Single Versus Bilateral Internal Thoracic Artery Grafting in Patients on Chronic Dialysis
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design and Population
2.2. Data Collection and Definitions
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Unmatched and Matched Cohorts
3.2. Early Outcomes
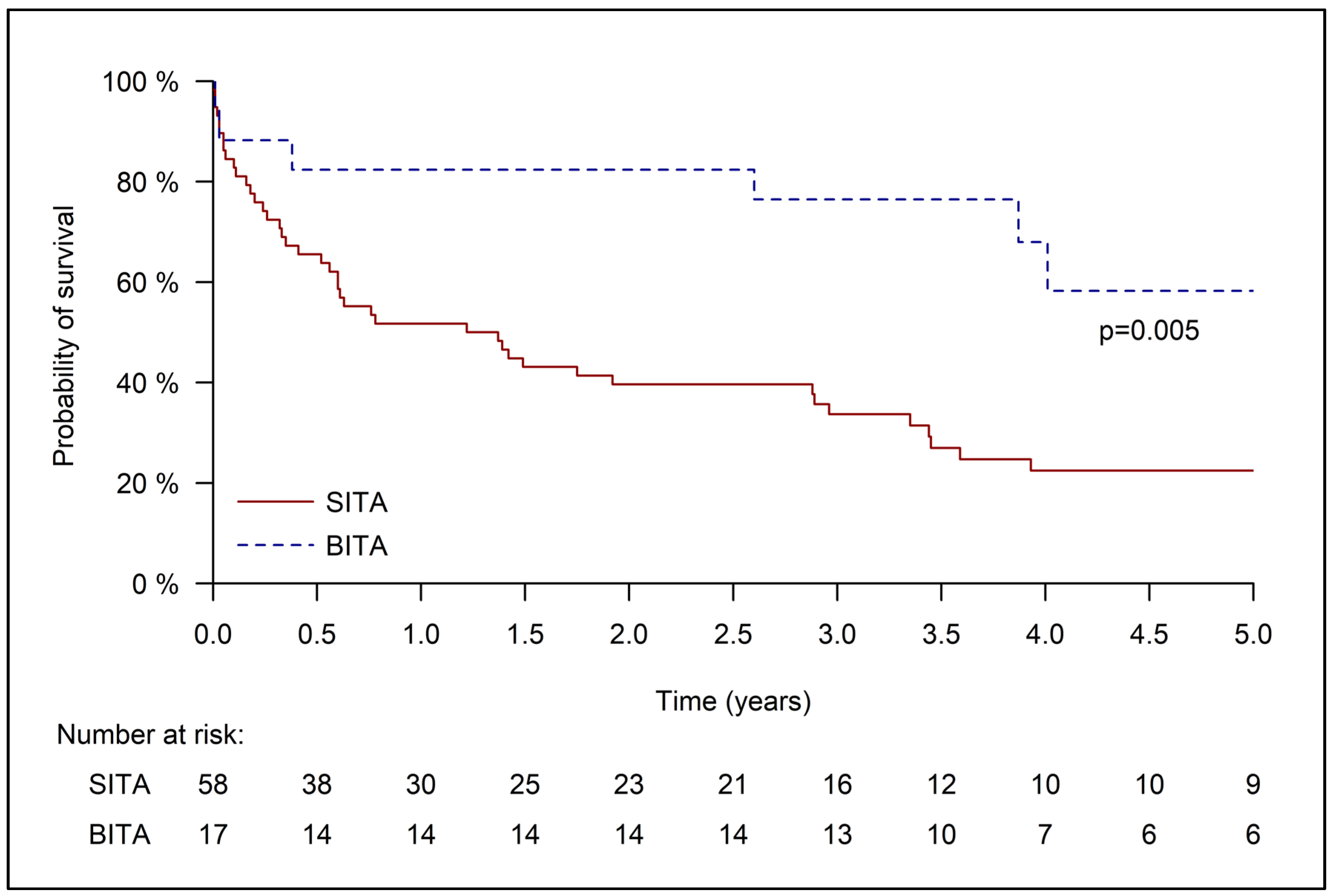
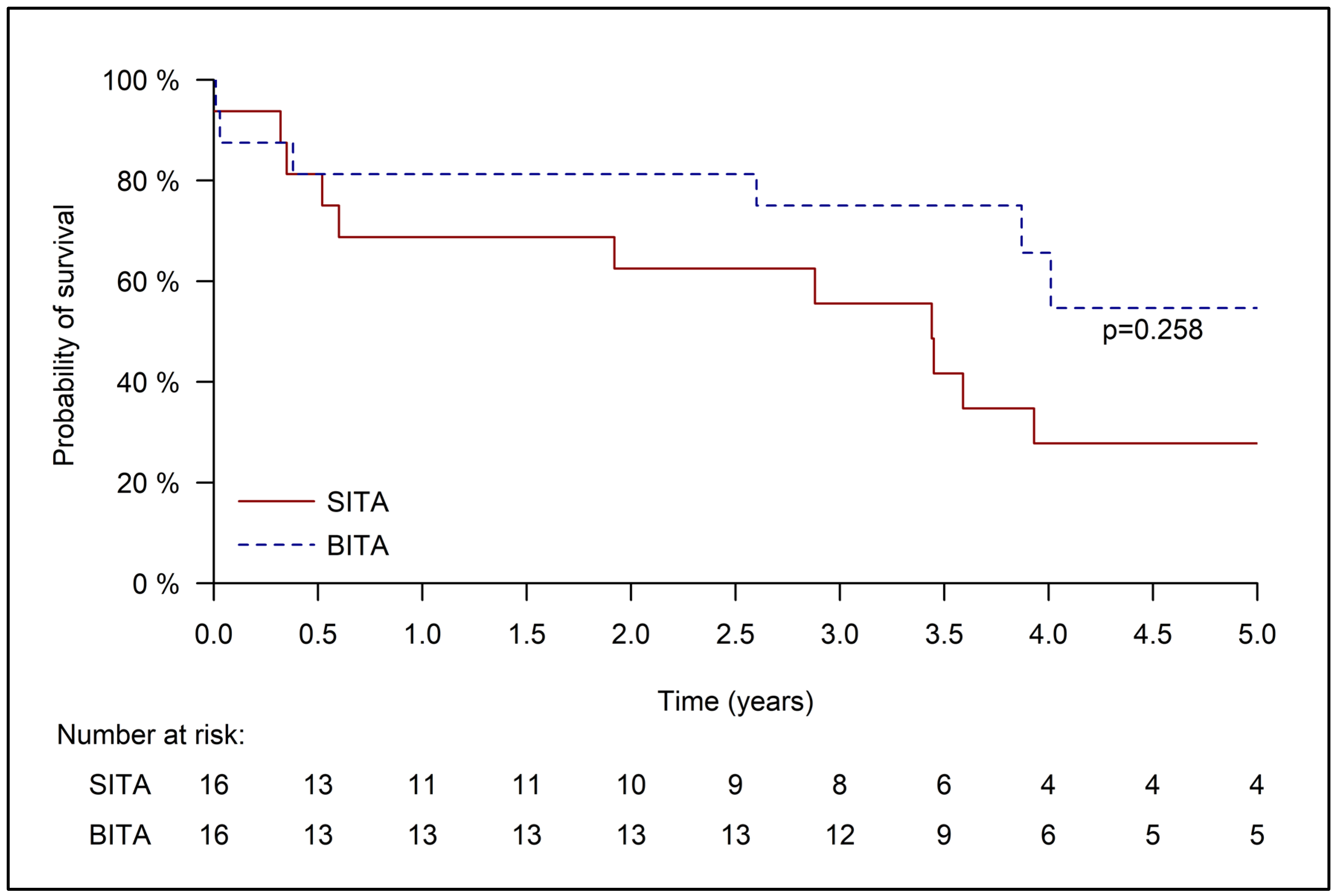
3.3. Late Outcomes
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SITA | Single internal thoracic artery |
| BITA | Bilateral internal thoracic artery |
| CABG | Coronary artery bypass graft surgery |
| ESRD | End stage renal disease |
References
- Johansen, K.L.; Chertow, G.M.; Foley, R.N.; Gilbertson, D.T.; Herzog, C.A.; Ishani, A.; Israni, A.K.; Ku, E.; Kurella Tamura, M.; Li, S.; et al. US Renal Data System 2020 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2021, 77 (Suppl. 1), A7–A8. [Google Scholar] [CrossRef] [PubMed]
- Wachterman, M.W.; O’Hare, A.M.; Rahman, O.K.; Lorenz, K.A.; Marcantonio, E.R.; Alicante, G.K.; Kelley, A.S. One-Year Mortality After Dialysis Initiation Among Older Adults. JAMA Intern. Med. 2019, 179, 987–990. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nishizawa, Y.; Koyama, H.; Inaba, M. Ages and cardiovascular diseases in patients with end-stage renal diseases. J. Ren. Nutr. 2012, 22, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Sarnak, M.J. Cardiovascular complications in chronic kidney disease. Am. J. Kidney Dis. 2003, 41 (Suppl. 5), 11–17. [Google Scholar] [CrossRef] [PubMed]
- Sarnak, M.J.; Amann, K.; Bangalore, S.; Cavalcante, J.L.; Charytan, D.M.; Craig, J.C.; Gill, J.S.; Hlatky, M.A.; Jardine, A.G.; Landmesser, U.; et al. Chronic Kidney Disease and Coronary Artery Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 74, 1823–1838. [Google Scholar] [CrossRef]
- Siddiqi, S.; Ravichandren, K.; Soltesz, E.G.; Johnston, D.R.; Roselli, E.E.; Tong, M.Z.; Navia, J.L.; Elgharably, H.; Ayyat, K.; Houghtaling, P.L.; et al. Coronary Artery Bypass Graft Patency and Survival in Patients on Dialysis. J. Surg. Res. 2020, 254, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, K.; Maeda, M.; Sakurai, H.; Nakayama, M.; Murayama, H.; Hasegawa, H. Cardiovascular