoatlantal decompression (OA-D) | Vagal tone modulation, cardiac autonomic control | RCT | Prolonged AV conduction and increased HRV, suggesting enhanced vagal tone after OA-D intervention |
| Rasmussen & Meulengracht (2021) [19] | Cranial rhythmic motion | Physiological mechanism | Biomechanical Study | Identified distinct cranial rhythm (~6.16 cpm), supports cranial osteopathy basis |
| Yao et al. (2014) [20] | Thoracic lymphatic pump, rib raising | Pneumonia | Experimental | Increased lymphatic flow, reduced congestion |
| Anwar et al. (2022) [7] | Breathing reeducation | Chronic neck pain & pulmonary outcomes | RCT | Improved cervical and pulmonary function |
| Chu et al. (2023) [6] | Cervical radiculopathy | Angina, dyspnea | Case Report | Linked cervical dysfunction to cardiopulmonary symptoms |
| Schend et al. (2020) [21] | Modular OMT techniques | Asthma management | Narrative Review | Outlined OMT principles, historical context, and practical techniques for asthma patients |
| Jones et al. (2021) [22] | Rib raising and suboccipital release | Pediatric asthma | RCT | Improved pulmonary function testing (PFT) values; however, changes not statistically significant |
| Lambrecht & Hammad (2015) [23] | Immunopathology of airway hypersensitivity | Asthma inflammation mechanisms | Review | Described Th2 and Th17 pathways, ILC2 cells, eosinophilic vs neutrophilic asthma heterogeneity |
| Hough et al. (2020) [24] | Epithelial-mesenchymal interactions | Asthmatic airway remodeling | Review | Described airway remodeling processes, matrix deposition, airway smooth muscle proliferation, inflammaging concept |
| Strada et al. (2025) [25] | Cranial dimensional motion | Cranial Rhythmic Impulse – systemic activity | Case Series | Subtle peripheral oscillations correlated with cardiopulmonary rhythms; CRI may reflect systemic physiological activity rather than cranial bone movement. |
| Rowane et al. (2024) [26] | Cranial and thoracic OMT | Chronic rhinosinusitis (CRS) | RCT | Statistically significant reduction in nasal congestion (p = 0.001), postnasal drainage (p = 0.002), and sinus pressure (p = 0.0004). |
| Stępnik et al. (2020) [27] | OMT techniques | Healthy subjects—pulmonary function | RCT | Increased peak expiratory flow (p < 0.001); no significant changes in FEV1 or FVC. |
| Zanotti et al. (2011) [28] | OMT adjunct to pulmonary rehab | Severe COPD | Pilot Study | Improved exercise capacity (+72.5 m, p = 0.01); decreased residual lung volume (–0.44 L, p = 0.001) vs. rehab alone. |
| Mériaux et al. (2024) [29] | Cranial rhythmic impulse (CRI) & PRM | PRM physiology and autonomic regulation | Systematic Review | Identified link between CRI, autonomic activity, and CSF flow; emphasized methodological heterogeneity across studies. |
| Sutherland (1939) [10] | Primary Respiratory Mechanism (PRM) | Osteopathic cranial model foundation | Foundational Educational Text | Introduced concept of cranial rhythmic motion and CSF dynamics forming a unified physiological system. |
| Rogers & Witt (1997) [30] | Cranial bone motion | Anatomical validity of cranial motion | Literature Review | Identified limited evidence for adult cranial bone mobility; called for more outcomes-based research. |
| Author (Year) | Technique Studied | Condition/Focus | Study Type | Key Outcomes |
|---|---|---|---|---|
| Hill et al. (2019) [31] | Larson’s maneuver | Laryngospasm during intubation | Case Technique Report | Improved intubation during laryngospasm |
| Yu et al. (2020) [32] | BURP maneuver | Difficult laryngoscopy/intubation | Observational Study | Reduced difficult laryngoscopy from 21.1% to 6.1% (p < 0.005) |
| Oh et al. (2021) [33] | BURP maneuver | Difficult intubation | Retrospective | Improved glottic visualization |
| Gattinoni et al. (2023) [34] | Prone positioning | * ARDS | Review | Improved oxygenation, reduced ventilation-perfusion mismatch |
| Harris et al. (2009) [35] | Prone positioning | Bronchoconstriction/Asthma | Experimental | Reduced ventilation defects, better lung expansion |
| Bose et al. (2013) [36] | * HFCWO | Refractory asthma | Case Study | Helped control symptoms not responsive to drugs |
| Chuang et al. (2017) [37] | * HFCWO | Pneumonia-induced respiratory failure | RCT | Improved secretion clearance and pulmonary mechanics |
| Yan et al. (2025) [38] | Prone positioning | * ARDS | Retrospective Cohort | Mortality benefit (HR 0.53; 95% CI 0.32–0.85; p = 0.033) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Benitez, L.; Hamanaka, A.; Abbas, G.H.; Faluade, E.; Pouwels, S.; Eller, J. Optimizing Airway Function Through Craniofacial and Cervical Manipulations and Emergency-Anesthesia Maneuvers: Applications in Airway Function Enhancement, Pneumonia, and Asthma—Narrative Review. J. Clin. Med. 2025, 14, 4494. https://doi.org/10.3390/jcm14134494
Park J, Benitez L, Hamanaka A, Abbas GH, Faluade E, Pouwels S, Eller J. Optimizing Airway Function Through Craniofacial and Cervical Manipulations and Emergency-Anesthesia Maneuvers: Applications in Airway Function Enhancement, Pneumonia, and Asthma—Narrative Review. Journal of Clinical Medicine. 2025; 14(13):4494. https://doi.org/10.3390/jcm14134494
Chicago/Turabian StylePark, Jason, Luz Benitez, Amethyst Hamanaka, Ghulam Husain Abbas, Emmanuel Faluade, Sjaak Pouwels, and Jamie Eller. 2025. "Optimizing Airway Function Through Craniofacial and Cervical Manipulations and Emergency-Anesthesia Maneuvers: Applications in Airway Function Enhancement, Pneumonia, and Asthma—Narrative Review" Journal of Clinical Medicine 14, no. 13: 4494. https://doi.org/10.3390/jcm14134494
APA StylePark, J., Benitez, L., Hamanaka, A., Abbas, G. H., Faluade, E., Pouwels, S., & Eller, J. (2025). Optimizing Airway Function Through Craniofacial and Cervical Manipulations and Emergency-Anesthesia Maneuvers: Applications in Airway Function Enhancement, Pneumonia, and Asthma—Narrative Review. Journal of Clinical Medicine, 14(13), 4494. https://doi.org/10.3390/jcm14134494