surgery in patients on chronic dialysis: Effect of intraoperative hemodialysis. Interact. Cardiovasc. Thorac. Surg. 2004, 3, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Bahrainwala, J.Z.; Gelfand, S.L.; Shah, A.; Abramovitz, B.; Hoffman, B.; Leonberg-Yoo, A.K. Preoperative Risk Assessment and Management in Adults Receiving Maintenance Dialysis and Those With Earlier Stages of CKD. Am. J. Kidney Dis. 2020, 75, 245–255. [Google Scholar] [CrossRef]
- Cooper, W.A.; O’Brien, S.M.; Thourani, V.H.; Guyton, R.A.; Bridges, C.R.; Szczech, L.A.; Petersen, R.; Peterson, E.D. Impact of renal dysfunction on outcomes of coronary artery bypass surgery: Results from the Society of Thoracic Surgeons National Adult Cardiac Database. Circulation 2006, 113, 1063–1070. [Google Scholar] [CrossRef]
- Chikwe, J.; Castillo, J.G.; Rahmanian, P.B.; Akujuo, A.; Adams, D.H.; Filsoufi, F. The impact of moderate–to–end-stage renal failure on outcomes after coronary artery bypass graft surgery. J. Cardiothorac. Vasc. Anesth. 2010, 24, 574–579. [Google Scholar] [CrossRef]
- Roques, F.; Nashef, S.A.; Michel, P.; Gauducheau, E.; de Vincentiis, C.; Baudet, E.; Cortina, J.; David, M.; Faichney, A.; Gabrielle, F.; et al. Risk factors and outcome in European cardiac surgery: Analysis of the EuroSCORE multinational database of 19030 patients. Eur. J. Cardiothorac. Surg. 1999, 15, 816–822; discussion 822–823. [Google Scholar] [CrossRef] [PubMed]
- Nashef, S.A.; Roques, F.; Sharples, L.D.; Nilsson, J.; Smith, C.; Goldstone, A.R.; Lockowandt, U. EuroSCORE II. Eur. J. Cardio-Thorac. Surg. 2012, 41, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Hemmelgarn, B.R.; Southern, D.; Culleton, B.F.; Mitchell, L.B.; Knudtson, M.L.; Ghali, W.A.; Alberta Provincial Project for Outcomes Assessment in Coronary Heart Disease (APPROACH) Investigators. Survival after coronary revascularization among patients with kidney disease. Circulation 2004, 110, 1890–1895. [Google Scholar] [CrossRef] [PubMed]
- Herzog, C.A.; Ma, J.Z.; Collins, A.J. Comparative survival of dialysis patients in the United States after coronary angioplasty, coronary artery stenting, and coronary artery bypass surgery and impact of diabetes. Circulation 2002, 106, 2207–2211. [Google Scholar] [CrossRef] [PubMed]
- Taggart, D.P.; D’Amico, R.; Altman, D.G. Effect of arterial revascularisation on survival: A systematic review of studies comparing bilateral and single internal mammary arteries. Lancet 2001, 358, 870–875. [Google Scholar] [CrossRef]
- Buttar, S.N.; Yan, T.D.; Taggart, D.P.; Tian, D.H. Long-term and short-term outcomes of using bilateral internal mammary artery grafting versus left internal mammary artery grafting: A meta-analysis. Heart 2017, 103, 1419–1426. [Google Scholar] [CrossRef]
- Lytle, B.W.; Blackstone, E.H.; Sabik, J.F.; Houghtaling, P.; Loop, F.D.; Cosgrove, D.M. The effect of bilateral internal thoracic artery grafting on survival during 20 postoperative years. Ann. Thorac. Surg. 2004, 78, 2005–2012; discussion 2012–2014. [Google Scholar] [CrossRef]
- Kinoshita, T.; Asai, T.; Suzuki, T. Off-pump bilateral skeletonized internal thoracic artery grafting in patients with chronic kidney disease. J. Thorac. Cardiovasc. Surg. 2015, 150, 315–321.e3. [Google Scholar] [CrossRef]
- Farkash, A.; Gordon, A.; Mohr, R.; Sela, O.; Pevni, D.; Ziv Baran, T.; Grupper, A.; Kfir, J.E.; Ben-Gal, Y. Single versus bilateral internal thoracic artery grafting in patients with impaired renal function. PLoS ONE 2024, 19, e0297194. [Google Scholar] [CrossRef]
- Tam, D.Y.; Rahouma, M.; An, K.R.; Gaudino, M.F.L.; Karkhanis, R.; Fremes, S.E. Bilateral versus single internal thoracic artery for coronary artery bypass grafting with end-stage renal disease: A systematic review and meta-analysis. J. Card. Surg. 2019, 34, 196–201. [Google Scholar] [CrossRef]
- Hachiro, K.; Kinoshita, T.; Suzuki, T.; Asai, T. Bilateral Internal Thoracic Artery Grafting in Hemodialysis Patients. Circ. J. 2021, 85, 2004–2010. [Google Scholar] [CrossRef]
- Fertouk, M.; Gordon, A.; Pevni, D.; Ziv-Baran, T.; Sela, O.; Mohr, R.; Farkash, A.; Kramer, A.; Teich, N.; Nesher, N.; et al. Early and late outcomes of single versus bilateral internal thoracic artery revascularization for patients in critical condition. PLoS ONE 2021, 16, e0255740. [Google Scholar] [CrossRef] [PubMed]
- Farkash, A.; Pevni, D.; Mohr, R.; Kramer, A.; Ziv-Baran, T.; Paz, Y.; Nesher, N.; Ben-Gal, Y. Single versus bilateral internal thoracic artery grafting in patients with low ejection fraction. Medicine 2020, 99, e22842. [Google Scholar] [CrossRef]
- Pevni, D.; Mohr, R.; Lev-Ran, O.; Paz, Y.; Kramer, A.; Frolkis, I.; Shapira, I. Technical aspects of composite arterial grafting with double skeletonized internal thoracic arteries. Chest 2003, 123, 1348–1354. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pevni, D.; Nesher, N.; Kramer, A.; Paz, Y.; Farkash, A.; Ben-Gal, Y. Does bilateral versus single thoracic artery grafting provide survival benefit in female patients? Interact. Cardiovasc. Thorac. Surg. 2019, 28, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Gurevitch, J.; Kramer, A.; Locker, C.; Shapira, I.; Paz, Y.; Matsa, M.; Mohr, R. Technical aspects of double-skeletonized internal mammary artery grafting. Ann. Thorac. Surg. 2000, 69, 841–846. [Google Scholar] [CrossRef]
- Roy, P.; de Labriolle, A.; Hanna, N.; Bonello, L.; Okabe, T.; Pinto Slottow, T.L.; Steinberg, D.H.; Torguson, R.; Kaneshige, K.; Xue, Z.; et al. Requirement for emergent coronary artery bypass surgery following percutaneous coronary intervention in the stent era. Am. J. Cardiol. 2009, 103, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Radford, M.J.; Arnold, J.M.; Bennett, S.J.; Cinquegrani, M.P.; Cleland, J.G.; Havranek, E.P.; Heidenreich, P.A.; Rutherford, J.D.; Spertus, J.A.; Stevenson, L.W.; et al. ACC/AHA key data elements and definitions for measuring the clinical management and outcomes of patients with chronic heart failure: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Heart Failure Clinical Data Standards): Developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: Endorsed by the Heart Failure Society of America. Circulation 2005, 112, 1888–1916. [Google Scholar] [CrossRef] [PubMed]
- Maisch, B.; Seferović, P.M.; Ristić, A.D.; Erbel, R.; Rienmüller, R.; Adler, Y.; Tomkowski, W.Z.; Thiene, G.; Yacoub, M.H. Task Force on the Diagnosis and Management of Pricardial Diseases of the European Society of Cardiology. Guidelines on the diagnosis and management of pericardial diseases executive summary; The Task force on the diagnosis and management of pericardial diseases of the European society of cardiology. Eur. Heart J. 2004, 25, 587–610. [Google Scholar] [CrossRef] [PubMed]
- Jablonski, K.L.; Chonchol, M. Vascular calcification in end-stage renal disease. Hemodial. Int. 2013, 17 (Suppl. 1), S17–S21. [Google Scholar] [CrossRef]
- Nakayama, Y.; Sakata, R.; Ura, M. Bilateral internal thoracic artery use for dialysis patients: Does it increase operative risk? Ann Thorac. Surg. 2001, 71, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Munakata, H.; Tajima, K.; Kato, W.; Tanaka, K.; Tokuda, Y.; Mutsuga, M.; Usui, A. Bilateral versus single internal thoracic artery grafting in hemodialysis patients. Gen. Thorac. Cardiovasc. Surg. 2020, 68, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Kai, M.; Okabayashi, H.; Hanyu, M.; Soga, Y.; Nomoto, T.; Nakano, J.; Matsuo, T.; Umehara, E.; Kawato, M. Long-term results of bilateral internal thoracic artery grafting in dialysis patients. Ann. Thorac. Surg. 2007, 83, 1666–1671. [Google Scholar] [CrossRef] [PubMed]
- Gatti, G.; Perrotti, A.; Fiore, A.; Bergoend, E.; Chocron, S.; Couetil, J.P.; Sinagra, G.; Pappalardo, A. Is bilateral internal thoracic artery grafting a safe option for chronic dialysis patients? Arch. Cardiovasc. Dis. 2017, 110, 646–658. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, M.; Serricchio, M.; Luciani, N.; Giungi, S.; Salica, A.; Pola, R.; Pola, P.; Luciani, G.; Possati, G. Risks of using internal thoracic artery grafts in patients in chronic hemodialysis via upper extremity arteriovenous fistula. Circulation 2003, 107, 2653–2655. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, J.; Nakajima, H.; Asakura, T.; Iguchi, A.; Tokunaga, C.; Takazawa, A.; Chubachi, F.; Hori, Y.; Yoshitake, A. Validity of Ipsilateral Internal Mammary Coronary Artery Bypass Graft of Arteriovenous Fistula. Heart Lung Circ. 2022, 31, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Johansen, K.L.; Gilbertson, D.T.; Li, S.; Li, S.; Liu, J.; Roetker, N.S.; Ku, E.; Schulman, I.H.; Greer, R.C.; Chan, K.; et al. US Renal Data System 2023 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2024, 83 (Suppl. 1), A8–A13. [Google Scholar] [CrossRef] [PubMed]
| All | Unmatched Cohort n (%) | Matched Cohort n (%) | |||||
|---|---|---|---|---|---|---|---|
| SITA | BITA | SMD | SITA | BITA | SMD | ||
| n = 75 | n = 58 | n = 17 | n = 16 | n = 16 | |||
| Male | 59 (78.7%) | 45 (77.6%) | 14 (82.4%) | 0.119 | 16 (100%) | 14 (87.5%) | 0.535 |
| Age (years), mean (SD) | 65.69 (10.07) | 67.78 (9.69) | 58.56 (8.05) | 1.035 | 63.88 (10.95) | 59.43 (7.43) | 0.476 |
| Age ≥ 70 | 29 (38.7%) | 28 (48.3%) | 1 (5.9%) | 1.085 | 6 (37.5%) | 1 (6.3%) | 0.816 |
| NIDDM | 32 (42.7%) | 26 (44.8%) | 6 (35.3%) | 0.195 | 6 (37.5%) | 6 (37.5%) | 0.000 |
| IDDM | 17 (22.7%) | 14 (24.1%) | 3 (17.6%) | 0.160 | 6 (37.5%) | 2 (12.5%) | 0.603 |
| DM | 46 (61.3%) | 38 (65.5%) | 8 (47.1%) | 0.379 | 12 (75%) | 7 (43.8%) | 0.671 |
| COPD | 11 (14.7%) | 10 (17.2%) | 1 (5.9%) | 0.361 | 4 (25.0%) | 1 (6.3%) | 0.535 |
| CHF | 31 (41.3%) | 23 (39.7%) | 8 (47.1%) | 0.150 | 6 (37.5%) | 7 (43.8%) | 0.128 |
| Recent MI | 17 (22.7%) | 17 (29.3%) | 0 (0%) | 0.911 | 5 (31.3%) | 0 (0%) | 0.953 |
| Old MI | 23 (30.7%) | 19 (32.8%) | 4 (23.5%) | 0.206 | 6 (37.5%) | 4 (25.0%) | 0.272 |
| Acute MI | 20 (26.7%) | 15 (25.9%) | 5 (29.4%) | 0.079 | 3 (18.8%) | 5 (31.3%) | 0.292 |
| MI | 43 (57.3%) | 34 (58.6%) | 9 (52.9%) | 0.115 | 8 (50.0%) | 9 (56.3%) | 0.125 |
| Unstable angina pectoris | 62 (82.7%) | 48 (82.8%) | 14 (82.4%) | 0.011 | 12 (75.0%) | 13 (81.3%) | 0.152 |
| EF < 30% | 15 (20.0%) | 13 (22.4%) | 2 (11.8%) | 0.286 | 3 (18.8%) | 1 (6.3%) | 0.385 |
| Intra-aortic balloon pump | 5 (6.7%) | 4 (6.9%) | 1 (5.9%) | 0.041 | 1 (6.3%) | 1 (6.3%) | 0.000 |
| Emergent operation * | 33 (44.0%) | 25 (43.1%) | 8 (47.1%) | 0.080 | 2 (12.5%) | 7 (43.8%) | 0.741 |
| Redo | 1 (1.3%) | 0 (0%) | 1 (5.9%) | 0.354 | 0 (0%) | 1 (6.3%) | 0.365 |
| PVD | 26 (34.7%) | 24 (41.4%) | 2 (11.8%) | 0.712 | 7 (43.8%) | 2 (12.5%) | 0.741 |
| Left main disease | 25 (33.3%) | 17 (29.3%) | 8 (47.1%) | 0.372 | 3 (18.8%) | 8 (50.0%) | 0.697 |
| Prior PCI | 33 (44%) | 26 (44.83%) | 7 (41.18%) | 0.074 | 7 (43.8%) | 7 (43.8%) | 0.000 |
| EuroSCORE II, median (IQR) | 3.15 (1.78–6.23) | 3.73 (1.95–7.1) | 1.78 (1.38–3.5) | 0.934 | 1.89 (1.51–3.08) | 1.68 (1.37–3.13) | 0.123 |
| Bypass number ≥ 3 | 41 (54.67%) | 30 (51.72%) | 11 (64.71%) | 0.266 | 9 (56.3%) | 11 (68.8%) | 0.260 |
| Sequential anastomoses | 33 (44%) | 24 (41.4%) | 9 (52.9%) | 0.233 | 8 (50%) | 9 (56.3%) | 0.125 |
| SVG | 54 (72%) | 47 (81%) | 7 (41.2%) | 0.896 | 13 (81.3%) | 7 (43.8%) | 0.840 |
| GEA | 6 (8%) | 4 (6.9%) | 2 (11.8%) | 0.168 | 2 (12.5%) | 2 (12.5%) | 0.000 |
| Right system revascularization | 33 (44%) | 26 (44.8%) | 7 (41.2%) | 0.074 | 9 (56.3%) | 7 (43.8%) | 0.252 |
| OPCAB | 16 (21.3%) | 14 (24.1%) | 2 (11.8%) | 0.327 | 6 (37.5%) | 2 (12.5%) | 0.603 |
| Operated after the year 2015 | 36 (48%) | 28 (48.3%) | 8 (47.1%) | 0.024 | 6 (37.5%) | 7 (43.8%) | 0.128 |
| All | Unmatched Cohort n(%) | |||
|---|---|---|---|---|
| SITA | BITA | p Value | ||
| n = 75 | n = 58 | n = 17 | ||
| Early mortality | 11 (14.7%) | 9 (15.5%) | 2 (11.8%) | >0.999 |
| Deep infection | 4 (5.3%) | 4 (6.9%) | 0 (0%) | 0.568 |
| Post CVA | 5 (6.7%) | 5 (8.6%) | 0 (0%) | 0.582 |
| Perioperative MI | 3 (4.0%) | 2 (3.4%) | 1 (5.9%) | 0.543 |
| Revision for bleeding | 4 (5.3%) | 3 (5.2%) | 1 (5.9%) | >0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farkash, A.; Gordon, A.; Teich, N.; Sela, O.; Kakoush, M.; Ziv Baran, T.; Pevni, D.; Ben-Gal, Y. Single Versus Bilateral Internal Thoracic Artery Grafting in Patients on Chronic Dialysis. J. Clin. Med. 2025, 14, 4451. https://doi.org/10.3390/jcm14134451
Farkash A, Gordon A, Teich N, Sela O, Kakoush M, Ziv Baran T, Pevni D, Ben-Gal Y. Single Versus Bilateral Internal Thoracic Artery Grafting in Patients on Chronic Dialysis. Journal of Clinical Medicine. 2025; 14(13):4451. https://doi.org/10.3390/jcm14134451
Chicago/Turabian StyleFarkash, Ariel, Amit Gordon, Nadav Teich, Orr Sela, Mohammad Kakoush, Tomer Ziv Baran, Dmitry Pevni, and Yanai Ben-Gal. 2025. "Single Versus Bilateral Internal Thoracic Artery Grafting in Patients on Chronic Dialysis" Journal of Clinical Medicine 14, no. 13: 4451. https://doi.org/10.3390/jcm14134451
APA StyleFarkash, A., Gordon, A., Teich, N., Sela, O., Kakoush, M., Ziv Baran, T., Pevni, D., & Ben-Gal, Y. (2025). Single Versus Bilateral Internal Thoracic Artery Grafting in Patients on Chronic Dialysis. Journal of Clinical Medicine, 14(13), 4451. https://doi.org/10.3390/jcm14134451






